Abstract
A mutation in the Schizosaccharomyces pombe sid4+ (septation initiation defective) gene was isolated in a screen for mutants defective in cytokinesis. We have cloned sid4+ and have found that sid4+ encodes a previously unknown 76.4-kDa protein that localizes to the spindle pole body (SPB) throughout the cell cycle. Sid4p is required for SPB localization of key regulators of septation initiation, including the GTPase Spg1p, the protein kinase Cdc7p, and the GTPase-activating protein Byr4p. An N-terminally truncated Sid4p mutant does not localize to SPBs and when overproduced acts as a dominant-negative mutant by titrating endogenous Sid4p and Spg1p from the SPB. Conversely, the Sid4p N-terminal 153 amino acids are sufficient for SPB localization. Biochemical studies demonstrate that Sid4p interacts with itself, and yeast two-hybrid analysis shows that its self-interaction domain lies within the C-terminal half of the protein. Our data indicate that Sid4p SPB localization is a prerequisite for the execution of the Spg1p signaling cascade.
To ensure proper segregation of genetic material and organelles to daughter cells during cell division, the onset of cytokinesis must be coordinated with the completion of mitosis. The regulation of cytokinesis has been extensively studied in Schizosaccharomyces pombe because it is amenable to both genetic and biochemical studies. Furthermore, Sz. pombe divides by using a medial actomyosin contractile ring, a process similar to cell division in animal cells (1–3).
Through genetic and biochemical studies in Sz. pombe, key components of a GTPase signaling pathway involved in regulating and initiating cytokinesis have been identified (reviewed in refs. 4–6). Mutations in many of these components give rise to the sid (septation initiation defective) phenotype. In sid mutants, the medial actomyosin ring forms, but it fails to constrict, and septum formation does not occur. However, nuclear division and growth continue, producing elongated multinucleate cells. The sid mutants display genetic interactions (7, 8) that are consistent with the Sid proteins acting in the same biochemical pathway. Several of the sid pathway components have now been analyzed at the molecular level, permitting positioning of components within a signaling cascade. The spg1+ gene encodes a small Ras-superfamily GTPase, whose activation is thought to trigger the sid pathway (8–10). The sid1+, sid2+, and cdc7+ genes encode protein kinases that act downstream of Spg1p activation to initiate septation (refs. 8, 10, and 11; D. Guertin and D. McCollum, personal communication). The two-component GTPase-activating protein (GAP) for Spg1p, composed of Cdc16p and Byr4p (12), works antagonistically to prevent septation.
Several proteins regulating the sid pathway have been shown to localize to the spindle pole body (SPB) during at least some portion of the cell cycle (10, 11, 13). Spg1p is present at the SPB throughout the cell cycle. It is in an inactive GDP-bound form on both SPBs during interphase, an active GTP-bound form on both SPBs during early mitosis, and an active GTP-bound form on one SPB in anaphase B (11). Both Cdc16p and Byr4p are present on the SPB during interphase (13), presumably inactivating Spg1p. During early mitosis, Cdc16p levels decrease at the SPBs, which permits the activation of Spg1p at both SPBs (11, 13). In late anaphase, Cdc16p and Byr4p levels increase at one SPB, resulting in asymmetric inactivation of Spg1p at one SPB (11, 13). Cdc7p kinase activity, which is not cell cycle regulated, is apparently spatially regulated by Spg1p such that its SPB localization is coincident with the activated GTP-bound form of Spg1p (11). Unlike Cdc7p, Sid2p is present at the SPB throughout the cell cycle, but its kinase activity is cell cycle regulated and peaks during medial ring constriction and septation (10). Sid2p activity depends on several components of the sid pathway, including cdc7+ and spg1+ (10). Unlike Cdc7p, Sid2p localizes to the actomyosin ring at late anaphase (10). Thus, it appears that Spg1p recruits Cdc7p to the SPB, which sets in motion the events leading to cell division.
The Spg1p signaling pathway is related to a pathway required for cyclin destruction and exit from mitosis in Saccharomyces cerevisiae. Spg1p, Cdc7p, and Sid2p are homologous with Sc. cerevisiae Tem1p (9, 14, 15), Cdc15p (16), and Dbf2p (8), respectively. In the absence of these protein functions, Sc. cerevisiae cells become arrested in telophase as large-budded cells with segregated chromosomes, an elongated spindle, and high Cdc28/Clb2 protein kinase activity (reviewed in ref. 17). In addition, Sz. pombe Cdc16p and Byr4p are homologues of Sc. cerevisiae Bub2p and Byr4p/Bfa1p, respectively (18–21). The existence of similar pathways in Sz. pombe and Sc. cerevisiae indicates that this signal transduction pathway is conserved, although its target(s) may vary between species.
In a previous screen for mutants defective in cytokinesis, we identified a mutation affecting the Sz. pombe sid pathway, sid4-SA1 (8). This temperature-sensitive (ts) mutation is synthetically lethal with spg1 and sid2 mutations, and Spg1p-induced septum formation requires Sid4p function (8). Here, we further characterize the sid4+ gene and its product and demonstrate that Sid4p localizes to the SPB. Sid4p localization is independent of other components required for septum formation, but all other characterized components of the Spg1p GTPase signaling pathway require Sid4p for their localization to the SPB.
Materials and Methods
Strains, Media, Genetic Methods, and Plasmid Manipulations.
Strains were grown in yeast extract (YE) or minimal medium with appropriate supplements (22). Crosses were performed on glutamate medium, and double-mutant strains were constructed by tetrad analysis (22). Unless otherwise indicated, yeast transformations were performed by electroporation (23). Plasmid manipulations and bacterial transformations used standard procedures (24). Strains and plasmids used in this study are listed in Tables 1 and 2 (supplementary material at www.pnas.org).
Cloning and Sequencing of sid4+.
sid4-SA1 mutant cells (KGY1234) were transformed with a pUR19-based Sz. pombe genomic library (25), and one plasmid, pKG986, permitted growth at restrictive temperature after isolation and retransformation in KGY1234. For integration mapping, the 4.0-kb genomic fragment was subcloned into the Sz. pombe ura4-based integrating vector pJK210 (26) to generate pKG1068. pKG1068 was linearized at the unique NdeI site in sid4+ and transformed into KGY1234. Sid4+ Ura+ transformants were picked and outcrossed to either sid4+ or sid4-SA1 strains to verify cosegregation of the sid4+ phenotype with the ura4+ marker. Approximately 2.7 kb of the 4.0-kb insert was sequenced; it corresponded to a sequenced region of the Sz. pombe genome on cosmid c244. The sid4+ ORF (GenBank accession no. AF153475) corresponds to g3184072 of the Sanger Center (SPBC244.01c). To sequence the sid4-SA1 allele, the sid4 locus was amplified by PCR from genomic DNA prepared from KGY1234. The PCR products were sequenced directly, and a single nucleotide change was detected, at position 2116 (T to A).
Deletion of sid4+.
pSK sid4+ (pKG1098) was cut with NdeI/StuI to release a fragment containing 1,256 nucleotides of sid4; this fragment was replaced with the ura4+ gene, creating pKG1269. This procedure left sequences encoding the first 153 and last 48 amino acids of Sid4p. A fragment containing ura4+, as well as 670 bp of 5′ and 1 kb of 3′ sid4+ flanking sequences, was isolated from pKG1269 and transformed into a diploid strain (KGY137). Transformants were selected on medium lacking uracil, and stable integrants were identified. Replacement of one copy of sid4+ with the ura4+ cassette was confirmed by Southern hybridization analysis in strain KGY1358. Tetrads segregated 2:2 for viability and all viable colonies were Ura−, indicating that deletion of sid4 was lethal.
Overexpression Analysis.
To study the overexpression phenotype of sid4+ constructs, strains were grown to mid-logarithmic phase in the presence of 2 μM thiamin and then grown in medium lacking thiamin to induce the thiamin-repressible nmt1 promoter at temperatures and times indicated.
Epitope Tagging of sid4+.
The sid4+ chromosomal locus was tagged at its 3′ end with sequences encoding either three copies of the hemagglutinin epitope (3xHA) or nine copies of the myc epitope (9xmyc) as described previously (27).
Immunoprecipitations and Immunoblots.
Denatured and native protein lysates were prepared from Sz. pombe as detailed previously (28, 29). Immunoprecipitations with anti-HA or anti-myc antibodies were performed as described (30). Proteins were resolved by SDS/PAGE on a 6–20% gradient gel and analyzed by immunoblotting as described (30).
Glycerol Gradient Analysis.
Native cell lysates were layered onto a 10–30% glycerol gradient and centrifuged at 40,000 rpm for 18 h in an SW-50 Ti rotor (Beckman). Gradient fractions were collected from the bottom and mixed with 5× sample buffer, and fractions were analyzed by SDS/PAGE and immunoblotting. Size standards were sedimented in a parallel gradient and analyzed by Coomassie blue staining.
Yeast Two-Hybrid Analysis.
YPB2 cells were cotransformed with bait (pBI770) and prey (pBI771) plasmids that either did or did not contain sid4+ fragments by the LiOAc/single-stranded DNA/PEG procedure (31) and plated on synthetic dextrose medium lacking leucine and tryptophan (32). Leu+ Trp+ transformants were scored for positive interactions by plating on synthetic dextrose medium lacking leucine, tryptophan, and histidine and containing 5 mM 3-amino-1,2,4-triazole (3AT; Sigma) at 25°C.
In Vitro Transcription and Translation.
pBluescript SK+ (Stratagene) containing sid4-myc (pKG1935) or sid4ΔN-HA (pKG1936) served as templates for in vitro transcription/translation reactions in the TNT coupled reticulocyte lysate system (Promega).
Microscopy.
All fluorescence microscopy was performed on a Zeiss Axioskop, and images were captured with a cooled charge-coupled device camera (Optronics ZVS47DEC). For staining the cell wall or for immunofluorescence, cells were fixed in formaldehyde or methanol, respectively, and processed as described previously (14). 4′,6-Diamidino-2-phenylindole (DAPI) was used to visualize DNA, Calcofluor was used to visualize cell wall material, TAT-1 antibody was used to visualize microtubules (33), polyclonal rabbit anti-Sad1p antibody was used to visualize SPBs (34), and Byr4p localization was determined by using affinity-purified polyclonal anti-Byr4p antibody (13, 18). To visualize Sid4p-HA, Cdc7p-HA, or Spg1p-HA, the 12CA5 monoclonal antibody (anti-HA) was used at 6 μg/ml. After incubation with primary antibody, cells were washed with PBAL as described previously (14). Secondary antibodies, Alexa488 goat anti-rabbit IgG or Alexa594 goat anti-mouse IgG (Molecular Probes), were used at 5 μg/ml.
Results
sid4+ Encodes an Essential Protein of ≈76.4 kDa.
A recessive ts mutant, sid4-SA1, was isolated in a screen for mutants defective in cytokinesis (8). At restrictive temperature, sid4-SA1 cells did not form septa but continued growth and underwent nuclear division, such that cells were elongated and contained multiple nuclei (ref. 8; Fig. 2). We cloned sid4+ by complementation of sid4-SA1 and confirmed by integration mapping that the rescuing plasmid encoded sid4+ and not a high-copy suppressor. The sid4+ genomic clone contains a single ORF interrupted by one intron and encodes a protein with a predicted molecular mass of 76.4 kDa. Database searches using the Basic Local Alignment Sequence Tool (blast; ref. 35) indicated that Sid4p has no obvious similarities to other proteins currently in the database. Sequence analysis of the sid4-SA1 mutant allele revealed a single mutation in nucleotide 2116 (T to A), resulting in a single amino acid change (L629P). By replacing one copy of sid4+ with ura4+ in a diploid background and performing tetrad analysis, we found that sid4+ encodes an essential protein (data not shown).
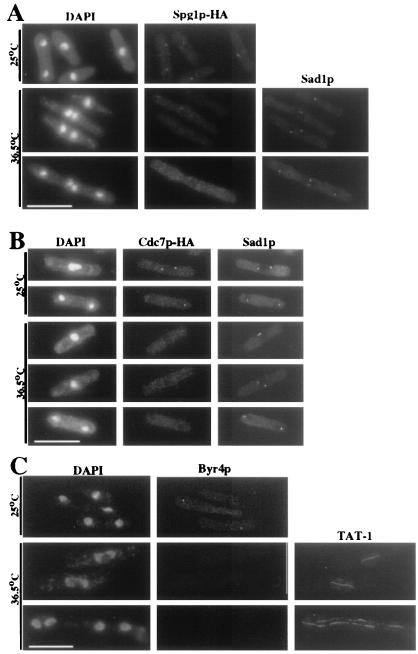
Figure 2.
Spg1p, Cdc7p, and Byr4p SPB localization requires Sid4p function. (A and B) spg1-HA sid4-SA1 (KGY1725; A) or cdc7-HA sid4-SA1 (KGY1600; B) cells were grown to mid-logarithmic phase at 25°C and then shifted to 36.5°C for 4 h (A) or 2 h (B). Cells were fixed and stained with DAPI, anti-HA, and anti-Sad1p. (C) sid4-SA1 (KGY1234) cells were grown to mid-logarithmic phase at 25°C and then shifted to 36.5°C for 4 h. Cells were fixed and stained with DAPI, anti-Byr4p, and TAT-1. (Bar = 10 μm.)
Sid4p Localizes to SPBs Independent of Microtubules and Other Sid Proteins.
To allow detection of Sid4p, we constructed sid4-HA and sid4-myc strains (see Materials and Methods). Both of these strains were morphologically indistinguishable from wild type at all temperatures, indicating that the epitope tags did not alter the function of Sid4p. Anti-HA antibody detected a protein of ≈80 kDa in the sid4-HA strain (Fig. 4 and data not shown), and anti-myc antibody detected a protein of ≈100 kDa in the sid4-myc strain (Fig. 4 and data not shown), as predicted.
Figure 4.
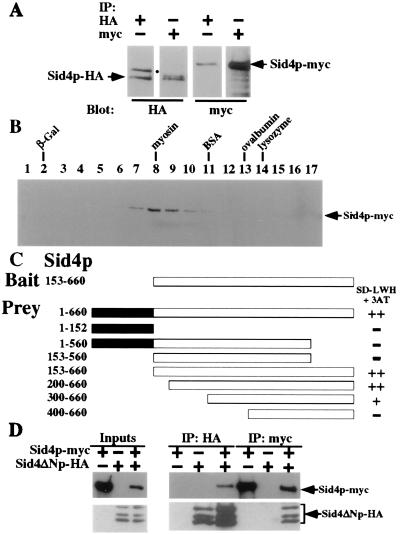
Sid4p interacts with itself. (A) Cell lysates were prepared under native conditions from a sid4-HA/sid4-myc heterozygous diploid strain (KGY2732), immunoprecipitated with either anti-HA or anti-myc, and analyzed by immunoblotting with either anti-HA or anti-myc. The positions of Sid4p-HA and Sid4p-myc are indicated. The position of a nonspecific HA-crossreactive protein is indicated (●). (B) Sid4p-myc sediments with an apparent mass of ≈200 kDa. Cell lysates were prepared under native conditions from a sid4-myc strain (KGY1340) and analyzed by sedimentation in a glycerol gradient. Fraction numbers are indicated. Peak fractions for molecular mass markers sedimented in a parallel gradient are indicated (lysozyme, 14 kDa; ovalbumin, 45 kDa; BSA, 66 kDa; myosin, 220 kDa; β-galactosidase (β-Gal), 464 kDa). (C) Sid4p dimerization domain. Sid4ΔNp in pBI770 (Bait) and Sid4p fragments in pBI771 (Prey) were cotransformed into strain YPB2 (KGY1400) and interactions were analyzed as described in the text. ++, Strong interaction; +, moderate interaction; −, no interaction. The amino acid residues encoded by each fragment are indicated. (D) Sid4p-myc binds directly to Sid4ΔNp-HA. Sid4p-myc and Sid4ΔNp-HA were translated in vitro either separately or together and immunoprecipitated with either anti-HA or anti-myc, and immunopellets were analyzed by immunoblot analysis with either anti-HA or anti-myc. Ten percent of the input into the immunoprecipitation reactions was also resolved by SDS/PAGE and immunoblotted. The positions of Sid4p-myc and Sid4ΔNp-HA (and its internal initiation products) are indicated.
The intracellular localization of Sid4p-HA was examined by immunofluorescence using antibodies to the HA and myc epitopes. In wild-type cells, no discrete staining was observed (data not shown); however, in sid4-HA and sid4-myc cells, dots were detected at the periphery of the nuclei throughout the cell cycle (Fig. 1A; data not shown). These dots coincided with those visualized by staining for Sad1p (Fig. 1A), a known constituent of the SPB (34). We conclude that Sid4p, like Spg1p, localizes to SPBs irrespective of cell cycle stage.
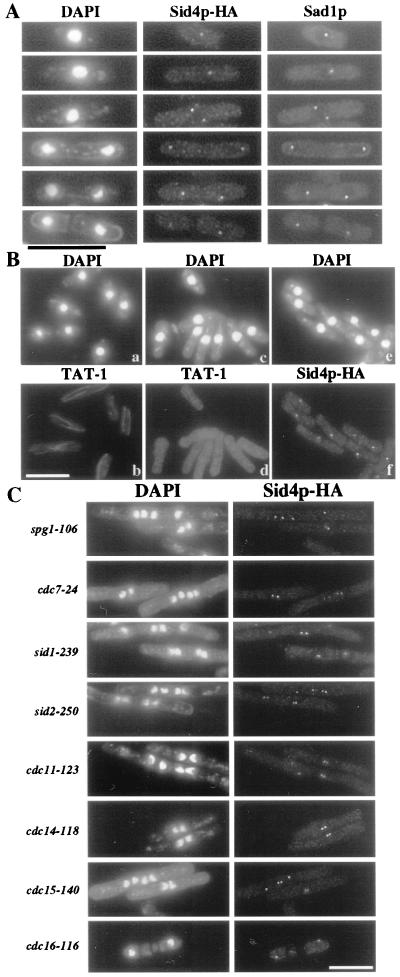
Figure 1.
(A) Sid4p-HA localizes to the SPBs throughout the cell cycle. sid4-HA cells (KGY1576) were fixed and stained with DAPI, anti-HA, and anti-Sad1p. (B) Sid4p-HA SPB localization does not require intact microtubules. Before (a and b) or after (c–f) cold shock, KGY1576 cells were fixed and stained with DAPI and either TAT-1 or anti-HA. (C) Sid4p SPB localization is independent of the known proteins required for cytokinesis. Endogenous sid4-HA was visualized in spg1–106 (KGY1593), cdc7–24 (KGY1594), sid1–239 (KGY1591), sid2–250 (KGY1592), cdc11–123 (KGY1595), cdc14-118 (KGY1596), cdc15-140 (KGY1597), or cdc16-116 (KGY1598). Cells were grown to mid-logarithmic phase at 25°C, shifted to 36°C for 4 h, and fixed and stained with DAPI and anti-HA. (Bar = 10 μm.)
To determine whether Sid4p localization is dependent upon microtubules, sid4-HA cells were placed on ice for 25 min to disrupt the microtubule array (34). After cold shock, Sid4p-HA was still detected at the SPBs (Fig. 1B). Similarly, Sid4p-HA localized to the SPBs when a strain carrying the β-tubulin mutation, nda3-KM311 (36, 37), was incubated at its restrictive temperature to disrupt microtubule arrays (data not shown).
Localization of the Cdc7p and Sid2p protein kinases to the SPB depends upon Spg1p (10, 11). To determine whether Sid4p localization also depends on Spg1p or other known proteins required for septum formation, Sid4p-HA localization was examined in various ts septation mutants. Sid4p-HA localized to SPBs in all of the ts mutants tested (Fig. 1C), as well as in a byr4+ shut-off strain (18) (data not shown).
Spg1p, Cdc7p, and Byr4p SPB Localization Depend on Sid4p.
We next asked whether the localization of Cdc7p, Spg1p, or Byr4p depends on Sid4p. Because Cdc7p localizes to SPBs only during mitosis, cells were stained with anti-Sad1p to identify cells in the early and late stages of anaphase. At permissive temperature, Spg1p-HA, Cdc7p-HA, and Byr4p localized to SPBs in the sid4-SA1 mutant (Fig. 2), but at restrictive temperature, these proteins were not detected at SPBs (Fig. 2), indicating that Sid4p is essential for their localization to the SPB.
Effects of Sid4p Overproduction.
Overproduction of proteins involved in cytokinesis can lead either to inhibition of septum formation as seen with Byr4p (18) or to uncontrolled septation as seen with Spg1p (8, 9) or Plo1p (38). To determine whether overproduction of Sid4p affects cytokinesis, we examined cells overproducing Sid4p from the nmt1 promoter (see Materials and Methods). Sid4p overproduction rescued the temperature sensitivity of the sid4-SA1 mutant (data not shown) and did not generate a detectable phenotype in wild-type cells. The overproduction of Sid4p in these strains was confirmed by immunoblot analysis (data not shown).
In attempts to map the functional domains of Sid4p, we found that a construct expressing the N-terminal 153 amino acids (pKG1386) or the N-terminal 153 amino acids fused to three copies of the HA epitope (pKG1534) did not rescue the sid4 ts or deletion mutants. In contrast, a construct expressing moderate levels of Sid4p lacking the N-terminal 153 amino acids (Sid4ΔNp, pKG1225) rescued the sid4 ts mutant although not the sid4 deletion mutant. Overproduction of either pKG1386 or pKG1534 in a wild-type background did not affect cell growth or morphology (data not shown). That N153Sid4p-HA was overproduced was confirmed by immunoblot analysis (data not shown). Interestingly, overproduction of Sid4ΔNp inhibited septum formation and produced elongated cells containing multiple nuclei. This was seen best when the nmt1-sid4ΔN construct was integrated in single copy (KGY1347) (Fig. 3A). The dominant-negative phenotype of Sid4ΔNp suggested that it may titrate out a factor required for septum formation.
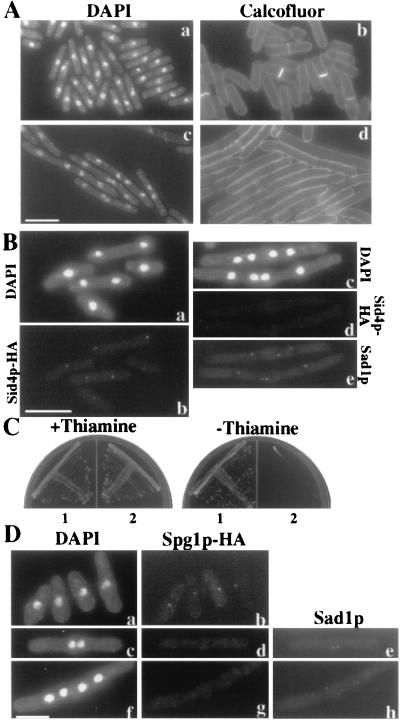
Figure 3.
(A) Overproduction of Sid4ΔNp inhibits cytokinesis. Wild-type cells carrying a single integrated copy of sid4ΔN under control of the thiamin-repressible nmt1 promoter (KGY1347) were grown to mid-logarithmic phase at 32°C in medium containing thiamin (a and b). To induce overexpression of sid4ΔN, cells were then grown in medium lacking thiamin for 22 h at 32°C (c and d). Cells were fixed and stained with DAPI or with Calcofluor. (B) Sid4ΔNp overproduction prevents Sid4p SPB localization. sid4-HA cells carrying a single integrated copy of nmt1-sid4ΔN (KGY1742) were grown to mid-logarithmic phase at 32°C under repressing conditions (a and b) or were shifted to inducing conditions for 20 h at 32°C (c–e). Cells were stained with DAPI, anti-HA, and anti-Sad1p. (C) Full-length Sid4p rescues Sid4ΔNp overproduction phenotype. KGY1347 was transformed with plasmid pUR19 sid4+ carrying full-length Sid4p (sector 1) or control plasmid pUR19 (sector 2) and grown on minimal medium with or without thiamin to repress or induce production of Sid4ΔNp. (D) Sid4ΔNp overproduction prevents Spg1p SPB localization. spg1-HA cells carrying a single integrated copy of nmt1-sid4ΔN (KGY1741) were grown to mid-logarithmic phase at 32°C under repressing (a and b) or inducing conditions (c–h). Cells were stained with DAPI, anti-HA, and anti-Sad1p to detect SPBs. (Bar = 10 μm.)
To test this possibility, the localization of endogenous Sid4p-HA was examined in cells overproducing untagged Sid4ΔNp under the control of the nmt1 promoter (KGY1742). When Sid4ΔNp was produced at moderate levels, Sid4p-HA SPB staining was strong (Fig. 3B). However, when Sid4ΔNp was overproduced, Sid4p-HA SPB staining was reduced and most often undetectable (Fig. 3B). In contrast, overproduction of Byr4p (18), which also inhibits septation, did not reduce the amount of Sid4p detectable at the SPBs (data not shown). If Sid4ΔNp titrates Sid4p-HA from the SPB, then compensatory overproduction of Sid4p might rescue the dominant-negative phenotype of Sid4ΔNp overproduction. Indeed, when sid4+ was expressed from a multicopy plasmid along with Sid4ΔNp, cell growth was restored (Fig. 3C).
Although the data of Fig. 2A showed that Spg1p localization to the SPB depends on Sid4p, it was not clear whether this depended upon Sid4p localizing to the SPB. To address this question, we removed Sid4p from the SPB by overproducing Sid4ΔNp. Under these conditions, Spg1p-HA staining was not detected at the SPBs (Fig. 3D). Sid4ΔNp overproduction also blocked Sid2p localization to the SPB (data not shown). We conclude that the function of Sid4p in recruiting other proteins to the SPB depends on its own localization to the SPB.
Dimerization of Sid4p.
Sid4ΔNp rescues the sid4 ts mutant even though it lacks a domain essential for function. This observation suggested that sid4ΔN and sid4-SA1 exhibit intragenic complementation that might depend on the formation of dimers or larger multimers; moreover, the domain allowing this interaction must be present within Sid4ΔNp. To examine this possibility, a heterozygous Sid4p-HA/Sid4p-myc diploid strain was constructed. When either anti-HA or anti-myc was used, both Sid4p-HA and Sid4p-myc were co-immunoprecipitated from native cell lysates (Fig. 4A). To examine the apparent size of the Sid4p complex, lysates containing Sid4p-myc were resolved by sedimentation on a glycerol gradient and analyzed by immunoblot analysis (Fig. 4B). Sid4p-myc was detected in fractions 7 through 11 and peaked in fractions 8 and 9, similar to myosin (220 kDa); these data suggest that Sid4p-myc (≈100 kDa) may form a homodimer.
The hypothesis that a Sid4p dimerization domain resided within Sid4ΔNp was supported by the ability of Sid4ΔNp-HA to be co-immunoprecipitated with myc antibodies when expressed in a sid4-myc strain (data not shown). To further define the interaction domain, the yeast two-hybrid system was used (32, 39). As expected, Sid4ΔNp did not interact with empty prey plasmid (data not shown) but was able to interact with itself (Fig. 4C). N- and C-terminal truncations of Sid4ΔNp demonstrated that the entire C-terminal half of the protein was important for Sid4p–Sid4p interaction in this assay (Fig. 4C).
To rule out the possibility that an unidentified yeast protein was bridging the interaction of Sid4p with itself in the assays just described, Sid4p-myc and Sid4ΔNp-HA were translated either separately or together in vitro. Immunoprecipitation and immunoblot analysis indicated that, when cotranslated, they were able to associate with one another (Fig. 4D). These results strongly suggest that Sid4p can interact directly with itself.
The N Terminus of Sid4p Is Sufficient for SPB Localization.
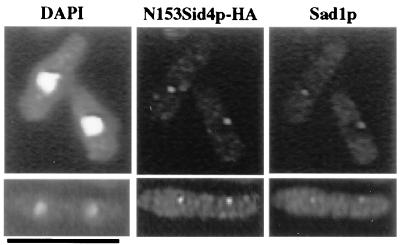
Because overproduction of Sid4ΔNp prevented assembly of other pathway components at the SPB, we inferred that the SPB localization signal within Sid4p resided in the N-terminal fragment that had been removed from Sid4ΔNp. To test this possibility directly, six copies of the HA epitope were appended to N153Sid4p and the localization of this fusion protein was determined. Anti-HA detected dots in cells expressing N153Sid4p-HA, and these dots colocalized with Sad1p (Fig. 5). Similarly, when green fluorescent protein (GFP) was appended to amino acids N153Sid4p at the C terminus and expressed from a plasmid, GFP was detected at SPBs in live cells (data not shown). Together these data indicate that the N-terminal 153 amino acids of Sid4p contain a SPB localization domain.
Figure 5.
Sid4p contains an SPB localization domain. Wild-type cells expressing the N-terminal 153 amino acids of Sid4p tagged with the HA epitope under control of the nmt1 promoter (pKG1534) were grown to mid-logarithmic phase under inducing conditions at 32°C. Cells were fixed and stained with DAPI, anti-HA to detect N153Sid4p-HA, and anti-Sad1p. (Bar = 10 μm.)
Discussion
Through genetic and biochemical studies in Sz. pombe, a signal transduction pathway involving Spg1p, Cdc7p, Cdc11p, Cdc14p, Cdc16p, Byr4p, Sid1p, Sid2p, and Sid4p proteins has been shown to regulate the onset of cytokinesis (4–6). Several components of this signaling cascade localize to the SPB. These include the GTPase Spg1p, the two-component GTPase-activating protein for Spg1p, and two protein kinases, Cdc7p and Sid2p, which act downstream of activated Spg1p (10, 11, 13). Like Spg1p and Sid2p, Sid4p-HA was detected at the SPB throughout the cell cycle. Using several tagged versions of Sid4p, we did not detect Sid4p at the medial ring (see above; additional data not shown). We and Sparks et al. (10) have found that Sid4p function is critical for the ability of at least four other proteins (Spg1p, Cdc7p, Bry4p, and Sid2p) to localize to the SPB, suggesting that Sid4p either acts upstream of Spg1p, Cdc7p, and Sid2p or perhaps serves as a scaffold for this pathway.
Like other proteins in this pathway, Sid4p is essential for viability, but unlike most other components thus far described, it does not have an obvious homologue in Sc. cerevisiae. The coils program (version 2.2, window 28; ref. 40) indicates the probable presence of two coiled-coil domains within the C-terminal half of Sid4p that may be involved in interactions of Sid4p with itself or with another protein(s). The sid4-SA1 mutant allele contains a single amino acid change from leucine to proline within the second putative coiled-coil domain. As a proline would interrupt the α-helical structure of the coiled-coil, the ts phenotype of sid4-SA1 might be explained by the disruption of a protein–protein interaction mediated by this domain.
Although overproduction of Sid4ΔNp is lethal to cells, moderate levels of Sid4ΔNp rescue the sid4 ts mutant but not the sid4 null mutant. This observation suggests that Sid4ΔNp lacks a domain essential for function. As tagged versions of the N-terminal 153 amino acids of Sid4p can localize to the SPB, it appears that the missing domain in Sid4ΔNp is the SPB localization domain. However, localization of the N-terminal Sid4p fragment to the SPB requires its overproduction (data not shown), suggesting that dimerization of Sid4p might improve the efficiency of localization. In future studies, it will be of interest to define the amino acids essential for SPB localization and to identify other SPB protein(s) with which these amino acids interact.
Although the Spg1p pathway is conserved between Sz. pombe and Sc. cerevisiae, the Sz. pombe guanine nucleotide exchange factor (GEF) for Spg1p has not yet been identified. It remains possible that Sid4p participates in the GEF activity for Spg1p, but this seems unlikely for several reasons. First, Sid4p has no sequence similarity to Sc. cerevisiae Lte1p, which is predicted to provide GEF activity for Tem1p (15, 41). Second, if Sid4p were the GEF for Spg1p, mutation of sid4+ might rescue mutations of cdc16+; this is not the case (data not shown). Similarly, overproduction of Sid4p might result in an increase in activated, GTP-bound Spg1p, which would in turn produce a phenotype similar to Spg1p-induced uncontrolled septum formation. However, overproduction of full-length Sid4p has no effect on cell growth or morphology.
Our data suggest that Sid4p might act as a scaffold at the SPB upon which other components of the Spg1p signaling pathway assemble. However, we have been unable to detect a direct physical interaction between Sid4p and Spg1p, Cdc7p, or Sid2p by either yeast two-hybrid or co-immunoprecipitation analyses (data not shown). Indeed, in yeast cell lysates, we can detect only interactions of Sid4p with itself. There are at least three possible explanations for these results. First, the form of Sid4p interacting with other pathway components might be embedded within the SPB, a structure well known for its resistance to solubilization. Perhaps pertinent to this hypothesis, Sid4p contains two predicted coiled-coil domains, as do several essential structural SPB components, including Cut12p (42), Spc110p (43, 44), Spc72p (45), Spc42p (46), and Mps2p (47). Second, there may exist an unidentified component of the pathway, such as the presumed GEF for Spg1p that links Sid4p to Spg1p. Third, Sid4p might not interact directly with any pathway component but instead be involved in organizing the SPB such that pathway components can bind to it. Further studies of the interaction between Sid4p and SPB components, as well as between Sid4p and the signaling pathway components, should not only enhance our understanding of the regulation of cytokinesis in Sz. pombe but also provide insight into the conserved features between the septation initiation pathway of Sz. pombe and the mitotic exit pathway of Sc. cerevisiae.
Supplementary Material
Acknowledgments
We thank J. Bähler, J. R. Pringle, and K. Nasmyth for providing epitope tagging cassettes; K. Gull for the TAT-1 monoclonal antibody; V. Simanis and C. Albright for Sz. pombe strains; C. Albright for Byr4p antibodies; I. Hagan for Sad1p antibodies; S. M. Hemmingsen for yeast two-hybrid vectors; and D. McCollum, M. Balasubramanian, W. H. McDonald, C. G. Burns, A. Feoktistova, J. L. Morrell, and an anonymous reviewer for critical reading and editing of our manuscript. We are grateful to all members of the Gould lab for their support and advice during the course of this work. This work was supported by the Howard Hughes Medical Institute, of which K.L.G. is an Associate Investigator.
Abbreviations
- GFP
green fluorescent protein
- HA
hemagglutinin
- SPB
spindle pole body
- ts
temperature-sensitive
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF153475).
References
- 1.Marks J, Hagan I M, Hyams J S. J Cell Sci Suppl. 1986;5:229–241. doi: 10.1242/jcs.1986.supplement_5.15. [DOI] [PubMed] [Google Scholar]
- 2.Kitayama C, Sugimoto A, Yamamoto M. J Cell Biol. 1997;137:1309–1319. doi: 10.1083/jcb.137.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCollum D, Balasubramanian M K, Pelcher L E, Hemmingsen S M, Gould K L. J Cell Biol. 1995;130:651–660. doi: 10.1083/jcb.130.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang F, Nurse P. Cell. 1996;84:191–194. doi: 10.1016/s0092-8674(00)80973-3. [DOI] [PubMed] [Google Scholar]
- 5.Le Goff X, Utzig S, Simanis V. Curr Genet. 1999;35:571–584. doi: 10.1007/s002940050455. [DOI] [PubMed] [Google Scholar]
- 6.Gould K L, Simanis V. Genes Dev. 1997;11:2939–2951. doi: 10.1101/gad.11.22.2939. [DOI] [PubMed] [Google Scholar]
- 7.Marks J, Fankhauser C, Simanis V. J Cell Sci. 1992;101:801–808. doi: 10.1242/jcs.101.4.801. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian M K, McCollum D, Chang L, Wong K C, Naqvi N I, He X, Sazer S, Gould K L. Genetics. 1998;149:1265–1275. doi: 10.1093/genetics/149.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt S, Sohrmann M, Hofmann K, Woollard A, Simanis V. Genes Dev. 1997;11:1519–1534. doi: 10.1101/gad.11.12.1519. [DOI] [PubMed] [Google Scholar]
- 10.Sparks C A, Morphew M, McCollum D. J Cell Biol. 1999;146:777–790. doi: 10.1083/jcb.146.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohrmann M, Schmidt S, Hagan I, Simanis V. Genes Dev. 1998;12:84–94. doi: 10.1101/gad.12.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furge K A, Wong K, Armstrong J, Balasubramanian M, Albright C F. Curr Biol. 1998;8:947–954. doi: 10.1016/s0960-9822(98)70394-x. [DOI] [PubMed] [Google Scholar]
- 13.Cerutti L, Simanis V. J Cell Sci. 1999;112:2313–2321. doi: 10.1242/jcs.112.14.2313. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramanian M K, McCollum D, Gould K L. Methods Enzymol. 1997;283:494–506. doi: 10.1016/s0076-6879(97)83039-x. [DOI] [PubMed] [Google Scholar]
- 15.Shirayama M, Matsui Y, Toh-E A. Mol Cell Biol. 1994;14:7476–7482. doi: 10.1128/mcb.14.11.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fankhauser C, Simanis V. EMBO J. 1994;13:3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshaies R J. Curr Opin Genet Dev. 1997;7:7–16. doi: 10.1016/s0959-437x(97)80103-7. [DOI] [PubMed] [Google Scholar]
- 18.Song K, Mach K E, Chen C Y, Reynolds T, Albright C F. J Cell Biol. 1996;133:1307–1319. doi: 10.1083/jcb.133.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R. Proc Natl Acad Sci USA. 1999;96:4989–4994. doi: 10.1073/pnas.96.9.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fankhauser C, Marks J, Reymond A, Simanis V. EMBO J. 1993;12:2697–2704. doi: 10.1002/j.1460-2075.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexandru G, Zachariae W, Schleiffer A, Nasmyth K. EMBO J. 1999;18:2707–2721. doi: 10.1093/emboj/18.10.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno S, Klar A, Nurse P. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 23.Prentice H L. Nucleic Acids Res. 1992;20:621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, New York: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 25.Barbet N, Muriel W J, Carr A M. Gene. 1992;114:59–66. doi: 10.1016/0378-1119(92)90707-v. [DOI] [PubMed] [Google Scholar]
- 26.Keeney J B, Boeke J D. Genetics. 1994;136:849–856. doi: 10.1093/genetics/136.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bähler J, Wu J Q, Longtine M S, Shah N G, McKenzie A R, Steever A B, Wach A, Philippsen P, Pringle J R. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Gould K L, Nurse P. Nature (London) 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 29.Gould K L, Moreno S, Owen D J, Sazer S, Nurse P. EMBO J. 1991;10:3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald W H, Ohi R, Smelkova N, Frendewey D, Gould K L. Mol Cell Biol. 1999;19:5352–5362. doi: 10.1128/mcb.19.8.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gietz R D, Schiestl R H, Willems A R, Woods R A. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 32.Kohalmi S E, Nowak J, Crosby W L. In: Differentially Expressed Genes in Plants: A Bench Manual. Hansen E, Harper G, editors. London: Taylor & Francis; 1997. pp. 63–82. [Google Scholar]
- 33.Woods A, Baines A J, Gull K. J Cell Sci. 1989;93:501–508. doi: 10.1242/jcs.93.3.501. [DOI] [PubMed] [Google Scholar]
- 34.Hagan I, Yanagida M. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 36.Umesono K, Toda T, Hayashi S, Yanagida M. J Mol Biol. 1983;168:271–284. doi: 10.1016/s0022-2836(83)80018-7. [DOI] [PubMed] [Google Scholar]
- 37.Toda T, Umesono K, Hirata A, Yanagida M. J Mol Biol. 1983;168:251–270. doi: 10.1016/s0022-2836(83)80017-5. [DOI] [PubMed] [Google Scholar]
- 38.Ohkura H, Hagan I M, Glover D M. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 39.Fields S, Song O. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 40.Lupas A. Curr Opin Struct Biol. 1997;7:388–393. doi: 10.1016/s0959-440x(97)80056-5. [DOI] [PubMed] [Google Scholar]
- 41.Shirayama M, Matsui Y, Tanaka K, Toh-E A. Yeast. 1994;10:451–461. doi: 10.1002/yea.320100404. [DOI] [PubMed] [Google Scholar]
- 42.Bridge A J, Morphew M, Bartlett R, Hagan I M. Genes Dev. 1998;12:927–942. doi: 10.1101/gad.12.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilmartin J V, Goh P Y. EMBO J. 1996;15:4592–4602. [PMC free article] [PubMed] [Google Scholar]
- 44.Kilmartin J V, Dyos S L, Kershaw D, Finch J T. J Cell Biol. 1993;123:1175–1184. doi: 10.1083/jcb.123.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soues S, Adams I R. J Cell Sci. 1998;111:2809–2818. doi: 10.1242/jcs.111.18.2809. [DOI] [PubMed] [Google Scholar]
- 46.Donaldson A D, Kilmartin J V. J Cell Biol. 1996;132:887–901. doi: 10.1083/jcb.132.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munoz-Centeno M C, McBratney S, Monterrosa A, Byers B, Mann C, Winey M. Mol Biol Cell. 1999;10:2393–2406. doi: 10.1091/mbc.10.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.