Abstract
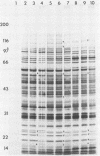
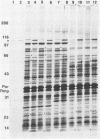
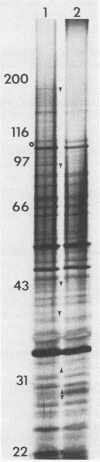
The immunoglobulin A1 (IgA1) proteases of Neisseria gonorrhoeae have been defined as having human IgA1 as their single permissive substrate. However, in recent years there have been reports of other proteins which are susceptible to the proteolytic activity of these enzymes. To examine the possibility that gonococcal membrane proteins are potential substrates for these enzymes, isolated outer and cytoplasmic membranes of N. gonorrhoeae were treated in vitro with exogenous pure IgA1 protease. Analysis of silver-stained sodium dodecyl sulfate-polyacrylamide gels of outer membranes indicated that there were two outer membrane proteins of 78 and 68 kDa which were cleaved by IgA1 protease in vitro in GCM 740 (a wild-type strain) and in two isogenic IgA1 protease-negative variants. Similar results were observed with a second gonococcal strain, F62, and its isogenic IgA1 protease-negative derivative. When GCM 740 cytoplasmic membranes were treated with protease, three minor proteins of 24.5, 23.5, and 21.5 kDa were cleaved. In addition, when outer membranes of Escherichia coli DH1 were treated with IgA1 protease, several proteins were hydrolyzed. While the identities of all of these proteolyzed proteins are unknown, the data presented indicate that there are several proteins found in the isolated membranes of gram-negative bacteria which are permissive in vitro substrates for gonococcal IgA1 protease.
Full text
PDF


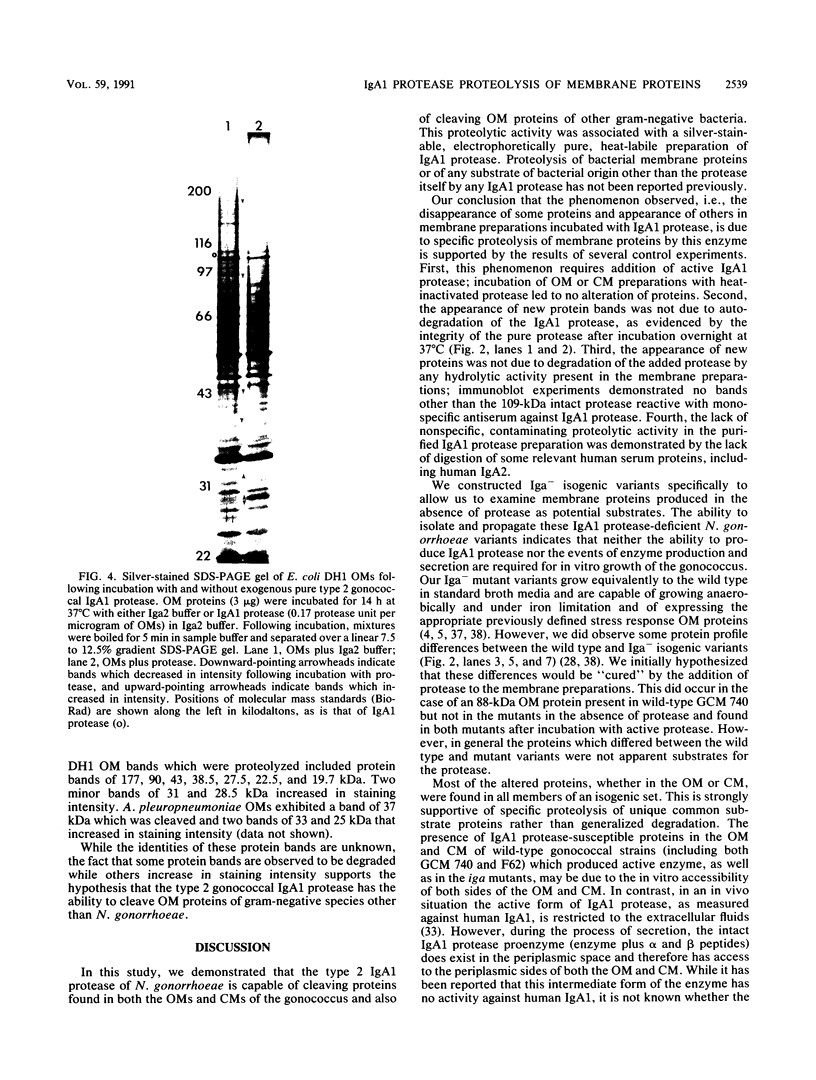


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bricker J., Mulks M. H., Plaut A. G., Moxon E. R., Wright A. IgA1 proteases of Haemophilus influenzae: cloning and characterization in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 May;80(9):2681–2685. doi: 10.1073/pnas.80.9.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Genco C. A., Rock J. P., Morse S. A. Physiology and metabolism of Neisseria gonorrhoeae and Neisseria meningitidis: implications for pathogenesis. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S35–S40. doi: 10.1128/cmr.2.suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Campbell L. A., Palermo D. A., Evans T. M., Klimpel K. W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect Immun. 1987 Jun;55(6):1359–1364. doi: 10.1128/iai.55.6.1359-1364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama Y., Kobayashi K., Senda S., Benno Y., Bamba T., Hosoda S. A novel IgA protease from Clostridium sp. capable of cleaving IgA1 and IgA2 A2m(1) but not IgA2 A2m(2) allotype paraproteins. J Immunol. 1985 Jan;134(1):573–576. [PubMed] [Google Scholar]
- Halter R., Pohlner J., Meyer T. F. IgA protease of Neisseria gonorrhoeae: isolation and characterization of the gene and its extracellular product. EMBO J. 1984 Jul;3(7):1595–1601. doi: 10.1002/j.1460-2075.1984.tb02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Unified nomenclature for pathogenic Neisseria species. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S64–S65. doi: 10.1128/cmr.2.suppl.s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Holmgren K. Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect Immun. 1981 Mar;31(3):868–873. doi: 10.1128/iai.31.3.868-873.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Russell M. W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988 Jun;52(2):296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Schrohenloher R. E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Gill R. E., Falkow S. Genetic and biochemical analysis of gonococcal IgA1 protease: cloning in Escherichia coli and construction of mutants of gonococci that fail to produce the activity. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7881–7885. doi: 10.1073/pnas.79.24.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Male C. J. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect Immun. 1979 Oct;26(1):254–261. doi: 10.1128/iai.26.1.254-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. K., Plaut A. G., Calvanico N. J., Tomasi T. B., Jr Human immunoglobulin A: production of an Fc fragment by an enteric microbial proteolytic enzyme. J Immunol. 1973 Oct;111(4):1274–1276. [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980 Jan;26(1):13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Mulks M. H., Kornfeld S. J., Frangione B., Plaut A. G. Relationship between the specificity of IgA proteases and serotypes in Haemophilus influenzae. J Infect Dis. 1982 Aug;146(2):266–274. doi: 10.1093/infdis/146.2.266. [DOI] [PubMed] [Google Scholar]
- Mulks M. H., Kornfeld S. J., Plaut A. G. Specific proteolysis of human IgA by Streptococcus pneumoniae and Haemophilus influenzae. J Infect Dis. 1980 Apr;141(4):450–456. doi: 10.1093/infdis/141.4.450. [DOI] [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G., Feldman H. A., Frangione B. IgA proteases of two distinct specificities are released by Neisseria meningitidis. J Exp Med. 1980 Nov 1;152(5):1442–1447. doi: 10.1084/jem.152.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulks M. H., Simpson D. A., Shoberg R. J. Restriction site polymorphism in genes encoding type 2 but not type 1 gonococcal IgA1 proteases. Antonie Van Leeuwenhoek. 1987;53(6):471–478. doi: 10.1007/BF00415505. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Heller I. Assay and properties of IgA protease of Streptococcus sanguis. Adv Exp Med Biol. 1978;107:489–495. doi: 10.1007/978-1-4684-3369-2_55. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Pohlner J., Halter R., Beyreuther K., Meyer T. F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. 1987 Jan 29-Feb 4Nature. 325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- Reinholdt J., Tomana M., Mortensen S. B., Kilian M. Molecular aspects of immunoglobulin A1 degradation by oral streptococci. Infect Immun. 1990 May;58(5):1186–1194. doi: 10.1128/iai.58.5.1186-1194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Stemler M. E., Stemke G. W. Immunoglobulin A protease activity of Ureaplasma urealyticum. J Clin Microbiol. 1984 Feb;19(2):255–258. doi: 10.1128/jcm.19.2.255-258.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. A., Hausinger R. P., Mulks M. H. Purification, characterization, and comparison of the immunoglobulin A1 proteases of Neisseria gonorrhoeae. J Bacteriol. 1988 Apr;170(4):1866–1873. doi: 10.1128/jb.170.4.1866-1873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Silver L. E., Clark V. L., Young F. E. Construction and characterization of a new shuttle vector, pLES2, capable of functioning in Escherichia coli and Neisseria gonorrhoeae. Gene. 1983 Nov;25(2-3):241–247. doi: 10.1016/0378-1119(83)90228-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]