Abstract
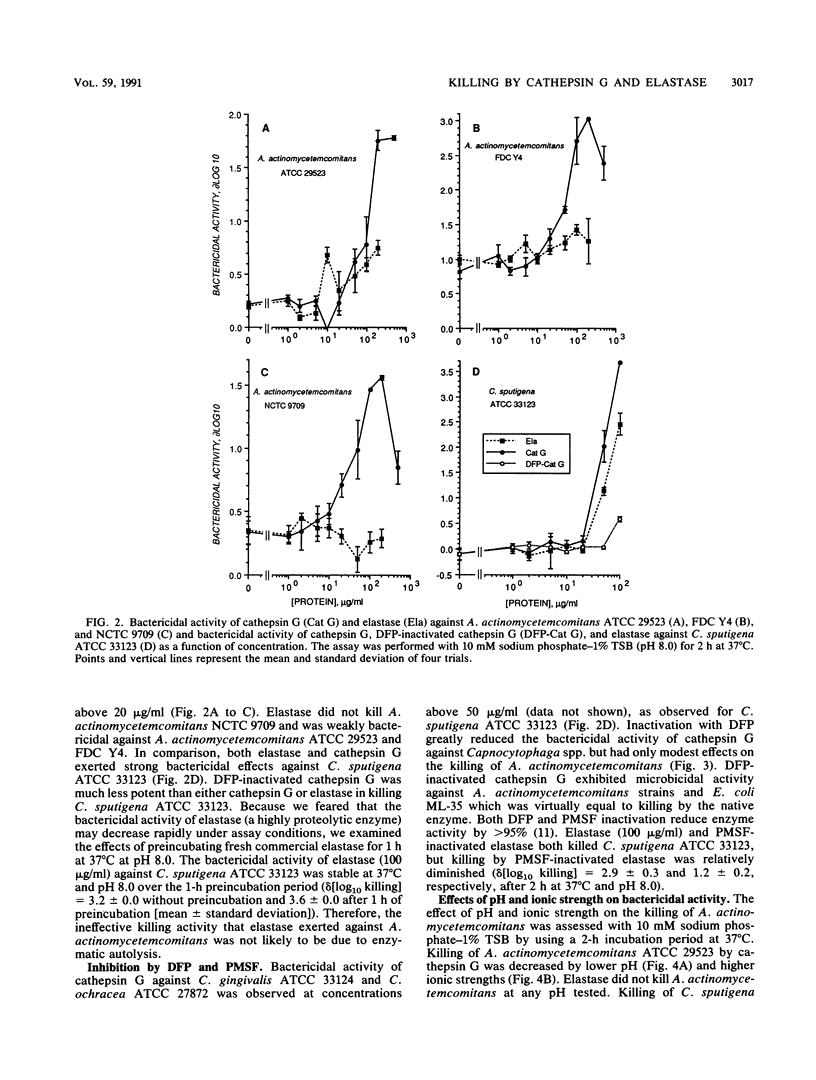
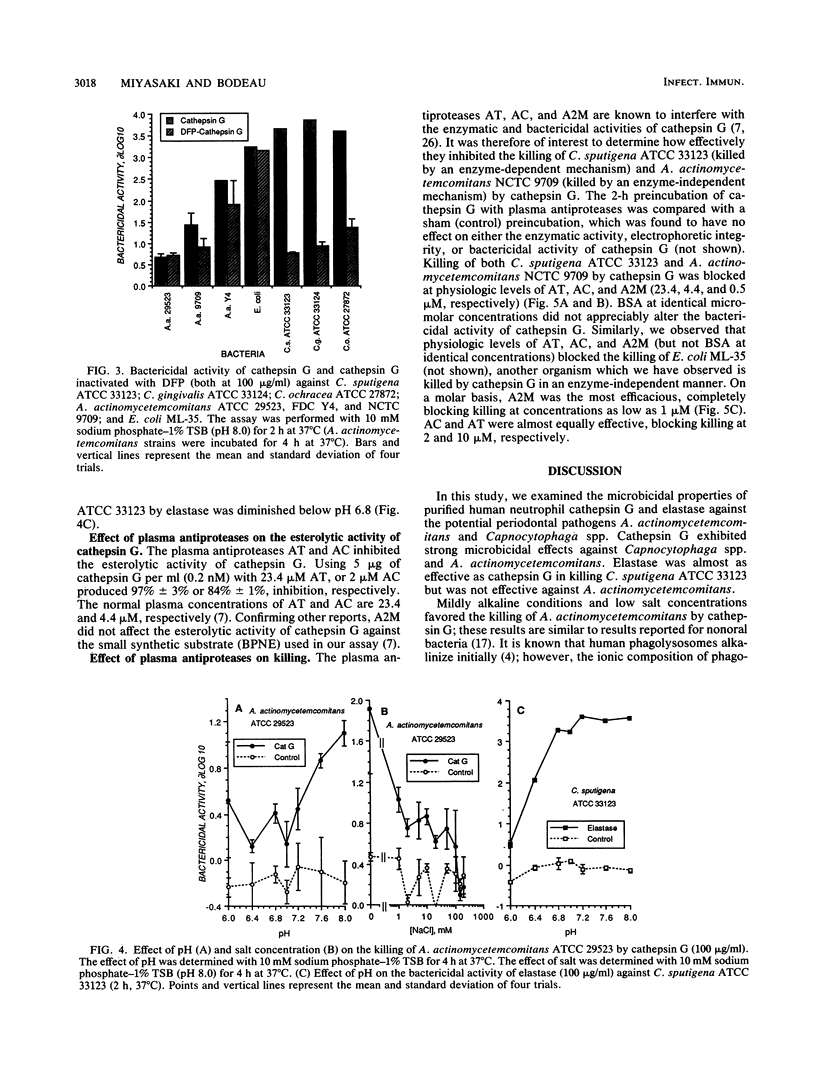
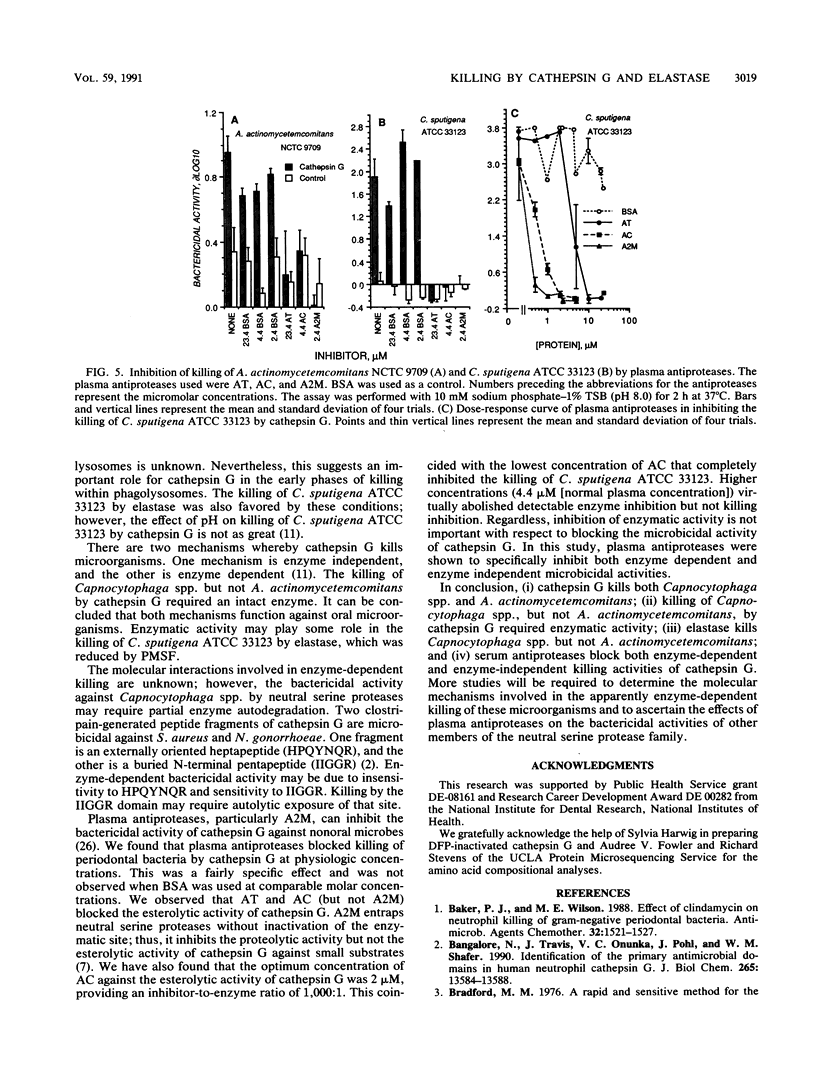
The purpose of this study was to compare the killing of Actinobacillus actinomycetemcomitans with that of Capnocytophaga spp. by purified cathepsin G and elastase in vitro. Both were sensitive to killing by purified cathepsin G, but only the Capnocytophaga spp. were killed by elastase. Killing by cathepsin G exhibited logarithmic kinetics, was enhanced slightly by alkaline pH, and was enhanced greatly under hypotonic conditions. Treatment of cathepsin G with diisopropyl fluorophosphate significantly reduced its bactericidal activity against Capnocytophaga spp. but not against Escherichia coli or A. actinomycetemcomitans. The bactericidal effects of cathepsin G against Capnocytophaga sputigena and A. actinomycetemcomitans were inhibited by alpha-1-antichymotrypsin, alpha-1-antitrypsin, and alpha-2-macroglobulin but not by bovine serum albumin. We conclude that (i) cathepsin G kills Capnocytophaga spp. and A. actinomycetemcomitans, (ii) elastase kills Capnocytophaga spp., (iii) the bactericidal activity of cathepsin G is enzyme dependent against Capnocytophaga spp. and enzyme independent against A. actinomycetemcomitans, and (iv) natural plasma antiproteases may control both enzyme-dependent and enzyme-independent bactericidal activities of cathepsin G.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Wilson M. E. Effect of clindamycin on neutrophil killing of gram-negative periodontal bacteria. Antimicrob Agents Chemother. 1988 Oct;32(10):1521–1527. doi: 10.1128/aac.32.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore N., Travis J., Onunka V. C., Pohl J., Shafer W. M. Identification of the primary antimicrobial domains in human neutrophil cathepsin G. J Biol Chem. 1990 Aug 15;265(23):13584–13588. [PubMed] [Google Scholar]
- Cech P., Lehrer R. I. Phagolysosomal pH of human neutrophils. Blood. 1984 Jan;63(1):88–95. [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn P. A., Popescu N. C., Hanson R. D., Salvesen G., Ley T. J. Genomic organization and chromosomal localization of the human cathepsin G gene. J Biol Chem. 1989 Aug 15;264(23):13412–13419. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K. T., Bodeau A. L., Ganz T., Selsted M. E., Lehrer R. I. In vitro sensitivity of oral, gram-negative, facultative bacteria to the bactericidal activity of human neutrophil defensins. Infect Immun. 1990 Dec;58(12):3934–3940. doi: 10.1128/iai.58.12.3934-3940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K. T., Bodeau A. L. In vitro killing of oral Capnocytophaga by granule fractions of human neutrophils is associated with cathepsin G activity. J Clin Invest. 1991 May;87(5):1585–1593. doi: 10.1172/JCI115172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K. T., Bodeau A. L., Selsted M. E., Ganz T., Lehrer R. I. Killing of oral, gram-negative, facultative bacteria by the rabbit defensin, NP-1. Oral Microbiol Immunol. 1990 Dec;5(6):315–319. doi: 10.1111/j.1399-302x.1990.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Miyasaki K. T., Wilson M. E., Brunetti A. J., Genco R. J. Oxidative and nonoxidative killing of Actinobacillus actinomycetemcomitans by human neutrophils. Infect Immun. 1986 Jul;53(1):154–160. doi: 10.1128/iai.53.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen G., Farley D., Shuman J., Przybyla A., Reilly C., Travis J. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987 Apr 21;26(8):2289–2293. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V. C. Mechanism of staphylococcal resistance to non-oxidative antimicrobial action of neutrophils: importance of pH and ionic strength in determining the bactericidal action of cathepsin G. J Gen Microbiol. 1989 Apr;135(4):825–830. doi: 10.1099/00221287-135-4-825. [DOI] [PubMed] [Google Scholar]
- Shurin S. B., Socransky S. S., Sweeney E., Stossel T. P. A neutrophil disorder induced by capnocytophaga, a dental micro-organism. N Engl J Med. 1979 Oct 18;301(16):849–854. doi: 10.1056/NEJM197910183011601. [DOI] [PubMed] [Google Scholar]
- Sinha S., Watorek W., Karr S., Giles J., Bode W., Travis J. Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Neutral proteinases of human spleen. Purification and criteria for homogeneity of elastase and cathepsin G. Biochem J. 1976 May 1;155(2):255–263. doi: 10.1042/bj1550255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman F., Ornstein L. Electrophoresis of elastase-like esterases from human neutrophils. J Histochem Cytochem. 1974 May;22(5):327–339. doi: 10.1177/22.5.327. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Nukiwa T., Yoshimura K., Quick C. D., States D. J., Holmes M. D., Whang-Peng J., Knutsen T., Crystal R. G. Structure of the human neutrophil elastase gene. J Biol Chem. 1988 Oct 15;263(29):14739–14747. [PubMed] [Google Scholar]
- Thompson H. L., Wilton J. M. Effects of anaerobiosis and aerobiosis on interactions of human polymorphonuclear leukocytes with the dental plaque bacteria Streptococcus mutans, Capnocytophaga ochracea, and Bacteroides gingivalis. Infect Immun. 1991 Mar;59(3):932–940. doi: 10.1128/iai.59.3.932-940.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Barrett A. J. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976 Aug;14(2):555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venge P., Olsson I., Odeberg H. Cationic proteins of human granulocytes. V. Interaction with plasma protease inhibitors. Scand J Clin Lab Invest. 1975 Dec;35(8):737–744. doi: 10.3109/00365517509095805. [DOI] [PubMed] [Google Scholar]
- Watorek W., Farley D., Salvesen G., Travis J. Neutrophil elastase and cathepsin G: structure, function, and biological control. Adv Exp Med Biol. 1988;240:23–31. doi: 10.1007/978-1-4613-1057-0_3. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Jonak-Urbanczyk J. T., Bronson P. M., Dudas K. C., Apicella M. A., Genco R. J. Capnocytophaga species: increased resistance of clinical isolates to serum bactericidal action. J Infect Dis. 1987 Jul;156(1):99–106. doi: 10.1093/infdis/156.1.99. [DOI] [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]


