Abstract
Exceptional progress has been made during the past two decades in identifying genes causing inherited retinal diseases such as retinitis pigmentosa. An inescapable consequence is that the relationship between genes, mutations, and clinical findings has become very complex. Success in identifying the causes of inherited retinal diseases has many implications, including a better understanding of the biological basis of vision and insights into the processes involved in retinal pathology. From a clinical point of view, there are two important questions arising from these developments: where do we stand today in finding disease-causing mutations in affected individuals, and what are the implications of this information for clinical practice? This perspective addresses these questions specifically for retinitis pigmentosa, but the observations apply generally to other forms of inherited eye disease.
The goal of this perspective is to summarize the current state of the molecular diagnosis of retinitis pigmentosa (RP) and its relevance to clinical practice. The comments are limited largely to nonsyndromic, nonsystemic forms of RP, using autosomal dominant RP (adRP) as an example. It is important to recognize, though, that what is true for simple RP is true in general for most other forms of inherited retinal degeneration. There has been rapid progress in identifying genes and mutations causing all forms of retinal disease, including multifactorial diseases such as age-related macular degeneration. Of course, the specific genes are different and the clinical findings are distinct, but the implications for clinical practice are similar. For example, what is true for RP alone is also true for Usher syndrome, Bardet-Biedl syndrome, and familial macular degeneration. That is, many genes and mutations are also known for these diseases and have relevance to clinical practice. A list of genes causing RP and other retinopathies can be found at the RetNet Web site.1 A number of recent reviews address the biological bases of RP.2-5
Retinitis pigmentosa encompasses many different diseases with many distinct causes and diverse biological pathways but with overlapping symptoms and similar consequences.4 It is no more a single disease than is “fever of unknown origin.” If one word more than any other comes to mind in describing RP, it is complicated. There are dominant, recessive, and X-linked forms of inheritance in addition to rare mitochondrial and digenic forms. Retinitis pigmentosa may occur alone or as part of a more complex syndrome. Even simple RP is strikingly complicated. Each genetic type is caused by mutations in several or many different genes. For most genes, many different mutations with similar consequences are known, yet other mutations in the same gene may cause different diseases. Perhaps most surprisingly, the same mutation in different individuals may cause distinctly different symptoms, even among individuals within the same family.
Ironically, the great success in identifying genes and mutations causing RP during the past 2 decades has revealed the extent of the complexity but also offers hope of taming it—by defining RP at a molecular level rather than clinically. Still, in spite of the progress in genetics, a careful clinical description is and will be an essential prerequisite for molecular diagnosis. Further, the molecular description of RP is intrinsically complicated. Molecular diagnosis alone will therefore neither replace clinical testing nor fully resolve the complexity. Nonetheless, clinical testing coupled with molecular diagnosis of RP is a powerful combination of approaches for diagnosing patients and families and will eventually lead to treatment and prevention.
SUMMARY OF GENES AND MUTATIONS CAUSING RP
Retinitis pigmentosa is a class of diseases involving progressive degeneration of the retina, typically starting in the midperiphery and advancing toward the macula and fovea.6 Typical symptoms include night blindness followed by decreasing visual fields, leading to tunnel vision and eventually legal blindness or, in many cases, complete blindness. Clinical hallmarks are an abnormal fundus with bone-spicule deposits and attenuated retinal vessels; abnormal, diminished, or absent electroretinographic findings; and reduced visual fields. Symptoms typically start in the early teenage years, and severe visual impairment occurs by ages 40 to 50 years. However, there are early-onset forms of RP (the earliest is indistinguishable from Leber congenital amaurosis [LCA]) and other late-onset or even nonpenetrant forms. The underlying genetic cause is a useful predictor of severity in some cases, but the inverse is usually not true: the phenotype alone is not a good predictor of the gene or mutation.
In addition to simple forms of RP, there are syndromic forms involving multiple organs and pleiotropic effects as well as systemic forms wherein the retinal disease is secondary to a systemwide pathology (although the distinction is more historic than biological). The most frequent form of syndromic RP is Usher syndrome, which manifests as early-onset or congenital hearing impairment followed by development of RP by the early teenage years.7,8 The second most common syndromic form is Bardet-Biedl syndrome, which includes RP, polydactyly, obesity, renal abnormalities, and mental retardation.9 In addition, many other complex, pleiotropic conditions include RP as a component.1
Table 1 shows the overall prevalence of RP and the proportions of the most common genetic subtypes. Retinitis pigmentosa, broadly defined to include simple, syndromic, and systemic disease, has a worldwide prevalence of 1 case per 3000 persons to 1 case per 7000 persons.10 This is a relatively narrow range of estimates given the inherent difficulty of counting RP cases in large populations. In contrast, estimates of the fractions of the various genetic subtypes vary 10-fold between studies (summarized by Haim10). Part of the reason is that definitions and clinical criteria differ significantly between surveys. However, there are also substantial differences between populations in the prevalence of specific mutations and, hence, in the proportion of specific genetic types. Therefore, the proportions in Table 1 should be taken with a grain of salt.
Table 1.
| Category | Type | % of Total* |
|---|---|---|
| Nonsyndromic RP | Autosomal dominant RP | 20 |
| Autosomal recessive RP | 13 | |
| X-linked RP | 8 | |
| Isolated or unknown RP | 20 | |
| Leber congenital amaurosis | 4 | |
| Subtotal | 65 | |
| Syndromic and systemic RP | Usher syndrome | 10 |
| Bardet-Biedl syndrome | 5 | |
| Other | 10 | |
| Subtotal | 25 | |
| Other or unknown types of RP | 10 | |
| Total | 100 |
Abbreviation: RP, retinitis pigmentosa.
The total prevalence is 1 case per 3100 persons (range, 1 case per 3000 persons to 1 case per 7000 persons), or 32.2 cases per 100 000 persons.10
Nonsyndromic, nonsystemic RP encompasses 65% of all cases, or about 65 000 people in the United States. Of the total number of nonsyndromic, nonsystemic cases, roughly 30% are adRP, 20% are autosomal recessive RP, 15% are X-linked RP, and 5% are early-onset forms of RP that are typically diagnosed as recessive LCA. The remaining cases, at least 30%, are isolated or simplex cases. The simplex cases are likely to include many individuals with recessive mutations, but dominant-acting de novo mutations are also found in these individuals.11,12
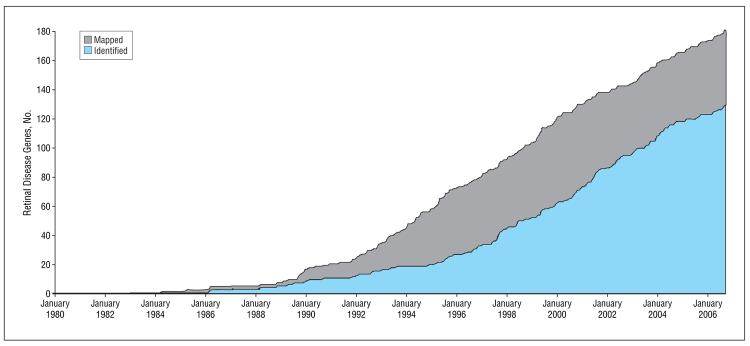
In the past few decades, rapid progress has been made in finding genes and mutations causing inherited retinal diseases. The Figure shows the progress in gene identification since 1980.1 Genes and the underlying mutations within these genes have been identified by a number of methods. Many genes were first localized to a chromosomal site by linkage mapping in families or, more recently, by homozygosity mapping.13,14 Once mapped, the underlying gene can be found by various targeted sequencing strategies. Other disease genes were identified by sequencing candidate genes in selected patient populations. A retinal gene may be a disease candidate because of its functional properties, because it is similar to a gene known to cause retinal disease, or because it is the cause of retinal disease in an animal model.
Figure.
Number of mapped and identified retinal disease genes from 1980 to 2006.
To date, 181 genes causing inherited retinal diseases have been mapped to a specific chromosomal site, and 129 of these have been identified at a sequence level. Also, at least 5 additional genes are known to contribute to the lifetime risk of multifactorial diseases such as age-related macular degeneration.15-22
What is true for retinal disease genes in general is especially true for RP. Currently, mutations in 17 different genes are known to cause adRP, mutations in 25 genes cause recessive RP, mutations in 13 genes cause recessive LCA, mutations in 2 genes cause dominant LCA, and mutations in 6 genes cause X-linked RP.1 Table 2 lists the genes that are currently known to cause nonsyndromic, nonsystemic RP. However, a simple listing of genes in each category is misleading because many genes can cause more than 1 form of disease. For example, although rhodopsin mutations usually cause dominant RP, other rare rhodopsin mutations cause recessive RP. Mutations in NRL can also be either dominant or recessive acting. Further, mutations in some genes, such as RDS, can cause dominant RP, dominant macular degeneration, or other distinct forms of retinopathy. Therefore, Table 2 also lists the alternate phenotypes that can arise for mutations in RP genes and lists some genes in more than 1 section.
Table 2.
Genes and Mapped Loci Causing Nonsyndromic, Nonsystemic Retinitis Pigmentosa*
| Symbol | Location | Protein | Other Diseases |
|---|---|---|---|
| Autosomal Dominant RP | |||
| CA4 | 17q23.2 | Carbonic anhydrase IV | None |
| CRX | 19q13.32 | Cone-rod homeobox | Recessive LCA, dominant LCA, dominant CORD |
| FSCN2 | 17q25.3 | Fascin homolog 2, actin-bundling protein, retinal | None |
| GUCA1B | 6p21.1 | Guanylate cyclase activator 1B (retina) | Dominant MD |
| IMPDH1 | 7q32.1 | IMP (inosine monophosphate) dehydrogenase 1 | Dominant LCA |
| NRL | 14q11.2 | Neural retina leucine zipper | Recessive RP |
| PRPF3 | 1q21.2 | PRP3 pre-mRNA processing factor 3 homolog (Saccharomyces cerevisiae) | None |
| PRPF8 | 17p13.3 | PRP8 pre-mRNA processing factor 8 homolog (S cerevisiae) | None |
| PRPF31 | 19q13.42 | PRP31 pre-mRNA processing factor 31 homolog (S cerevisiae) | None |
| RDS | 6p21.2 | Retinal degeneration, slow (peripherin 2) | Digenic RP with retinal outer segment membrane protein 1, dominant MD |
| RHO | 3q22.1 | Rhodopsin | Recessive RP, dominant CSNB |
| ROM1 | 11q12.3 | Retinal outer segment membrane protein 1 | Digenic RP with retinal degeneration, slow |
| RP1 | 8q12.1 | RP-1 protein | Recessive RP |
| RP9 | 7p14.3 | RP-9 (autosomal dominant) | None |
| RP31 | 9p22-p13 | Unknown | None |
| RP33 | 2cen-q12.1 | Unknown | None |
| SEMA4A | 1q22 | Sema domain, immunoglobulin domain (Ig), transmembrane domain (TM), and short cytoplasmic domain (semiphorin) 4A | Dominant CORD |
|
Autosomal Recessive RP | |||
| ABCA4 | 1p22.1 | ATP-binding cassette, subfamily A (ABC1), member 4 | Recessive MD, recessive CORD |
| CERKL | 2q31.3 | Ceramide kinase–like protein | None |
| CNGA1 | 4p12 | Cyclic nucleotide gated channel α1 | None |
| CNGB1 | 16q13 | Cyclic nucleotide gated channel β1 | None |
| CRB1 | 1q31.3 | Crumbs homolog 1 | Recessive LCA |
| LRAT | 4q32.1 | Lecithin retinol acyltransferase | Recessive LCA |
| MERTK | 2q13 | C-mer proto-oncogene tyrosine kinase | None |
| NR2E3 | 15q23 | Nuclear receptor subfamily 2, group E, member 3 | Recessive enhanced S-cone syndrome |
| NRL | 14q11.2 | Neural retina leucine zipper | Dominant RP |
| PRCD | 17q25.1 | Progressive rod-cone degeneration gene | None |
| PDE6A | 5q33.1 | Phosphodiesterase 6A, cGMP-specific, rod, α | None |
| PDE6B | 4p16.3 | Phosphodiesterase 6B, cGMP-specific, rod, β | Dominant CSNB |
| RGR | 10q23.1 | Retinal G protein–coupled receptor | Dominant choroidal sclerosis |
| RHO | 3q22.1 | Rhodopsin | Dominant RP |
| RLBP1 | 15q26.1 | Retinaldehyde-binding protein 1 | Recessive Bothnia dystrophy |
| RP1 | 8q12.1 | RP-1 protein | Dominant RP |
| RP22 | 16p12.3-p12.1 | Unknown | None |
| RP25 | 6cen-q15 | Unknown | None |
| RP28 | 2p16-p11 | Unknown | None |
| RP29 | 4q32-q34 | Unknown | None |
| RP32 | 1p34.3-p13.3 | Unknown | None |
| RPE65 | 1p31.2 | RPE-specific 65-kd protein | Recessive LCA |
| SAG | 2q37.1 | S-antigen; retina and pineal gland (arrestin) | Recessive Oguchi disease |
| TULP1 | 6p21.31 | Tubby-like protein 1 | Recessive LCA |
| USH2A | 1q41 | Usher syndrome 2A | Recessive Usher syndrome |
|
Autosomal Recessive LCA | |||
| AIPL1 | 17p13.2 | Arylhydrocarbon-interacting receptor protein-like 1 | Dominant CORD |
| CEP290 | 12q21.32 | Centrosomal 290-kd protein | Recessive Senior-Loken syndrome, recessive Joubert syndrome |
| CRB1 | 1q31.3 | Crumbs homolog 1 | Recessive RP |
| CRX | 19q13.32 | Cone-rod homeobox | Dominant CORD, dominant LCA, dominant RP |
| GUCY2D | 17p13.1 | Guanylate cyclase 2D, membrane (retina-specific) | Dominant CORD |
| LRAT | 4q32.1 | Lecithin retinol acyltransferase | Recessive RP |
| LCA3 | 14q24 | Unknown | None |
| LCA5 | 6q11-q16 | Unknown | None |
| LCA9 | 1p36 | Unknown | None |
| RDH12 | 14q24.1 | Retinol dehydrogenase 12 | None |
| RPE65 | 1p31.2 | RPE-specific 65-kd protein | Recessive RP |
| RPGRIP1 | 14q11.2 | RP GTPase regulator interacting protein 1 | None |
| TULP1 | 6p21.31 | Tubby-like protein 1 | Recessive RP |
|
Autosomal Dominant LCA | |||
| CRX | 19q13.32 | Cone-rod homeobox | Dominant CORD, recessive LCA, dominant RP |
| IMPDH1 | 7q32.1 | IMP (inosine monophosphate) dehydrogenase 1 | Dominant RP |
|
X-Linked RP | |||
| RP2 | Xp11.23 | RP-2 protein | None |
| RP6 | Xp21.3-p21.2 | Unknown | None |
| RP23 | Xp22 | Unknown | None |
| RP24 | Xq26-q27 | Unknown | None |
| RP34 | Xq28-qter | Unknown | None |
| RPGR | Xp11.4 | RP GTPase regulator | X-linked COD, X-linked CSNB |
Abbreviations: ATP, adenosine triphosphate; cGMP, cyclic guanosine monophosphate; COD, cone dystrophy; CORD, cone-rod dystrophy; CSNB, congenital stationary night blindness; GTPase, guanosine triphosphatase; LCA, Leber congenital amaurosis; MD, macular dystrophy; mRNA, messenger RNA; RP, retinitis pigmentosa; RPE, retinal pigment epithelium.
References are in RetNet (http://www.sph.uth.tmc.edu/RetNet/).
In total, mutations in 53 genes are known to cause nonsyndromic, nonsystemic RP or LCA (counting each gene once only, even if it causes more than 1 type of retinopathy). As stunning as this number may be, a question more important than the number of genes is the total fraction of patients in whom disease-causing mutations can be detected. In other words, how close are we to knowing all of the RP genes?
One way to answer this question is to summarize the fraction of mutations detected in each gene based on surveys of appropriate patient populations. Table 3 is a compilation of the percentage of patients with detectable mutations in each major RP gene as reported in representative surveys. A gene is “major” if it accounts for at least 1% of cases. In summary, with a number of simplifying assumptions, it is now possible to detect disease-causing mutations in 56% of patients with adRP, roughly 30% of patients with recessive RP, more than 70% of patients with recessive LCA, and nearly 90% of patients with X-linked RP. This is a remarkable achievement given that the first gene known to cause RP, the rhodopsin gene, was described only 17 years ago.40,41
Table 3.
Mutations in Genes That Cause an Appreciable Fraction of Retinitis Pigmentosa Cases
| Symbol | % of All Cases in Disease Category |
Source |
|---|---|---|
| Autosomal Dominant RP | ||
| CRX | 1.0 | Sullivan et al,23 2006 |
| IMPDH1 | 2.5 | Sullivan et al,23 2006 |
| PRPF3 | 1.0 | Sullivan et al,23 2006 |
| PRPF8 | 3.0 | Sullivan et al,23 2006 |
| PRPF31 | 8.0 | Sullivan et al,23 2006; Sullivan et al,24 2006 |
| RDS | 9.5* | Sullivan et al,23 2006 |
| RHO | 26.5 | Sullivan et al,23 2006 |
| RP1 | 3.5 | Sullivan et al,23 2006 |
| RPGR | 1.0 | Sullivan et al,23 2006 |
| Total | 56.0 | |
|
Autosomal Recessive RP | ||
| ABCA4 | 2.9 | Klevering et al,25 2004 |
| CNGA1 | 2.3 | Dryja et al,26 1995 |
| CRB1 | 6.5† | Bernal et al,27 2003 |
| CRX | 1.0 | Rivolta et al,28 2001 |
| PDE6A | 4.0 | Dryja et al,29 1999 |
| PDE6B | 4.0 | McLaughlin et al,30 1995 |
| RPE65 | 2.0 | Morimura et al,31 1998 |
| USH2A | 10.0 | Seyedahmadi et al,32 2004 |
| Total | 32.7 | |
|
Autosomal Recessive LCA | ||
| AIPL1 | 3.4 | Hanein et al,33 2004 |
| CEP290 | 21.0 | den Hollander et al,34 2006 |
| CRB1 | 10.0 | Hanein et al,33 2004 |
| GUCY2D | 21.2 | Hanein et al,33 2004 |
| RDH12 | 4.1 | Perrault et al,35 2004 |
| RPE65 | 6.1 | Hanein et al,33 2004 |
| RPGRIP1 | 4.5 | Hanein et al,33 2004 |
| TULP1 | 1.7 | Hanein et al,33 2004 |
| Total | 72.0 | |
|
Autosomal Dominant LCA | ||
| CRX | ≈1 | Perrault et al,36 2003; Sohocki et al,37 1998 |
| IMPDH1 | ≈1 | Bowne et al,11 2006 |
| Total | Unknown | |
|
X-Linked RP | ||
| RP2 | 15.1 | Pelletier et al,38 2006 |
| RPGR | 74.2 | Pelletier et al,38 2006‡ |
| Total | 89.3 | |
Abbreviations: adRP, autosomal dominant retinitis pigmentosa; LCA, Leber congenital amaurosis; RP, retinitis pigmentosa.
Includes 1 family with digenic RDS-ROM1 mutations.
Up to 50% of recessive RP with Coats disease or para-arteriolar preservation of the retinal pigment epithelium.39
Includes families with X-linked retinitis pigmentosa not linked to RP2 or RPGR.
The percentages in Table 3 come with several caveats. First, many of the numbers are “soft” because disease definitions are not consistent between reports, sample sizes may be small, different segments of the gene may have been screened, and the definition of a mutation differs significantly from study to study. In fact, very few published percentages include confidence intervals, which are usually large. Further, most of these studies are of Americans of European origin and Europeans. Other ethnic and geographic groups have different fractions of disease-causing mutations.42,43 Finally, these are the fractions of mutations detected in carefully designed studies with optimal methods; screening in practice may be less efficient.
But caveats aside, across all of the categories of inherited retinopathy, careful screening of known disease genes leads to detection of pathogenic mutations in 25% to 90% of patients, an extraordinary accomplishment. At the same time, however, linkage studies and other evidence show that that there are more, perhaps many more, RP genes to be found.
GENES AND MUTATIONS CAUSING adRP
To give a more detailed perspective, what follows is a look at the genes and mutations causing just 1 form of retinal disease, adRP. However, many of the conclusions from the study of adRP are broadly applicable to other inherited retinal diseases. Therefore, this section ends with observations that apply generally to all forms of RP.
In a recent survey, we tested a panel of affected individuals from 200 families with adRP for mutations in most of the known dominant RP genes (Table 2).23 To be included in the study, a family had to have a diagnosis of adRP by a knowledgeable clinical specialist and either 3 affected generations with affected females or 2 affected generations with male-to-male transmission. The latter requirement was to reduce the likelihood of including families with X-linked RP. This possibility arises because some mutations in the X-linked gene RPGR affect female carriers; thus, the disease in these families can be misinterpreted as adRP.44-46
The cohort of patients with adRP was screened (largely by DNA sequencing) for mutations in the protein-coding regions and intronexon junctions of all adRP genes or gene regions causing at least 1% of cases. Open reading frame 15 (ORF15), the “hot spot” for dominant-acting mutations in RPGR, was also tested in families without male-to-male transmission. Determining whether a novel, rare variant is pathogenic can be challenging.47 We used several computational and genetic tools for this purpose.23 Generally, once a definite disease-causing mutation was identified in a family, other genes were not tested further in these individuals.
We found definite or probable mutations in 53.5% of the families with adRP. In subsequent studies, we tested several of the remaining families for linkage to genetic markers within or close to the known adRP genes and to RPGR.24 The logic here was to uncover mutations that might have been missed by sequencing or to locate genes that have been mapped but not identified yet. In 1 large family, we found linkage to the PRPF31 gene, even though careful resequencing failed to disclose a DNA change. Further testing revealed that affected members of the family have a complex deletion and insertion in PRPF31. This rearrangement was not detected earlier because only the nondeleted, homologous chromosome was sequenced; that is, the deletion is “invisible” to sequencing.
We then tested the remaining families for deletions in PRPF31 using multiplex ligation-dependent probe amplification (MLPA).48,49 Surprisingly, we found 4 large deletions, including 2 that encompass genes adjacent to PRPF31.24 This brings the fraction of detected mutations to 56% (Table 3).
These studies have a number of implications that go beyond just adRP. First, 14 different, common mutations account for up to 30% of the families with adRP in this survey; that is, each of these mutations accounts for at least 1% of the cases.23 Thus, screening for this handful of mutations alone will resolve at least 30% of the cases. Common mutations are found in other RP genes, and numerous inexpensive, high-throughput techniques exist for detecting these variants.50,51
Second, another 20% of mutations were novel and could only be detected by sequencing entire genes. Further, each novel mutation requires careful evaluation of pathogenicity. As a consequence, the main bottleneck in genetic testing of patients with RP is the need to screen and analyze many genes by expensive, time-consuming methods. Fortunately, promising high-throughput resequencing techniques, such as microarray gene chips, may relieve this bottleneck.52 Nonetheless, interpretation of novel, rare variants will still require professional evaluation.47
Third, some families thought to have adRP actually have digenic or X-linked mutations. Digenic RP is the result of 1 mutation in RDS and a second in ROM1.53 Different individual mutations in RDS and ROM1 can cause adRP, but each of the digenic mutations alone is not pathogenic. Digenic and polygenic inheritance is true of other forms of retinal disease, such as Bardet-Biedl syndrome, which can be “triallelic.”9 Another misleading mode of inheritance among families diagnosed with “adRP” is X-linked inheritance of RPGR mutations with significant disease in carrier females.44-46 Both of these phenomena are important reminders that the molecular diagnosis can radically change genetic counseling.
Fourth, at least 2.5% of adRP mutations are genomic rearrangements or deletions in PRPF31 that are not detectable by conventional screening methods.24 Whether there are disease-causing deletions in other adRP genes or in recessive or X-linked genes is an active area of research. This is likely, though, because deletions are a common cause of other inherited and acquired diseases.54-56 For example, large deletions cause up to 17% of familial breast cancer.57
The existence of disease-causing deletions has significant implications for molecular testing of patients with RP. For one, routine testing methods may miss deletions (eg, sequencing does not detect the breast cancer deletions). For another, deletions may explain reported anomalies in the frequency and segregation of RP mutations. If so, here again, the molecular diagnosis will affect counseling. Finally, this finding suggests that there may be other subtle mutations in known RP genes that are missed by standard methods.
Fifth, there are definitely additional, unknown adRP genes. We failed to detect mutations in 40% of the families we tested. Some, but not all, of the remaining mutations may be deletions or subtle changes in known genes that have not been detected to date.58 Linkage mapping continues to locate new adRP genes—most recently RP31 and RP33.59,60 Likewise, new recessive and X-linked genes are reported regularly.1
It is impossible to predict whether there are several or many more RP genes that have yet to be discovered. Completion of the Human Genome Project, new high-throughput screening methods, and development of powerful bioinformatic approaches have dramatically reduced the time it will take to find new genes. In spite of these technical advances, the need for thorough, knowledgeable, innovative clinical characterization of patients and families has never been greater.
RELEVANCE TO CLINICAL PRACTICE AND FUTURE DIRECTIONS
What does the current state of RP genetics say of the future? A reasonable hope is that within 5 years, molecular testing of newly diagnosed patients with RP will be a routine part of clinical practice and will uncover the underlying disease-causing mutation (or mutations) in at least 90% of cases. For this hope to come true, 4 conditions must be met:
Most of the genes causing RP must be identified.
It must be possible to detect nearly all of the disease-causing mutations within these genes.
Mutation testing must become inexpensive, reliable, and widely available.
We must be able to understand, interpret, and explain the molecular information.
Before addressing these necessary conditions, it is worth asking why finding the underlying disease-causing mutation should matter to the patient or the clinician. After all, RP is currently an untreatable condition, so wouldn't the molecular information be of no use?
There are several compelling reasons why molecular testing is important for clinical care. For one, identifying the underlying mutation(s) can establish the diagnosis, which may be problematic otherwise. This is particularly important for childhood retinopathies wherein the molecular diagnosis may portend distinctly different clinical outcomes.33,61 Also, knowing the genetic cause is essential for family counseling and for predicting recurrence risk and prognosis. In addition, each new mutation that is found contributes to a better understanding of ocular biology. Finally and of the most importance, the era of gene-specific and mutation-specific treatments for inherited retinal diseases is quickly approaching.62,63 Knowing the underlying genetic cause will be essential for enrolling patients in clinical trials, a few of which have begun already or will begin shortly.64,65 It is a safe prediction that in the near future, there will be many more treatment and prevention strategies based on knowledge of the underlying mutation(s) in affected individuals and families.
Then, how close are we to routine molecular diagnosis of RP? Identification of new RP genes is proceeding swiftly. An educated guess (at best) is that most of the major genes, at least in Americans of European origin and Europeans, will be found within 5 to 10 years. Whether current screening methods, such as sequencing or microarray testing, can detect all or even most of the mutations in known genes is debatable. Not all of the gene regions that could harbor mutations are tested routinely. For example, large intervening sequences and noncoding regulatory regions are usually ignored. Also, current methods do not detect large deletions or rearrangements. Venturing another educated guess, though, existing methods and methods under development will be able to detect most mutations, ie, more than 90%, within 5 years.
Currently, the greatest road-block to molecular diagnosis of RP is the availability of genetic testing. Large commercial interests have not yet entered the field, primarily because there are so many genes to test and so many inherent complications. However, methods for rapid, inexpensive detection of known RP mutations exist today and will be routinely available soon.51,66 Also, targeted screening of genes and gene regions that are frequent causes of inherited retinal diseases is being offered on a fee-for-service basis by a few institutions in the United States and Europe (see the GeneTests Web site for further information67). In addition, the National Eye Institute has recently developed a program, eyeGENE™,68 to facilitate genetic testing of inherited eye diseases. Finally, it is reasonable to expect that new high-throughput sequencing methods will make genetic testing of all diseases affordable and efficient within 10 years.69
In our opinion, the major impediment to routine molecular diagnosis of RP is not technical or commercial but rather informational. No aspect of understanding, interpreting, and explaining the molecular causes of RP is routine. Skilled, informed clinical diagnosis must precede testing. Even if genetic testing is standardized, interpretation of novel variants will require sophisticated analysis. Understanding the results of genetic testing will be challenging, especially if novel findings such as polygenic inheritance are involved. Making sense of this to patients and families in a helpful and supportive way will require good counseling skills. Finally, when gene-specific and mutation-specific treatments become available, which is inevitable, even greater levels of knowledge and understanding will be demanded.
None of this is unique to RP: molecular diagnostics will enrich all aspects of medical care in future years. What is unusual, though, is the extent of the current knowledge of the molecular causes of inherited retinal diseases and the recognition of the underlying complexity. Thus, clinical ophthalmology has the unique opportunity to prepare for the near future by enhancing training in genetics, incorporating genetic counseling at all levels of care, and developing specialized centers for the diagnosis and treatment of inherited eye diseases. Another reasonable prediction is that the ophthalmology profession will lead the way for other branches of medicine.
Acknowledgments
Funding/Support: This study was supported by grants from the Foundation Fighting Blindness, the William Stamps Farish Foundation, the Gustavus and Louise Pfeiffer Research Foundation, and the Hermann Eye Fund as well as by grants EY007142 and EY014170 from the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Daiger SP. The University of Texas Health Science Center. RetNet: Retinal Information Network. http://www.sph.uth.tmc.edu/RetNet/. Accessed December 1, 2006.
- 2.Bessant DA, Ali RR, Bhattacharya SS. Molecular genetics and prospects for therapy of the inherited retinal dystrophies. Curr Opin Genet Dev. 2001;11:307–316. doi: 10.1016/s0959-437x(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 3.Dryja TP. Retinitis pigmentosa and stationary night blindness. In: Scriver CR, Beaudet AL, Sly WS, Vale D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGraw-Hill; New York, NY: 2001. pp. 5903–5934. [Google Scholar]
- 4.Heckenlively JR, Daiger SP. Hereditary retinal and choroidal degenerations. In: Rimoin DL, Connor JM, Pyeritz RE, editors. Emery and Rimoin's Principles and Practice of Medical Genetics. 4th ed. Vol. 1. Churchill Livingston; New York, NY: 2002. pp. 2255–2576. [Google Scholar]
- 5.Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- 6.Heckenlively JR, editor. Retinitis Pigmentosa. JB Lippincott; Philadelphia, Pa: 1988. [Google Scholar]
- 7.Keats BJ, Savas S. Genetic heterogeneity in Usher syndrome. Am J Med Genet A. 2004;130:13–16. doi: 10.1002/ajmg.a.30052. [DOI] [PubMed] [Google Scholar]
- 8.Kimberling WJ, Orten D, Pieke-Dahl S. Genetic heterogeneity of Usher syndrome. Adv Otorhinolaryngol. 2000;56:11–18. doi: 10.1159/000059077. [DOI] [PubMed] [Google Scholar]
- 9.Katsanis N. The oligogenic properties of Bardet-Biedl syndrome. Hum Mol Genet. 2004;13:R65–R71. doi: 10.1093/hmg/ddh092. [DOI] [PubMed] [Google Scholar]
- 10.Haim M. Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl. 2002;233:1–34. doi: 10.1046/j.1395-3907.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 11.Bowne SJ, Sullivan LS, Mortimer SE, et al. Spectrum and frequency of mutations in IMPDH1 associated with autosomal dominant retinitis pigmentosa and Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2006;47:34–42. doi: 10.1167/iovs.05-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz SB, Aleman TS, Cideciyan AV, Swaroop A, Jacobson SG, Stone EM. De novo mutation in the RP1 gene (Arg677ter) associated with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:3593–3597. doi: 10.1167/iovs.03-0155. [DOI] [PubMed] [Google Scholar]
- 13.Lathrop GM, Lalouel JM, Julier C, Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984;81:3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheffield VC, Nishimura DY, Stone EM. Novel approaches to linkage mapping. Curr Opin Genet Dev. 1995;5:335–341. doi: 10.1016/0959-437x(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 15.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 16.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77:389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 21.Stone EM, Braun TA, Russell SR, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 22.Zareparsi S, Buraczynska M, Branham KE, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet. 2005;14:1449–1455. doi: 10.1093/hmg/ddi154. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan LS, Bowne SJ, Birch DG, et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa (adRP): a screen of known genes in 200 families. Invest Ophthalmol Vis Sci. 2006;47:3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan LS, Bowne SJ, Seaman R, et al. Genomic rearrangements of the PRPF31 gene account for 2.5% of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2006;47:4579–4588. doi: 10.1167/iovs.06-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klevering BJ, Yzer S, Rohrschneider K, et al. Microarray-based mutation analysis of the ABCA4 (ABCR) gene in autosomal recessive cone-rod dystrophy and retinitis pigmentosa. Eur J Hum Genet. 2004;12:1024–1032. doi: 10.1038/sj.ejhg.5201258. [DOI] [PubMed] [Google Scholar]
- 26.Dryja TP, Finn JT, Peng YW, McGee TL, Berson EL, Yau KW. Mutations in the gene encoding the alpha subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995;92:10177–10181. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernal S, Calaf M, Garcia-Hoyos M, et al. Study of the involvement of the RGR, CRPB1, and CRB1 genes in the pathogenesis of autosomal recessive retinitis pigmentosa. J Med Genet. 2003;40:e89. doi: 10.1136/jmg.40.7.e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivolta C, Peck NE, Fulton AB, Fishman GA, Berson EL, Dryja TP. Novel frameshift mutations in CRX associated with Leber congenital amaurosis. Hum Mutat. 2001;18:550–551. doi: 10.1002/humu.1243. [DOI] [PubMed] [Google Scholar]
- 29.Dryja TP, Rucinski DE, Chen SH, Berson EL. Frequency of mutations in the gene encoding the alpha subunit of rod cGMP-phosphodiesterase in autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40:1859–1865. [PubMed] [Google Scholar]
- 30.McLaughlin ME, Ehrhart TL, Berson EL, Dryja TP. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci U S A. 1995;92:3249–3253. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seyedahmadi BJ, Rivolta C, Keene JA, Berson EL, Dryja TP. Comprehensive screening of the USH2A gene in Usher syndrome type II and non-syndromic recessive retinitis pigmentosa. Exp Eye Res. 2004;79:167–173. doi: 10.1016/j.exer.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Hanein S, Perrault I, Gerber S, et al. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum Mutat. 2004;23:306–317. doi: 10.1002/humu.20010. [DOI] [PubMed] [Google Scholar]
- 34.den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrault I, Hanein S, Gerber S, et al. Retinal dehydrogenase 12 (RDH12) mutations in Leber congenital amaurosis. Am J Hum Genet. 2004;75:639–646. doi: 10.1086/424889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrault I, Hanein S, Gerber S, et al. Evidence of autosomal dominant Leber congenital amaurosis (LCA) underlain by a CRX heterozygous null allele. J Med Genet. 2003;40:e90. doi: 10.1136/jmg.40.7.e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohocki MM, Sullivan LS, Mintz-Hittner HA, et al. A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am J Hum Genet. 1998;63:1307–1315. doi: 10.1086/302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelletier V, Jambou M, Delphin N, et al. Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies: genotype-phenotype correlations and impact on genetic counseling [published online ahead of print September 12, 2006] Hum Mutat. 2006;27 doi: 10.1002/humu.20417. doi:10.1002/humu.20417. [DOI] [PubMed] [Google Scholar]
- 39.den Hollander AI, Davis J, van der Velde-Visser SD, et al. CRB1 mutation spectrum in inherited retinal dystrophies. Hum Mutat. 2004;24:355–369. doi: 10.1002/humu.20093. [DOI] [PubMed] [Google Scholar]
- 40.Dryja TP, McGee TL, Hahn LB, et al. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990;323:1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- 41.Dryja TP, McGee TL, Reichel E, et al. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990;343:364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- 42.Pang CP, Lam DS. Differential occurrence of mutations causative of eye diseases in the Chinese population. Hum Mutat. 2002;19:189–208. doi: 10.1002/humu.10053. [DOI] [PubMed] [Google Scholar]
- 43.Wada Y, Tamai M. Molecular genetic analysis for Japanese patients with autosomal dominant retinitis pigmentosa. Nippon Ganka Gakkai Zasshi. 2003;107:687–694. [PubMed] [Google Scholar]
- 44.Mears AJ, Hiriyanna S, Vervoort R, et al. Remapping of the RP15 locus for X-linked cone-rod degeneration to Xp11.4-p21.1, and identification of a de novo insertion in the RPGR exon ORF15. Am J Hum Genet. 2000;67:1000–1003. doi: 10.1086/303091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozet JM, Perrault I, Gigarel N, et al. Dominant X-linked retinitis pigmentosa is frequently accounted for by truncating mutations in exon ORF15 of the RPGR gene. J Med Genet. 2002;39:284–285. doi: 10.1136/jmg.39.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vervoort R, Lennon A, Bird AC, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet. 2000;25:462–466. doi: 10.1038/78182. [DOI] [PubMed] [Google Scholar]
- 47.Stone EM. Finding and interpreting genetic variations that are important to ophthalmologists. Trans Am Ophthalmol Soc. 2003;101:437–484. [PMC free article] [PubMed] [Google Scholar]
- 48.Stern RF, Roberts RG, Mann K, Yau SC, Berg J, Ogilvie CM. Multiplex ligation-dependent probe amplification using a completely synthetic probe set. Biotechniques. 2004;37:399–405. doi: 10.2144/04373ST04. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz M, Duno M. Improved molecular diagnosis of dystrophin gene mutations using the multiplex ligation-dependent probe amplification method. Genet Test. 2004;8:361–367. doi: 10.1089/gte.2004.8.361. [DOI] [PubMed] [Google Scholar]
- 50.Kwok PY, Chen X. Detection of single nucleotide variations. Genet Eng (N Y) 1998;20:125–134. doi: 10.1007/978-1-4899-1739-3_6. [DOI] [PubMed] [Google Scholar]
- 51.Yzer S, Leroy BP, De Baere E, et al. Microarray-based mutation detection and phenotypic characterization of patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2006;47:1167–1176. doi: 10.1167/iovs.05-0848. [DOI] [PubMed] [Google Scholar]
- 52.Mandal MN, Heckenlively JR, Burch T, et al. Sequencing arrays for screening multiple genes associated with early-onset human retinal degenerations on a high-throughput platform. Invest Ophthalmol Vis Sci. 2005;46:3355–3362. doi: 10.1167/iovs.05-0007. [DOI] [PubMed] [Google Scholar]
- 53.Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264:1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- 54.Canning S, Dryja TP. Short, direct repeats at the breakpoints of deletions of the retinoblastoma gene. Proc Natl Acad Sci U S A. 1989;86:5044–5048. doi: 10.1073/pnas.86.13.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- 56.Sharp AJ, Locke DP, McGrath SD, et al. Segmental duplications and copy-number variation in the human genome. Am J Hum Genet. 2005;77:78–88. doi: 10.1086/431652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh T, Casadei S, Coats KH, et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA. 2006;295:1379–1388. doi: 10.1001/jama.295.12.1379. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan LS, Bowne SJ, Shankar SP, et al. Autosomal dominant retinitis pigmentosa: exclusion of known and mapped genes in three families. Invest Ophthalmol Vis Sci. 2004;45 E-abstract 4747. [Google Scholar]
- 59.Papaioannou M, Chakarova CF, Prescott DC, et al. A new locus (RP31) for autosomal dominant retinitis pigmentosa maps to chromosome 9p. Hum Genet. 2005;118:501–503. doi: 10.1007/s00439-005-0063-3. [DOI] [PubMed] [Google Scholar]
- 60.Zhao C, Lu S, Zhou X, Zhang X, Zhao K, Larsson C. A novel locus (RP33) for autosomal dominant retinitis pigmentosa mapping to chromosomal region 2cen-q12.1. Hum Genet. 2006;119:617–623. doi: 10.1007/s00439-006-0168-3. [DOI] [PubMed] [Google Scholar]
- 61.Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol. 2004;49:379–398. doi: 10.1016/j.survophthal.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Bennett J. Gene therapy for Leber congenital amaurosis. Novartis Found Symp. 2004;255:195–202. [PubMed] [Google Scholar]
- 63.Chader GJ. Beyond basic research for inherited and orphan retinal diseases: successes and challenges. Retina. 2005;25:S15–S17. doi: 10.1097/00006982-200512001-00006. [DOI] [PubMed] [Google Scholar]
- 64.Hauswirth WW. The consortium project to treat RPE65 deficiency in humans. Retina. 2005;25:S60. doi: 10.1097/00006982-200512001-00027. [DOI] [PubMed] [Google Scholar]
- 65.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaakson K, Zernant J, Kulm M, et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum Mutat. 2003;22:395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 67.GeneTests. University of Washington; Seattle: http://www.GeneTests.org. Accessed December 1, 2006. [Google Scholar]
- 68.National Eye Institute National Ophthalmic Disease Genotyping Network (eyeGENE™) http://www.nei.nih.gov/resources/eyegene.asp. Accessed December 1, 2006.
- 69.Service RF. Gene sequencing: the race for the $1000 genome. Science. 2006;311:1544–1546. doi: 10.1126/science.311.5767.1544. [DOI] [PubMed] [Google Scholar]