Abstract
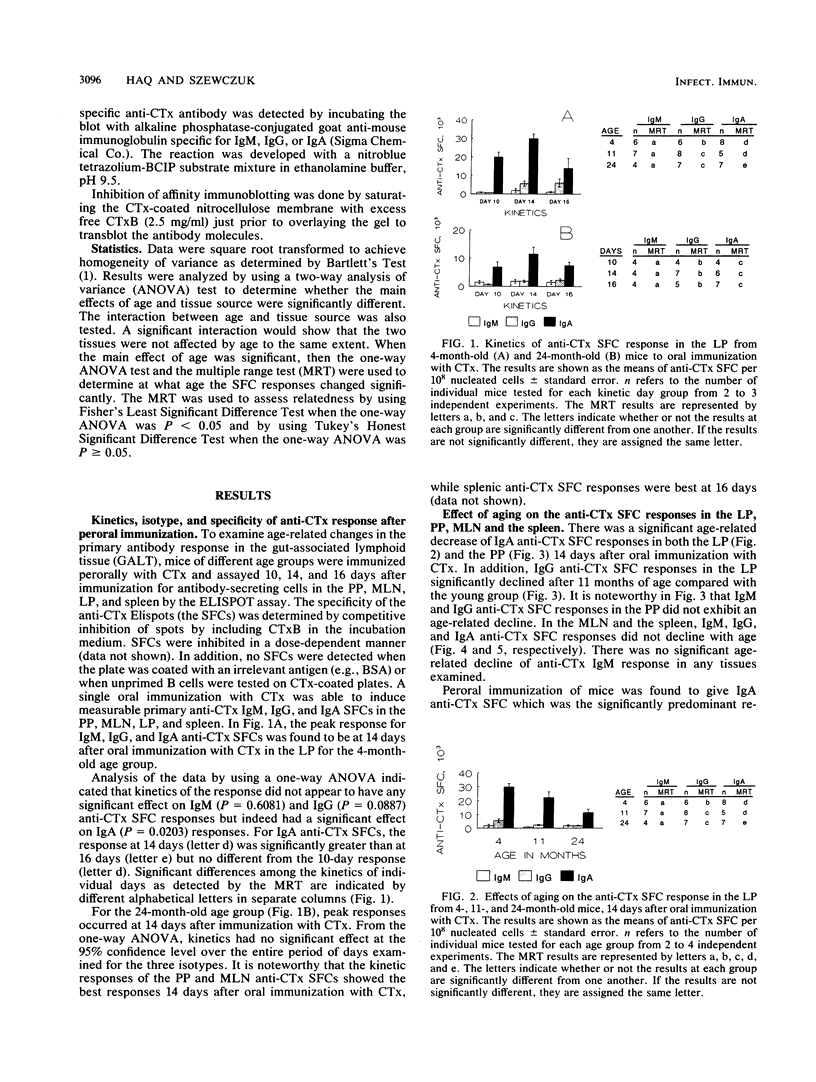
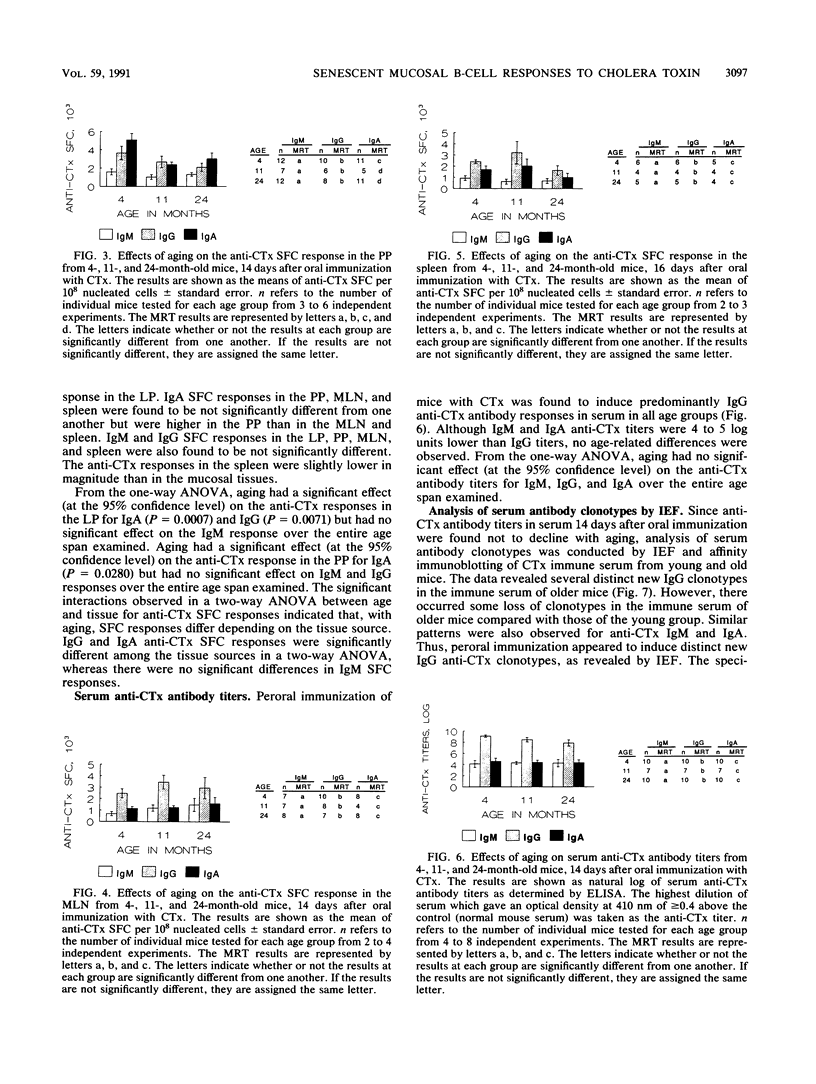
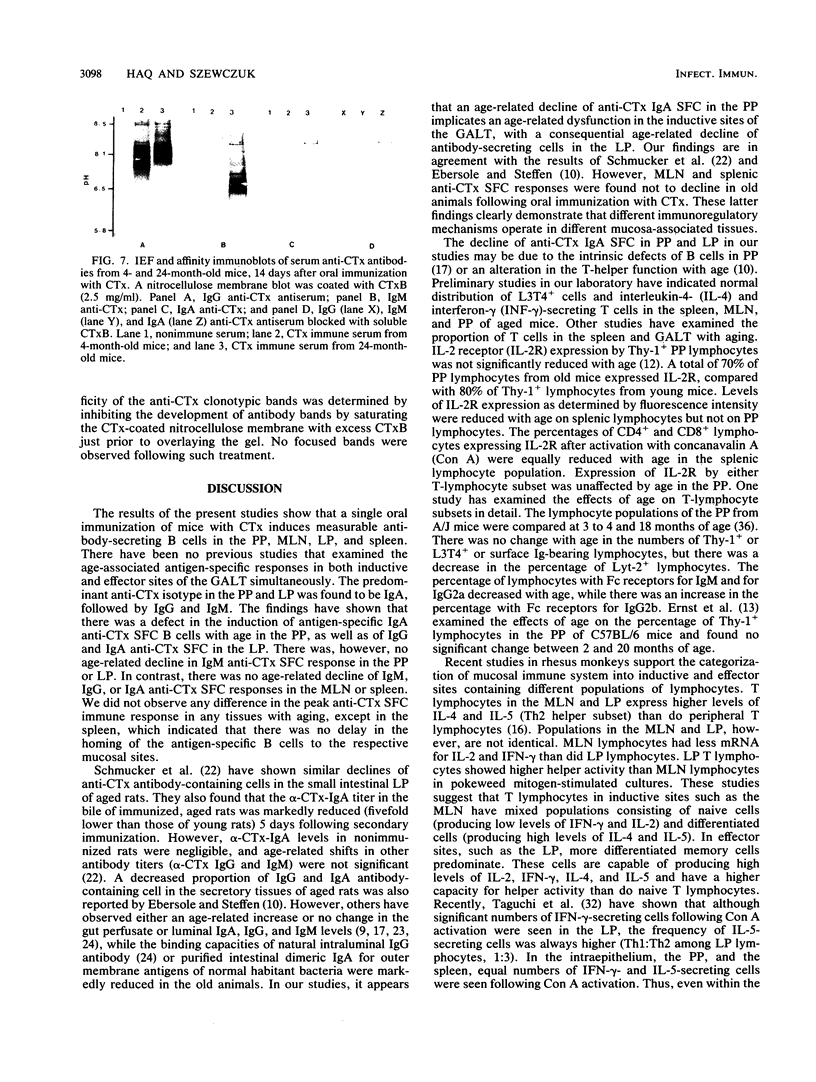
The age-associated primary immune response of B cells from the Peyer's patches (PP), the lamina propria (LP), the mesenteric lymph nodes (MLN), and the spleen of mice following oral immunization with cholera toxin (CTx) was investigated. The induction of immune responses was assessed in 4-, 11-, and 24-month-old, individual C57BL/6J male mice by determining the number and isotype of anti-CTx ELISPOT-forming cells (SFC) in the PP, LPL, MLN, and spleen and the titer and isotype of serum anti-CTx antibody. The data indicate a significant age-associated decline in immunoglobulin G (IgG) and IgA anti-CTx SFC in the LP B cells but only in IgA anti-CTx SFC in the PP. No decline was seen in the anti-CTx SFC response in the MLN and spleen. Peroral immunization of mice with CTx resulted in a serum anti-CTx antibody response which was predominantly of the IgG class in all three age groups of mice tested. There was no age-associated decline in anti-CTx IgM, IgG, or IgA titers in serum. Isoelectric focusing and affinity immunoblotting revealed several distinct new antibody clonotypes in the immune serum of old mice following oral immunization with CTx. The results indicate a loss of immune responsiveness to CTx following oral immunization in senescent PP and LP B cells. The MLN and spleen B-cell responses were found to be refractory to the loss of immune function with aging. These findings suggest a differential effect of aging in the inductive and effector sites of the mucosal immune system, and the loss of antigen-specific IgA responses at mucosal sites may have adverse effects on the host's defense against potential pathogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beagley K. W., Eldridge J. H., Lee F., Kiyono H., Everson M. P., Koopman W. J., Hirano T., Kishimoto T., McGhee J. R. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989 Jun 1;169(6):2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D., McDermott M., Mirski S., Rosenthal K., Tagliabue A. The mucosal immunological network: compartmentalization of lymphocytes, natural killer cells, and mast cells. Ann N Y Acad Sci. 1983 Jun 30;409:164–170. doi: 10.1111/j.1749-6632.1983.tb26866.x. [DOI] [PubMed] [Google Scholar]
- Bienenstock J. The mucosal immunologic network. Ann Allergy. 1984 Dec;53(6 Pt 2):535–540. [PubMed] [Google Scholar]
- Czerkinsky C. C., Nilsson L. A., Nygren H., Ouchterlony O., Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Dec 16;65(1-2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981 Jun;22(6):481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria G., Adorini L., Sabbadini E., Mancini C., Frasca D. Immunoregulation in aging. Ann N Y Acad Sci. 1988;521:182–188. doi: 10.1111/j.1749-6632.1988.tb35277.x. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Smith D. J., Taubman M. A. Secretory immune responses in ageing rats. I. Immunoglobulin levels. Immunology. 1985 Oct;56(2):345–350. [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Steffen M. J. Aging effects on secretory IgA immune responses. Immunol Invest. 1989 Jan-May;18(1-4):59–68. doi: 10.3109/08820138909112227. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Steffen M. J., Pappo J. Secretory immune responses in ageing rats. II. Phenotype distribution of lymphocytes in secretory and lymphoid tissues. Immunology. 1988 Jun;64(2):289–294. [PMC free article] [PubMed] [Google Scholar]
- Ernst D. N., Weigle W. O., McQuitty D. N., Rothermel A. L., Hobbs M. V. Stimulation of murine T cell subsets with anti-CD3 antibody. Age-related defects in the expression of early activation molecules. J Immunol. 1989 Mar 1;142(5):1413–1421. [PubMed] [Google Scholar]
- Ernst D. N., Weigle W. O., Thoman M. L. Retention of T cell reactivity to mitogens and alloantigens by Peyer's patch cells of aged mice. J Immunol. 1987 Jan 1;138(1):26–31. [PubMed] [Google Scholar]
- James S. P., Kwan W. C., Sneller M. C. T cells in inductive and effector compartments of the intestinal mucosal immune system of nonhuman primates differ in lymphokine mRNA expression, lymphokine utilization, and regulatory function. J Immunol. 1990 Feb 15;144(4):1251–1256. [PubMed] [Google Scholar]
- Kawanishi H., Kiely J. Immune-related alterations in aged gut-associated lymphoid tissues in mice. Dig Dis Sci. 1989 Feb;34(2):175–184. doi: 10.1007/BF01536048. [DOI] [PubMed] [Google Scholar]
- Knisley K. A., Rodkey L. S. Affinity immunoblotting. High resolution isoelectric focusing analysis of antibody clonotype distribution. J Immunol Methods. 1986 Dec 4;95(1):79–87. doi: 10.1016/0022-1759(86)90320-0. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- McDermott M. R., Clark D. A., Bienenstock J. Evidence for a common mucosal immunologic system. II. Influence of the estrous cycle on B immunoblast migration into genital and intestinal tissues. J Immunol. 1980 Jun;124(6):2536–2539. [PubMed] [Google Scholar]
- Schibeci A., Wade A. W., Depew W. T., Szewczuk M. R. Analysis of serum antibody repertoires by isoelectric focusing and capillary blotting onto nitrocellulose paper. J Immunol Methods. 1986 May 22;89(2):201–205. doi: 10.1016/0022-1759(86)90358-3. [DOI] [PubMed] [Google Scholar]
- Schmucker D. L., Daniels C. K., Wang R. K., Smith K. Mucosal immune response to cholera toxin in ageing rats. I. Antibody and antibody-containing cell response. Immunology. 1988 Aug;64(4):691–695. [PMC free article] [PubMed] [Google Scholar]
- Senda S., Cheng E., Kawanishi H. Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scand J Immunol. 1988 Feb;27(2):157–164. doi: 10.1111/j.1365-3083.1988.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Senda S., Cheng E., Kawanishi H. IgG in murine intestinal secretions. Aging effect and possible physiological role. Scand J Immunol. 1989 Jan;29(1):41–47. doi: 10.1111/j.1365-3083.1989.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Luther E. A., Marshall J. D., Nguyen K. A., Roopenian D. C., Worthen S. M. Increased expression of major histocompatibility complex antigens on lymphocytes from aged mice. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7624–7628. doi: 10.1073/pnas.84.21.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Ebersole J. L., Taubman M. A. Local and systemic immune response in aged hamsters. Immunology. 1983 Nov;50(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Stoolman L. M. Adhesion molecules controlling lymphocyte migration. Cell. 1989 Mar 24;56(6):907–910. doi: 10.1016/0092-8674(89)90620-x. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. The effect of aging on the secretory immune system of the eye. Immunology. 1988 Mar;63(3):403–410. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Hann L. E., Allansmith M. R. The influence of age on the ocular secretory immune system of the rat. Adv Exp Med Biol. 1987;216B:1395–1407. [PubMed] [Google Scholar]
- Szewczuk M. R., Campbell R. J., Jung L. K. Lack of age-associated immune dysfunction in mucosal-associated lymph nodes. J Immunol. 1981 Jun;126(6):2200–2204. [PubMed] [Google Scholar]
- Szewczuk M. R., Campbell R. J. Lack of age-associated auto-anti-idiotypic antibody regulation in mucosal-associated lymph nodes. Eur J Immunol. 1981 Aug;11(8):650–656. doi: 10.1002/eji.1830110811. [DOI] [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990 Jul 1;145(1):68–77. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Van der Heijden P. J., Bianchi A. T., Stok W., Bokhout B. A. Background (spontaneous) immunoglobulin production in the murine small intestine as a function of age. Immunology. 1988 Oct;65(2):243–248. [PMC free article] [PubMed] [Google Scholar]
- Vetvicka V., Tlaskalová-Hogenová H., Fornůsek L., Ríhová B., Holán V. Membrane and functional characterization of lymphoid and macrophage populations of Peyer's patches from adult and aged mice. Immunology. 1987 Sep;62(1):39–43. [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bartlett A., Bidwell D. E. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978 Jun;31(6):507–520. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade A. W., Szewczuk M. R. Aging and compartmentalization of the mucosal immune system. Adv Exp Med Biol. 1987;216B:1383–1393. [PubMed] [Google Scholar]
- Wade A. W., Szewczuk M. R. Aging, idiotype repertoire shifts, and compartmentalization of the mucosal-associated lymphoid system. Adv Immunol. 1984;36:143–188. doi: 10.1016/s0065-2776(08)60901-3. [DOI] [PubMed] [Google Scholar]



