Abstract
The lysin motif (LysM) is a ubiquitous protein module that binds peptidoglycan and structurally related molecules. Here, we used single-molecule force spectroscopy (SMFS) to measure and localize individual LysM-peptidoglycan interactions on both model and cellular surfaces. LysM modules of the major autolysin AcmA of Lactococcus lactis were bound to gold-coated atomic force microscopy tips, while peptidoglycan was covalently attached onto model supports. Multiple force curves recorded between the LysM tips and peptidoglycan surfaces yielded a bimodal distribution of binding forces, presumably reflecting the occurrence of one and two LysM-peptidoglycan interactions, respectively. The specificity of the measured interaction was confirmed by performing blocking experiments with free peptidoglycan. Next, the LysM tips were used to map single LysM interactions on the surfaces of L. lactis cells. Strikingly, native cells showed very poor binding, suggesting that peptidoglycan was hindered by other cell wall constituents. Consistent with this notion, treatment of the cells with trichloroacetic acid, which removes peptidoglycan-associated polymers, resulted in substantial and homogeneous binding of the LysM tip. These results provide novel insight into the binding forces of bacterial LysMs and show that SMFS is a promising tool for studying the heterologous display of proteins or peptides on bacterial surfaces.
The lysin motif (LysM), originally identified in enzymes that degrade bacterial cell walls (21), is present in many bacterial proteins, among which the well-characterized N-acetylglucosaminidase AcmA of Lactococcus lactis (7, 8), which binds in a noncovalent manner to the cell wall and is responsible for cell lysis. Several LysM-containing proteins are involved in bacterial pathogenesis and symbiosis. For instance, the major extracellular murease of Listeria monocytogenes, p60, which contains LysM modules, may be involved in bacterial adherence and in the invasion of non-professional phagocytic cells (12). Intimin, an outer membrane protein in enteropathogenic Escherichia coli, containing also a LysM motif, is involved in the attachment to mammalian cells (1). The protein A from Staphylococcus aureus, an immunoglobulin-binding protein, contains an immunoglobulin G-binding region at the N terminus and a LysM at the C terminus (16). LysM also appears to be a key motif in the recognition of symbiotic bacteria and fungi by plants (30). Many proteins contain only one LysM module, such as the prophage amidase XlyA of Bacillus subtilis (23), while others show several LysM repeats, such as the cell wall-bound γ-d-glutamate-meso-diaminopimelate muropeptidases LytE and LytF of B. subtilis (20, 24, 25, 29), muramidase-2 produced by Enterococcus hirae, and the major autolysin AcmA of L. lactis (32, 33).
There is increasing evidence supporting the notion that LysM is a general peptidoglycan-binding module (5, 8, 19, 20, 22-25, 29, 31-33). Partially purified muramidase-2 of E. hirae, a protein similar to AcmA and containing six LysM repeats, binds to peptidoglycan fragments of the same strain (22). The p60 protein of L. monocytogenes contains two LysM repeats and was shown to be associated with the cell surface (31). The γ-d-glutamate-meso-diaminopimelate muropeptidases LytE and LytF of B. subtilis have three and five repeats, respectively, in their N termini and are both cell wall bound (20, 24, 25, 29). Notably, Steen et al. (32) showed that the LysM domain of AcmA binds to many gram-positive bacteria with different peptidoglycan structures, including L. lactis, Enterococcus faecalis, Streptococcus thermophilus, B. subtilis, Lactobacillus sake, and Lactobacillus casei. The LysM domain showed similar affinity for both A-type and B-type peptidoglycan. As repetition of the disaccharide N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) is the only part common to both A-type and B-type peptidoglycan, LysM modules most likely bind to this component (8). Immunofluorescence microscopy revealed that the LysM domain interacts with specific loci on the cell surface (32). In addition, specific chemical treatments of cells and cell walls indicated that a cell wall component, extractable with trichloroacetic acid (TCA), is responsible for hindering LysM recognition, thereby causing this localized binding (32).
Despite the multiple important roles played by LysM domains (8), the forces and dynamics underlying their interaction with peptidoglycan as well as the spatial distribution of LysM binding sites on bacterial cell walls remain essentially unknown. Here, we used atomic force microscopy (AFM) (10) in the single-molecule force spectroscopy (SMFS) mode (18, 27) for exploring specific LysM-peptidoglycan interactions between AFM tips modified with the recombinant AcmA cell wall-binding domain (three LysM repeats) and model surfaces coated with peptidoglycan. Moreover, AcmA tips were used to detect and localize single LysM interactions on the surfaces of L. lactis cells. Major differences were found between native and TCA-treated cells, demonstrating that the binding of LysM to native L. lactis surfaces is hindered by cell surface constituents.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. lactis wild-type strain NZ3900(pNZ8048) (conferring resistance to chloramphenicol) was grown in M17 (Difco) medium at 30°C with the addition of 1% of glucose (Sigma, St Louis, MO) and 10 μg·ml−1 of chloramphenicol to avoid contamination. For cell experiments, bacteria were harvested by centrifugation from exponentially growing cultures (optical density at 600 nm, 0.3), washed three times with Tris-maleate (50 mM maleic acid, 40 mM Tris, pH 5.3), and mechanically immobilized onto porous polycarbonate membranes (Millipore, Billerica, MA).
Chemical treatment of the cells.
For some experiments, cells were treated with TCA as previously described (6, 32). Briefly, cells were harvested from exponentially growing culture by centrifugation, washed two times with Tris-maleate, resuspended in a 10% solution of TCA (Sigma), and boiled for 10 min. Treated cells were then washed three times with Tris-maleate and directly used for AFM experiments.
Preparation of LysM-modified supports and tips.
Recombinant AcmA cell wall-binding domains (PA3), purified as described before (6) and each bearing a cysteine residue near the extremity of its N-terminal end, were immobilized onto gold-coated supports and AFM tips. Silicon nitride AFM cantilevers (Microlevers; Veeco Metrology Group, Santa Barbara, CA) and silicon wafers (Siltronix, France) were coated, by thermal evaporation, with a 5-nm-thick Cr layer followed by a 30-nm-thick Au layer. Before use, gold-coated cantilevers and supports were cleaned for 5 min by UV-ozone treatment (Jelight Co., Irvine, CA), rinsed with ethanol, and dried with a gentle nitrogen flow. They were immersed overnight in phosphate-buffered saline (PBS) solutions (10 mM PBS, 150 mM NaCl, pH 7.4) containing 10 μg·ml−1 of recombinant peptide for 12 h, rinsed three times with PBS, and briefly sonicated to remove aggregates that may be adsorbed.
Preparation of peptidoglycan-modified surfaces.
Peptidoglycan solutions were prepared by dissolving peptidoglycan from B. subtilis (Fluka) in PBS solution at a concentration of 10 μg·ml−1 and sonicated for several minutes. Gold supports (see above) were immersed for 12 h in ethanol solution containing 1 mM of mercaptododecahexanoic acid (Sigma), rinsed three times with ethanol, and briefly sonicated. The supports were then immersed for 30 min into a solution containing 20 g/liter N-hydroxysuccinimide (NHS) and 50 g/liter 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) (Sigma), rinsed with MilliQ water (Millipore), incubated with 10 μg·ml−1 of peptidoglycan solution for 1 h, rinsed further with PBS, and then immediately used.
Surface characterization.
The chemical composition of the modified surfaces was assessed using X-ray photoelectron spectroscopy (XPS). Supports were rinsed with water and dried by flushing with a gentle nitrogen flow and then immediately introduced in the XPS vacuum chamber. The analyses were performed on a Kratos Axis Ultra spectrometer (Kratos Analytical, United Kingdom) equipped with a monochromatized aluminum X-ray source. The samples were fixed on a stainless steel multispecimen holder by using double-sided conductive tape. The angle between the normal to the sample surface and the electrostatic lens axis was 0°. The analyzed area was approximately 700 μm by 300 μm. The constant pass energy of the hemispherical analyzer was set at 40 eV. The following sequence of spectra was recorded: survey spectrum, C1s, N1s, O1s, Au4f, S2p, and C1s again to check the stability of charge compensation as a function of time and the absence of degradation of the sample during the analyses. The binding energies were calculated with respect to the C-(C,H) component of the C1s peak of adventitious carbon fixed at 284.8 eV. Following subtraction of a linear baseline, molar fractions were calculated (CasaXPS program; Casa Software Ltd., United Kingdom) using peak areas normalized on the basis of acquisition parameters, sensitivity factors, and the transmission function provided by the manufacturer.
AFM measurements.
AFM images and force-distance curves were obtained either in PBS (for supports) or in Tris-maleate (for cells) at room temperature using a Nanoscope IV multimode AFM (Veeco Metrology Group, Santa Barbara, CA). Native and treated cells were immobilized by mechanical trapping into porous polycarbonate membranes (Millipore) with a pore size similar to the bacterial cell size (10). After filtering a concentrated cell suspension, the filter was gently rinsed with PBS, carefully cut (sample size, 1 cm by 1 cm) and attached to a steel sample puck (Veeco Metrology Group) by use of a small piece of double-face adhesive tape, and the mounted sample was transferred into the AFM liquid cell while avoiding dewetting. All force curves were recorded with a maximum applied force of ∼450 pN. The spring constants of the cantilevers were measured using the thermal noise method (Picoforce, Veeco Metrology Group), yielding values (0.011 N/m) that were slightly larger than those announced by the manufacturer (0.01 N/m). Control experiments on the model surface were conducted by incubating tips in a solution of peptidoglycan (10 μg·ml−1) for 30 min and by injecting a peptidoglycan solution in the fluid cell. To account for the flexibility of the biomolecules, loading rates (pN·s−1) were estimated by multiplying the tip retraction velocity (nm·s−1) by the slope of the rupture peaks (pN·nm−1). Adhesion maps were obtained by recording 32-by-32 force-distance curves on areas of given size, calculating the adhesion force for each force curve and displaying the value as a gray pixel.
RESULTS AND DISCUSSION
Surface modifications for preparing AFM tips and supports.
The C-terminal region of AcmA contains three LysM modules of 45 amino acid residues which bind specifically to peptidoglycan. Peptidoglycan is composed of glycan strands with a disaccharide repeating unit of GlcNAc and MurNAc, cross-linked via pentapeptides of various compositions (9). Binding of AcmA LysM repeats to many different bacteria, including L. lactis and B. subtilis, has been observed, suggesting that LysM modules recognize a common glycan part, presumably GlcNAc, in all peptidoglycans (8, 32, 33).
To measure LysM-peptidoglycan interactions, AcmA LysM modules terminated with cysteine residues (Fig. 1A) were attached onto gold-coated AFM tips (Fig. 1B), while peptidoglycan from B. subtilis was covalently attached onto carboxyl-terminated surfaces via NHS/EDC chemistry (Fig. 1B). The chemical composition of the functionalized surfaces was assessed using XPS. Table 1 presents the XPS data obtained for gold supports prior to and after LysM and peptidoglycan immobilization. After incubation with LysM peptides, the gold samples showed a large increase of the nitrogen concentration, indicating the presence of a significant amount of peptide at the surface. Following treatment with COOH-terminated alkanethiols, the gold surfaces showed significant oxygen, sulfur, and nitrogen concentrations, consistent with the presence of an alkanethiol monolayer. Treatment with NHS/EDC and peptidoglycan led to an increase of the nitrogen, the oxygen, and the carbon concentration, essentially reflecting the presence of covalently bond peptidoglycan.
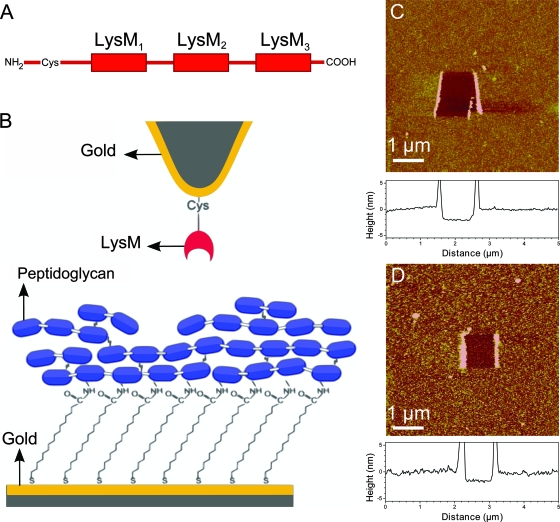
FIG. 1.
Strategy for measuring the LysM-peptidoglycan interaction forces by use of AFM. (A) Schematic representation of the recombinant AcmA cell wall-binding domain. (B) Schematics of the surface chemistry used to functionalize AFM tips and supports with LysM and peptidoglycan. AcmA LysM modules terminated with cysteine residues were attached onto gold-coated AFM tips, while peptidoglycan was covalently attached onto carboxyl-terminated surfaces via NHS/EDC chemistry. The blue boxes represent the GlcNAc and MurNAc disaccharide repeating units of peptidoglycan and are cross-linked by pentapeptides. (C and D) AFM images, and cross-sections taken in the middle of the images (graphs below images), of the biologically modified supports in PBS, confirming the presence of smooth, homogeneous LysM (C) and peptidoglycan (D) layers. To determine the layer thicknesses, small square areas were first scanned under large forces (>10 nN), and this was followed by recording 5-μm by 5-μm images of the same areas under smaller forces.
TABLE 1.
Surface chemical composition of solid supports functionalized with either LysM peptides or peptidoglycan
| Sample | Mole fraction (%) fora:
|
||||
|---|---|---|---|---|---|
| Au | C | S | O | N | |
| Au | 47.4 | 43.7 | /b | 8.5 | /b |
| Au/LysMc | 27.9 | 46.1 | /b | 14.5 | 11.6 |
| Au/COOHd | 36.5 | 54.5 | 1.8 | 6.6 | 0.13 |
| Au/COOH/PGe | 30.5 | 57.5 | 1.3 | 8.0 | 2.7 |
Mole fractions of elements excluding hydrogen.
/, below the detection limit.
Au/LysM, AcmA cell wall binding domain immobilized on a gold-coated surface.
Au/COOH, mercaptododecahexanoic acid on a gold-coated surface.
Au/COOH/PG, peptidoglycan immobilized on Au/COOH via NHS/EDC chemistry.
The modified supports were further characterized using AFM topographic imaging in aqueous solution. Figure 1C and D show that, respectively, the LysM and peptidoglycan surfaces were rather smooth (root mean square roughness values on 1-μm by 1-μm areas of 0.5 ± 0.01 nm and 1.6 ± 0.1 nm, respectively) and stable upon repeated scanning. To confirm the presence of peptide layers, a small area was first recorded at large forces (>10 nN) for short periods of time, followed by imaging a larger image of the same area under normal load. Figure 1C and D show that imaging at high forces resulted in the grafted material being pushed aside, thereby revealing the underlying support. The thicknesses of the removed films were found to be 2.2 ± 0.1 nm and 1.9 ± 0.2 nm, confirming the presence of LysM and peptidoglycan layers, respectively, on the surface.
Forces and dynamics of the LysM-peptidoglycan interaction.
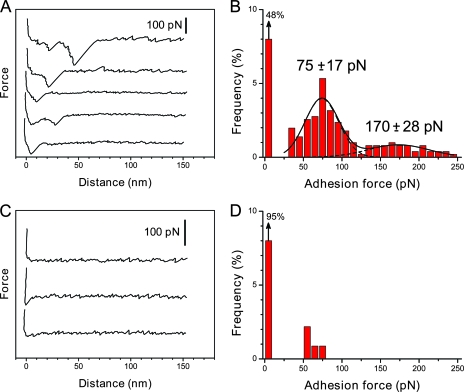
Having validated the functionalization strategies, the LysM-peptidoglycan binding forces were measured by recording force-distance curves between LysM-terminated tip and peptidoglycan-terminated support at a loading rate of 6,700 pN·s−1 (Fig. 2). As shown in Fig. 2A and B, 52% of a total of 750 curves displayed single or multiple binding forces, with the remaining curves exhibiting no adhesion. The corresponding histogram of binding forces showed two maxima centered at 75 ± 17 pN and 170 ± 28 pN. Several observations suggest that the 75-pN binding force reflects the rupture of a single LysM-peptidoglycan complex. First, the specificity of the interaction was confirmed by showing a dramatic reduction of adhesion probability when the same experiment was performed with a silicon nitride tip (data not shown) or in a solution containing 10 μg·ml−1 of peptidoglycan (Fig. 2C and D). Second, the observation of two maxima at 75 and 170 pN in the histogram suggests that the value of ∼75 pN corresponds to the adhesion strength quantum between individual molecules. Third, this value is in the range of those obtained at fairly comparable loading rates for other receptor-ligand complexes (18).
FIG. 2.
Force spectroscopy of the LysM-peptidoglycan interaction. (A and B) Representative retraction force curves (A) and adhesion force histograms (n = 750) (B) measured in PBS between a LysM tip and a peptidoglycan surface. Data were obtained using five independent samples and eight different tips. All curves were obtained using a retraction speed of 1,000 nm·s−1. (C and D) Force curves (C) and adhesion force histograms (n = 250) (B) obtained after the injection of free peptidoglycan (10 μg·ml−1).
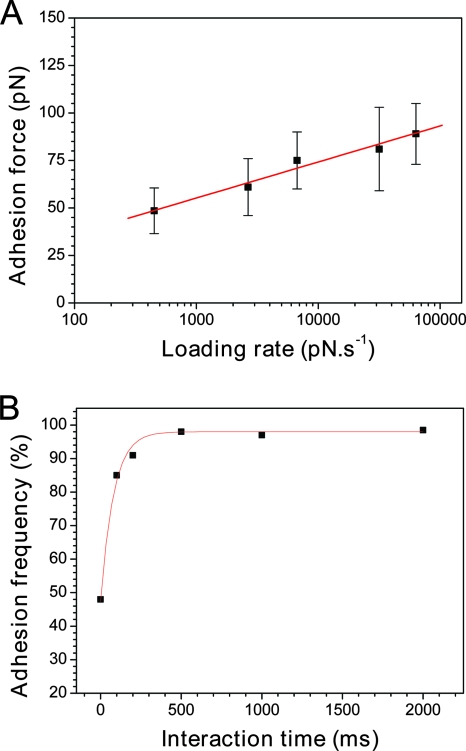
Unbinding forces between receptors and ligands measured at constant pulling rates represent only a single point in a continuous spectrum of bond strengths, since these depend on the rate at which the load is applied (4, 13, 14, 18, 26, 28). We therefore explored the dynamics of the LysM-peptidoglycan interaction by measuring the unbinding force as a function of the loading rate (dynamic force spectroscopy). Figure 3A shows that the mean adhesion force (F) increased linearly with the logarithm of the loading rate (r), as observed for other receptor-ligand systems (13-15, 26). Kinetic parameters on the unbinding process were extracted from these dynamic measurements (18). First, the length scale of the energy barrier was obtained from the fβ slope of the F versus ln(r) plot. This slope represents the characteristic force scale for a specific energy barrier and is defined as the ratio of thermal energy (kBT) (∼4.1 pN nm at room temperature) to the projected bond displacement, xβ, along the direction of the applied force. A slope of 8.2 pN was found, yielding a length scale of the energy barrier xβ of approximately 0.5 nm. Second, extrapolation to zero forces yielded the kinetic off-rate constant of dissociation at zero force, koff = rF = 0 xβ/kBT = 0.15 s−1. Note that given the error bars and distribution of the data points, the uncertainty on this extrapolation is fairly large, meaning the obtained koff value should be taken only as a rough estimate.
FIG. 3.
Dynamics of the LysM-peptidoglycan interaction. (A) Dependence of the adhesion force on the loading rate applied during retraction (mean ± standard error of the mean), measured between a LysM tip and a peptidoglycan surface at a constant approach speed (1,000 nm·s−1). (B) Dependence of the adhesion frequency on the interaction time, measured at a constant approach and a retracting speed of 1,000 nm·s−1.
The interaction time is another parameter that may affect specific biomolecular forces. For instance, the adhesion probabilities of cadherin, bacterial adhesin, and vancomycin interactions were shown to increase dramatically with interaction time (2, 3, 11, 15). To determine whether this applies to the LysM-peptidoglycan interaction, we varied the interaction time while keeping the loading rate constant (Fig. 3B). Interestingly, we found that the adhesion frequency increased exponentially with contact time to reach a plateau after only 0.2 to 0.5 s. This value, similar to that of the cadherin system (0.2 s) (2, 3), is much smaller than those of the adhesin (11) and vancomycin interactions (2 to 5 s) (15), indicating that the formation of the LysM-peptidoglycan bond is relatively fast. Such a fast binding may play a crucial role in defining the biological function of the L. lactis AcmA. The peptidoglycan binding and catalytic activity of AcmA are known to increase with the number of LysM repeats to up to three, suggesting that LysM is needed to properly position the active-site domain(s) toward their substrate (33). Moreover, the peptidoglycan binding mechanism could allow “scooting” of the enzyme along its polymeric substrate. It is therefore tempting to speculate that the fast formation of LysM-peptidoglycan bonds is important to ensure optimum catalytic activity and thus cell wall hydrolysis.
From these data, we can estimate the interaction time needed for a half-maximal probability of binding (t0.5) of 5.5 × 10−3 s, and, in turn, the association rate constant, kon, of t0.5−1 NA Veff equal to 2.4 103 M−1 s−1, where Veff is the effective volume explored by the tip-tethered LysM, approximated here to a half-sphere of 2.2-nm radius (17), and NA is Avogadro's constant. Here again, we note that the accuracy of the estimated t0.5 and kon values is rather limited and should be considered with caution. Nevertheless, considering the above rate constant values, a rough estimate of the equilibrium dissociation constant can be obtained, namely, KD equals koff/kon equals 61 μM, which is on the order of that estimated for cadherin-cadherin interactions (2, 3). Taken together, the above data indicate that the LysM domain binds to peptidoglycan with high specificity and affinity.
Detection of single LysM-peptidoglycan interactions on L. lactis.
Immunofluorescence microscopy studies have revealed that peptidoglycan of L. lactis and other gram-positive bacteria bind the AcmA LysM domain (8, 32). With this in mind, we used LysM tips to probe the surfaces of L. lactis cells (Fig. 4 and 5). Bacteria were immobilized in porous polymer filters, a method allowing AFM analysis of living cells while preserving their native macromolecular architecture. Topographic images of living L. lactis cells (Fig. 4C) revealed a smooth morphology, consistent with an earlier report (15). Interestingly, most force curves recorded either over the cell surface or over the filter with a LysM tip did not show any binding events (Fig. 5C and E). The very poor binding on the native L. lactis surface suggests that peptidoglycan is hindered by other cell wall constituents. These AFM data differ from earlier immunofluorescence results in that the latter showed some binding of the LysM domain on very specific locations of L. lactis (32). Presumably, this behavior reflects differences in the probing depths of the two techniques: while fluorescence microscopy probes peptidoglycan from the entire cell wall, AFM detects only those peptidoglycan molecules exposed on the outermost cell surface.
FIG. 4.
AFM imaging of single Lactococcus lactis cells, either in the native state or after treatment with TCA. (A) Schematic representation of the cell wall of L. lactis. Abbreviations: TA, teichoic acids; PR, proteins; PS, polysaccharides; PG, peptidoglycan; PM, plasma membrane. (B) Schematic of the cell wall after treatment with TCA, which is expected to remove peptidoglycan-associated polymers. (C) AFM deflection image in Tris-maleate buffer showing two dividing L. lactis cells trapped into a porous polymer filter for in situ imaging. (D) AFM deflection image in Tris-maleate buffer showing dividing L. lactis cells after treatment with TCA.
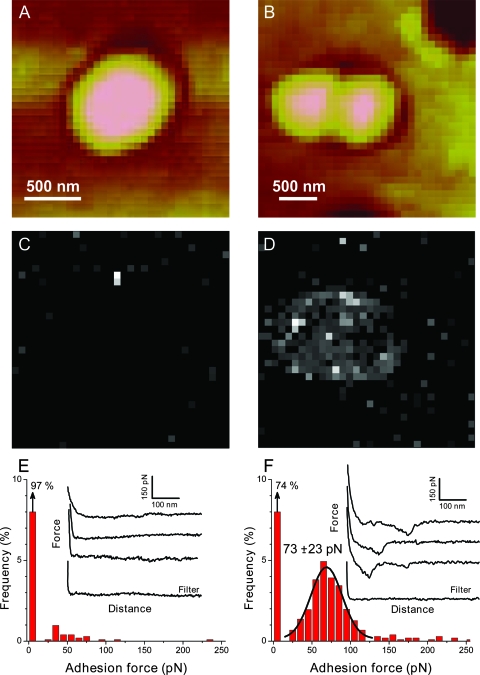
FIG. 5.
Detecting single LysM-peptidoglycan interactions on L. lactis cells. (A and B) Low-resolution AFM images with Tris-maleate buffer obtained for native (A) and TCA-treated (B) L. lactis cells. (C to F) Adhesion force maps (gray scale, 300 pN) (C and D) and adhesion force histograms (n = 1,024) (E and F) together with representative retraction force curves recorded with a LysM tip on the native (C and E) and TCA-treated (D and F) cell surfaces by use of a constant retraction speed (1,000 nm/s). The bright pixels, observed frequently on top of the treated cell but very rarely on the native cell or on the filter, document substantial binding of the LysM tip due to the exposure of peptidoglycan. Approach and retraction force curves were similar near the contact region.
To support the hypothesis that peptidoglycan in native L. lactis cells is indeed hindered by other cell wall constituents, we performed similar experiments following treatment of the cells with TCA, which is believed to remove peptidoglycan-associated polymers (32) (Fig. 4B). As shown in Fig. 4D, TCA-treated cells could be imaged by AFM by use of the same immobilization procedure as for native cells. Although the cell surface was slightly rougher, it could be imaged repeatedly without significant alteration by the scanning tip. Notably, most force curves recorded with a LysM tip on the treated cell surface showed single or multiple binding forces (Fig. 5D and 5F), the resulting force distribution being reminiscent of that observed for the model peptidoglycan surfaces (Fig. 2B). The observed force distribution (Fig. 5F) showed a single well-pronounced maximum at 73 ± 23 pN; thus, it was rather close to the force values found for model surfaces. This strongly suggests that the measured 73-pN forces originate from specific LysM-peptidoglycan interactions and therefore provides direct evidence that peptidoglycan is homogeneously exposed on TCA-treated cells. These data also demonstrate that cell wall components, presumably teichoic acids (32), hinder peptidoglycan on the surfaces of L. lactis cells.
In summary, using SMFS (i) we measured the specific binding forces of single AcmA LysM-peptidoglycan interactions on model surfaces, thereby providing an estimate of association and dissociation rate constants; and (ii) we detected these interactions on L. lactis cell walls. Clearly, it would be most interesting in future research to further explore the force and dynamics of LysM interactions using other purified peptidoglycans and other bacterial strains. A crucial question is to clarify whether other moieties besides GlcNAc are also recognized (8), which could be achieved by SMFS using various possible peptidoglycan-based substrates. SMFS experiments could also help our understanding of how the number of LysM repeats in AcmA modulates its binding forces, since this factor is known to affect the binding efficiency and, consequently, the in vivo activity of the enzyme (33). In addition to providing novel insight into the structure-function relationships of bacterial surfaces, these single-molecule analyses may find promising applications for studying the heterologous display of proteins or peptides. Indeed, Bosma et al. (6) recently proposed a novel display system that allows a highly efficient immobilization of heterologous proteins on L. lactis surfaces. Nonliving gram-positive enhancer matrix particles were obtained from gram-positive bacterial cells by use of TCA treatment, e.g., and then used as substrates to bind externally added heterologous proteins by means of the high-affinity LysM binding domains. Hence, AFM may be powerful method in future research for assessing the quality of such novel surface display systems.
Acknowledgments
This work was supported by the National Foundation for Scientific Research (FNRS), the Foundation for Training in Industrial and Agricultural Research (FRIA), the Université catholique de Louvain (Fonds Spéciaux de Recherche), the Federal Office for Scientific, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme), and the Research Department of the Communauté française de Belgique (Concerted Research Action). Y.F.D. and P.H. are research associates of the FNRS.
Footnotes
Published ahead of print on 29 August 2008.
REFERENCES
- 1.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 2991113-1119. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner, W., H. Gruber, P. Hinterdorfer, and D. Drenckhahn. 2000. Affinity of trans-interacting VE-cadherin determined by atomic force microscopy. Single Mol. 1119-122. [Google Scholar]
- 3.Baumgartner, W., P. Hinterdorfer, W. Ness, A. Raab, D. Vestweber, H. Schindler, and D. Drenckhahn. 2000. Cadherin interaction probed by atomic force microscopy. Proc. Natl. Acad. Sci. USA 974005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, G. I. 1978. Models for the specific adhesion of cells to cells. Science 200618-627. [DOI] [PubMed] [Google Scholar]
- 5.Birkeland, N. K. 1994. Cloning, molecular characterization, and expression of the genes encoding the lytic functions of lactococcal bacteriophage-Phi-Lc3—a dual lysis system of modular design. Can. J. Microbiol. 40658-665. [DOI] [PubMed] [Google Scholar]
- 6.Bosma, T., R. Kanninga, J. Neef, S. A. L. Audouy, M. L. van Roosmalen, A. Steen, G. Buist, J. Kok, O. P. Kuipers, G. Robillard, and K. Leenhouts. 2006. Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl. Environ. Microbiol. 72880-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buist, G., J. Kok, K. J. Leenhouts, M. Dabrowska, G. Venema, and A. J. Haandrikman. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 1771554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buist, G., A. Steen, J. Kok, and O. P. Kuipers. 2008. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol. Microbiol. 68838-847. [DOI] [PubMed] [Google Scholar]
- 9.Delcour, J., T. Ferain, M. Deghorain, E. Palumbo, and P. Hols. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie van Leeuwenhoek 76159-184. [PubMed] [Google Scholar]
- 10.Dufrêne, Y. F. 2004. Using nanotechniques to explore microbial surfaces. Nat. Rev. Microbiol. 2451-460. [DOI] [PubMed] [Google Scholar]
- 11.Dupres, V., F. D. Menozzi, C. Locht, B. H. Clare, N. L. Abbott, S. Cuenot, C. Bompard, D. Raze, and Y. F. Dufrene. 2005. Nanoscale mapping and functional analysis of individual adhesins on living bacteria. Nat. Methods 2515-520. [DOI] [PubMed] [Google Scholar]
- 12.Dussurget, O., J. Pizarro-Cerda, and P. Cossart. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58587-610. [DOI] [PubMed] [Google Scholar]
- 13.Evans, E., and K. Ritchie. 1997. Dynamic strength of molecular adhesion bonds. Biophys. J. 721541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz, J., A. G. Katopodis, F. Kolbinger, and D. Anselmetti. 1998. Force-mediated kinetics of single P-selectin/ligand complexes observed by atomic force microscopy. Proc. Natl. Acad. Sci. USA 9512283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert, Y., M. Deghorain, L. Wang, B. Xu, P. D. Pollheimer, H. J. Gruber, J. Errington, B. Hallet, X. Haulot, C. Verbelen, P. Hols, and Y. F. Dufrêne. 2007. Single-molecule force spectroscopy and imaging of the vancomycin/d-Ala-d-Ala interaction. Nano Lett. 7796-801. [DOI] [PubMed] [Google Scholar]
- 16.Goward, C. R., M. D. Scawen, J. P. Murphy, and T. Atkinson. 1993. Molecular evolution of bacterial cell-surface proteins. Trends Biochem. Sci. 18136-140. [DOI] [PubMed] [Google Scholar]
- 17.Hinterdorfer, P., W. Baumgartner, H. J. Gruber, K. Schilcher, and H. Schindler. 1996. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc. Natl. Acad. Sci. USA 933477-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinterdorfer, P., and Y. F. Dufrêne. 2006. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods 3347-355. [DOI] [PubMed] [Google Scholar]
- 19.Hourdou, M. L., M. Guinand, M. J. Vacheron, G. Michel, L. Denoroy, C. Duez, S. Englebert, B. Joris, G. Weber, and J. M. Ghuysen. 1993. Characterization of the sporulation-related γ-d-glutamyl-(L)meso-diaminopimelic-acid-hydrolyzing peptidase-I of Bacillus sphaericus Nctc-9602 as a member of the metallo(zinc) carboxypeptidase A family—modular design of the protein. Biochem. J. 292563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa, S., Y. Hara, R. Ohnishi, and J. Sekiguchi. 1998. Regulation of a new cell wall hydrolase gene, cwlF, which affects cell separation in Bacillus subtilis. J. Bacteriol. 1802549-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joris, B., S. Englebert, C. P. Chu, R. Kariyama, L. Daneo-Moore, G. D. Shockman, and J. M. Ghuysen. 1992. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus faecalis autolysin. FEMS Microbiol. Lett. 91257-264. [DOI] [PubMed] [Google Scholar]
- 22.Kariyama, R., and G. D. Shockman. 1992. Extracellular and cellular distribution of muramidase-2 and muramidase-1 of Enterococcus hirae ATCC 9790. J. Bacteriol. 1743236-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longchamp, P. F., C. Mauel, and D. Karamata. 1994. Lytic enzymes associated with defective prophages of Bacillus subtilis: sequencing and characterization of the region comprising the N-acetylmuramoyl-l-alanine amidase gene of prophage PBSX. Microbiology 1401855-1867. [DOI] [PubMed] [Google Scholar]
- 24.Margot, P., M. Wahlen, A. Gholamhuseinian, P. Piggot, and D. Karamata. 1998. The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase. J. Bacteriol. 180749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margot, P., M. Pagni, and D. Karamata. 1999. Bacillus subtilis 168 gene lytF encodes a γ-d-glutamate-meso-diaminopimelate muropeptidase expressed by the alternative vegetative sigma factor, σd. Microbiology 14557-65. [DOI] [PubMed] [Google Scholar]
- 26.Merkel, R., P. Nassoy, A. Leung, K. Ritchie, and E. Evans. 1999. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 39750-53. [DOI] [PubMed] [Google Scholar]
- 27.Müller, D. J., and Y. F. Dufrêne. 2008. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nat. Nanotechnol. 3261-269. [DOI] [PubMed] [Google Scholar]
- 28.Nevo, R., C. Stroh, F. Kienberger, D. Kaftan, V. Brumfeld, M. Elbaum, Z. Reich, and P. Hinterdorfer. 2003. A molecular switch between alternative conformational states in the complex of Ran and importin β 1. Nat. Struct. Biol. 10553-557. [DOI] [PubMed] [Google Scholar]
- 29.Ohnishi, R., S. Ishikawa, and J. Sekiguchi. 1999. Peptidoglycan hydrolase LytF plays a role in cell separation with CwlF during vegetative growth of Bacillus subtilis. J. Bacteriol. 1813178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radutoiu, S., L. H. Madsen, E. B. Madsen, H. H. Felle, Y. Umehara, M. Gronlund, S. Sato, Y. Nakamura, S. Tabata, N. Sandal, and J. Stougaard. 2003. Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425585-592. [DOI] [PubMed] [Google Scholar]
- 31.Ruhland, G. J., M. Hellwig, G. Wanner, and F. Fiedler. 1993. Cell-surface location of Listeria-specific protein P60. Detection of Listeria cells by indirect immunofluorescence. J. Gen. Microbiol. 139609-616. [DOI] [PubMed] [Google Scholar]
- 32.Steen, A., G. Buist, K. J. Leenhouts, M. E. Khattabi, F. Grijpstra, A. L. Zomer, G. Venema, O. P. Kuipers, and J. Kok. 2003. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J. Biol. Chem. 27823874-23881. [DOI] [PubMed] [Google Scholar]
- 33.Steen, A., G. Buist, G. J. Horsburgh, G. Venema, O. P. Kuipers, S. J. Foster, and J. Kok. 2005. AcmA of Lactococcus lactis is an N-acetylglucosaminidase with an optimal number of LysM domains for proper functioning. FEBS J. 2722854-2868. [DOI] [PubMed] [Google Scholar]