Abstract
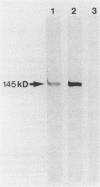
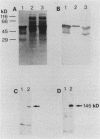
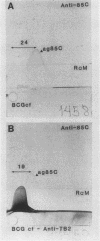
Recombinant phage clones, TB1 and TB2, were selected from a Mycobacterium tuberculosis lambda gt11 DNA expression library by screening with a polyclonal antiserum raised against the antigen 85 complex of Mycobacterium bovis BCG. Analysis of recombinant DNA inserts and expressed fusion proteins showed that two new genes had been isolated. The product of clone TB2 was identified as a member of the 30/31-kDa antigen 85 complex. Restriction enzyme analysis showed that this gene differs from previously cloned members of this antigen complex, with detailed serological analysis indicating that it may encode the 85C component. Antisera raised against the expressed product of clone TB1 recognized a 55-kDa protein in M. tuberculosis extracts. The 55-kDa protein also has fibronectin-binding activity and, like the 30/31-kDa family, is a prominent target of the antibody response in patients with mycobacterial disease. Although the clones were selected by using the same antiserum, detailed analysis by serology and by DNA hybridization showed that they represent two quite distinct types of fibronectin-binding activities expressed by M. tuberculosis. Further analysis of the fibronectin-binding antigens of M. tuberculosis may provide important insights into their role in mediating the interaction with the host immune system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Zeid C., Smith I., Grange J. M., Ratliff T. L., Steele J., Rook G. A. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988 Feb;134(2):531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- Andersen A. B., Yuan Z. L., Hasløv K., Vergmann B., Bennedsen J. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Mar;23(3):446–451. doi: 10.1128/jcm.23.3.446-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Cornette J., Capron A., Ouaissi M. A. Trypanosoma cruzi: fibronectin promotes uptake of epimastigote culture forms by human neutrophils and monocytes. Int Arch Allergy Appl Immunol. 1988;86(2):139–146. doi: 10.1159/000234563. [DOI] [PubMed] [Google Scholar]
- De Wit L., de la Cuvellerie A., Ooms J., Content J. Nucleotide sequence of the 32 kDa-protein gene (antigen 85 A) of Mycobacterium bovis BCG. Nucleic Acids Res. 1990 Jul 11;18(13):3995–3995. doi: 10.1093/nar/18.13.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Kavoussi L. R., Brown E. J., Ritchey J. K., Ratliff T. L. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990 Jan;85(1):62–67. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Terasaka K., Yamada T. Cloning and expression of the gene for the cross-reactive alpha antigen of Mycobacterium kansasii. Infect Immun. 1990 Feb;58(2):550–556. doi: 10.1128/iai.58.2.550-556.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessolani M. C., Rumjanek F. D., Marques M. A., de Melo F. S., Sarno E. N. Serological response of patients with leprosy to a 28- to 30-kilodalton protein doublet from early cultures of Mycobacterium bovis BCG. J Clin Microbiol. 1989 Oct;27(10):2184–2189. doi: 10.1128/jcm.27.10.2184-2189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff T. L., McGarr J. A., Abou-Zeid C., Rook G. A., Stanford J. L., Aslanzadeh J., Brown E. J. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988 May;134(5):1307–1313. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- Rumschlag H. S., Shinnick T. M., Cohen M. L. Serological responses of patients with lepromatous and tuberculoid leprosy to 30-, 31-, and 32-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Oct;26(10):2200–2202. doi: 10.1128/jcm.26.10.2200-2202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- SHEPARD C. C. A comparison of the growth of selected mycobacteria in HeLa, monkey kidney, and human amnion cells in tissue culture. J Exp Med. 1958 Feb 1;107(2):237–246. doi: 10.1084/jem.107.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M. Integration of monoclonal antibodies in quantitative immunoelectrophoresis by indirect immunoprecipitation. J Immunol Methods. 1990 Aug 28;132(1):127–135. doi: 10.1016/0022-1759(90)90406-l. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Lea T. E. Purification and characterization of two protein antigens from the heterogeneous BCG85 complex in Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):298–306. doi: 10.1159/000234153. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Sletten K., Nagai S., Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990 Jan;58(1):272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]