Abstract
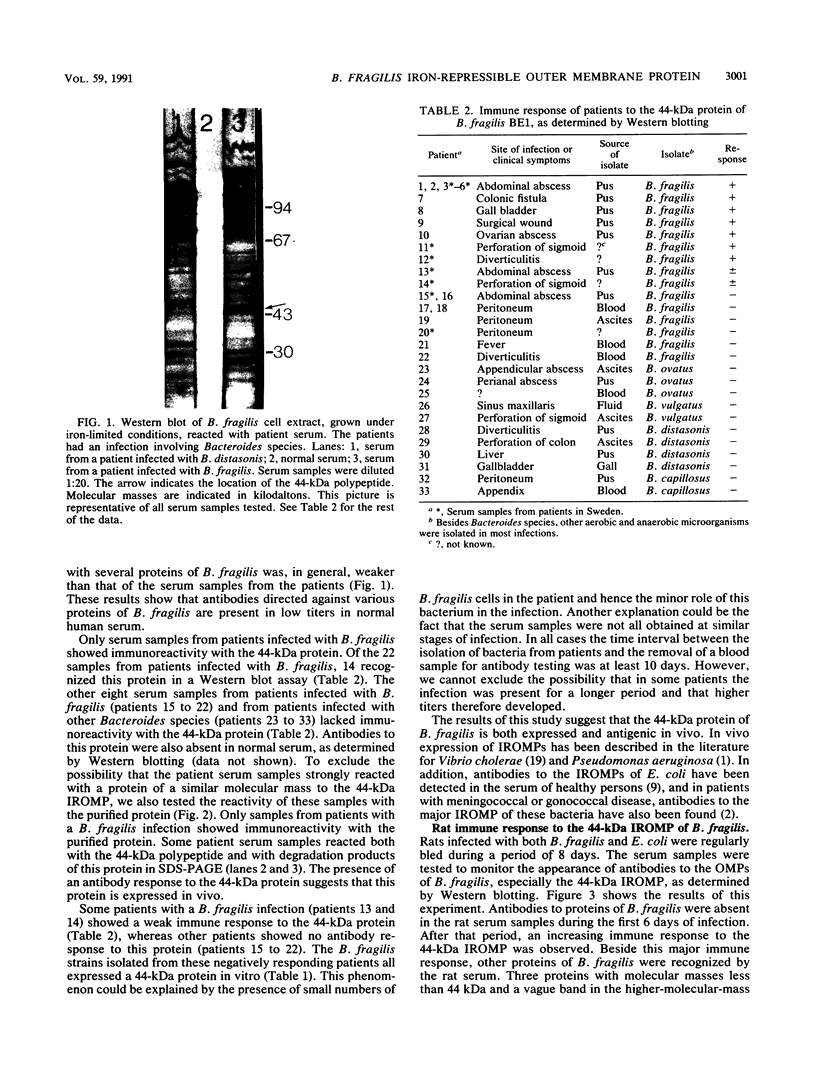
Under conditions of iron starvation, Bacteroides fragilis expresses various iron-repressible outer membrane proteins (IROMPs). A 44-kDa protein appears to be one of the major outer membrane proteins (OMPs) in B. fragilis under iron stress and plays a role in heme uptake by this bacterium. To determine whether the 44-kDa IROMP of B. fragilis is expressed in vivo and whether this protein is immunogenic, we used Western immunoblotting to examine serum samples from patients with an infection caused by Bacteroides species. All the serum samples from patients and from normal controls showed reactivity with several proteins of B. fragilis. Only serum samples from patients infected with B. fragilis showed immunoreactivity with the 44-kDa protein. We also used a rat infection model to study the immune response against this protein during the process of an intra-abdominal infection in these animals. During the first 8 days of infection a gradual increase of antibodies to the 44-kDa protein in the rat was detected. These results suggest that the 44-kDa IROMP is expressed in vivo, since it induces an antibody response in patients and animals. We also analyzed 85 strains of the B. fragilis group for the presence of proteins antigenically related to the B. fragilis 44-kDa protein. The data indicate that this protein was conserved in B. fragilis strains and was absent in the other bacterial strains tested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. R., Dyer D. W., Thompson M. K., Sparling P. F. Human immune response to iron-repressible outer membrane proteins of Neisseria meningitidis. Infect Immun. 1986 Dec;54(3):710–713. doi: 10.1128/iai.54.3.710-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouza E., Reig M., Garcia de la Torre M., Rodríguez-Créixems M., Romero J., Cercenado E., Baquero F. Retrospective analysis of two hundred and twelve cases of bacteremia due to anaerobic microorganisms. Eur J Clin Microbiol. 1985 Jun;4(3):262–267. doi: 10.1007/BF02013649. [DOI] [PubMed] [Google Scholar]
- Brook I. A 12 year study of aerobic and anaerobic bacteria in intra-abdominal and postsurgical abdominal wound infections. Surg Gynecol Obstet. 1989 Nov;169(5):387–392. [PubMed] [Google Scholar]
- Brook I. Pathogenicity of the Bacteroides fragilis group. Ann Clin Lab Sci. 1989 Sep-Oct;19(5):360–376. [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Stevenson P., Griffiths E. Iron-regulated outer-membrane proteins of Escherichia coli strains associated with enteric or extraintestinal diseases of man and animals. J Gen Microbiol. 1988 Jun;134(6):1549–1559. doi: 10.1099/00221287-134-6-1549. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Stevenson P., Thorpe R., Chart H. Naturally occurring antibodies in human sera that react with the iron-regulated outer membrane proteins of Escherichia coli. Infect Immun. 1985 Mar;47(3):808–813. doi: 10.1128/iai.47.3.808-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heseltine P. N., Appleman M. D., Leedom J. M. Epidemiology and susceptibility of resistant Bacteroides fragilis group organisms to new beta-lactam antibiotics. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S254–S259. doi: 10.1093/clinids/6.supplement_1.s254. [DOI] [PubMed] [Google Scholar]
- Immune response in patients with intraabdominal infections treated with imipenem. Swedish Study Group. Infection. 1989 Nov-Dec;17(6):369–373. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maskell J. P. The pathogenicity of Bacteroides fragilis and related species estimated by intracutaneous infection in the guinea-pig. J Med Microbiol. 1981 Feb;14(1):131–140. doi: 10.1099/00222615-14-1-131. [DOI] [PubMed] [Google Scholar]
- Otto B. R., Sparrius M., Verweij-van Vught A. M., MacLaren D. M. Iron-regulated outer membrane protein of Bacteroides fragilis involved in heme uptake. Infect Immun. 1990 Dec;58(12):3954–3958. doi: 10.1128/iai.58.12.3954-3958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B. R., Verweij-Van Vught A. M., Maclaren D. M. Purification and partial characterization of an iron regulated outer membrane protein of B. fragilis under non-denaturing conditions. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):285–290. doi: 10.1016/0378-1097(90)90298-5. [DOI] [PubMed] [Google Scholar]
- Otto B. R., Verweij-van Vught A. M., van Doorn J., Maclaren D. M. Outer membrane proteins of Bacteroides fragilis and Bacteroides vulgatus in relation to iron uptake and virulence. Microb Pathog. 1988 Apr;4(4):279–287. doi: 10.1016/0882-4010(88)90088-5. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Simmons R. L. Mechanisms of microbial synergy in polymicrobial surgical infections. Rev Infect Dis. 1985 Mar-Apr;7(2):151–170. doi: 10.1093/clinids/7.2.151. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Wells C. L., Simmons R. L. The role of Bacteroides encapsulation in the lethal synergy between Escherichia coli and Bacteroides species studied in a rat fibrin clot peritonitis model. J Infect. 1987 Sep;15(2):135–146. doi: 10.1016/s0163-4453(87)93113-6. [DOI] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpenning M. S. Anaerobic bacteremia in the elderly. Gerontology. 1989;35(2-3):130–136. doi: 10.1159/000213011. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij-van Vught A. M., Namavar F., Vel W. A., Sparrius M., MacLaren D. M. Pathogenic synergy between Escherichia coli and Bacteroides fragilis or B. vulgatus in experimental infections: a non-specific phenomenon. J Med Microbiol. 1986 Feb;21(1):43–47. doi: 10.1099/00222615-21-1-43. [DOI] [PubMed] [Google Scholar]
- Verweij-van Vught A. M., Namavar F., Vel W. A., Sparrius M., MacLaren D. M. Pathogenic synergy between Escherichia coli and Bacteroides fragilis or B. vulgatus in experimental infections: a non-specific phenomenon. J Med Microbiol. 1986 Feb;21(1):43–47. doi: 10.1099/00222615-21-1-43. [DOI] [PubMed] [Google Scholar]