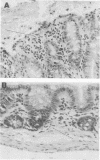
Abstract
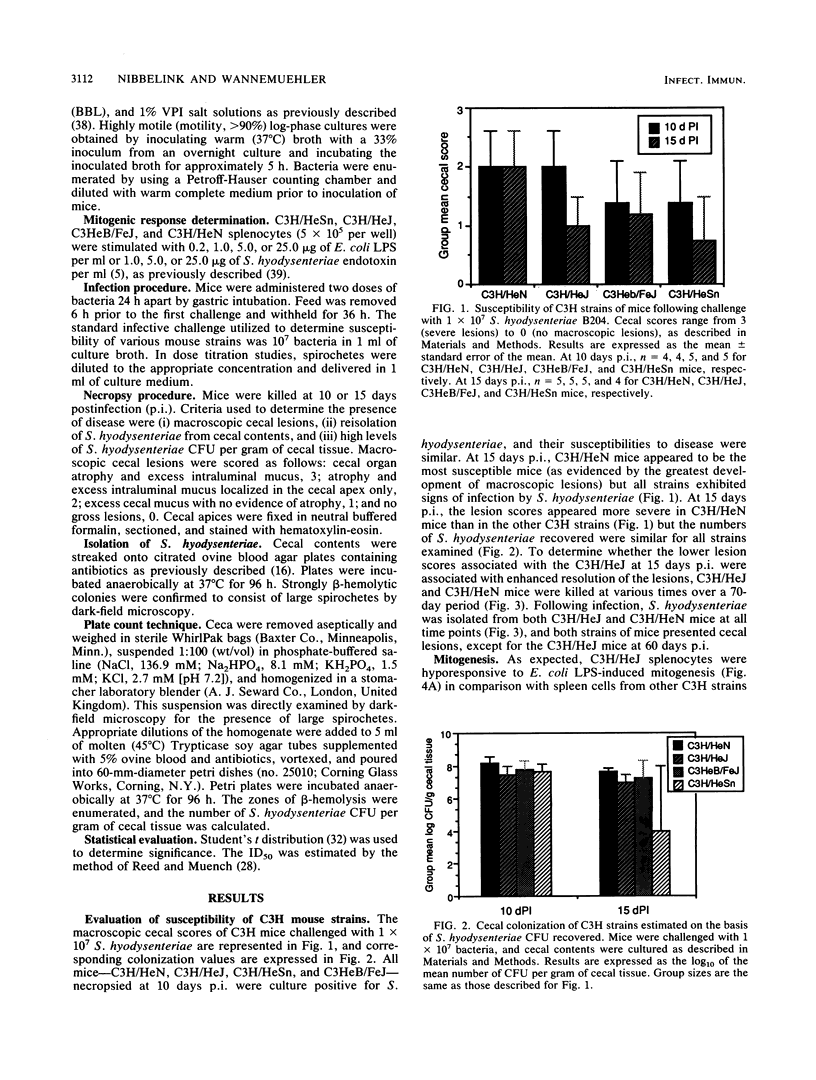
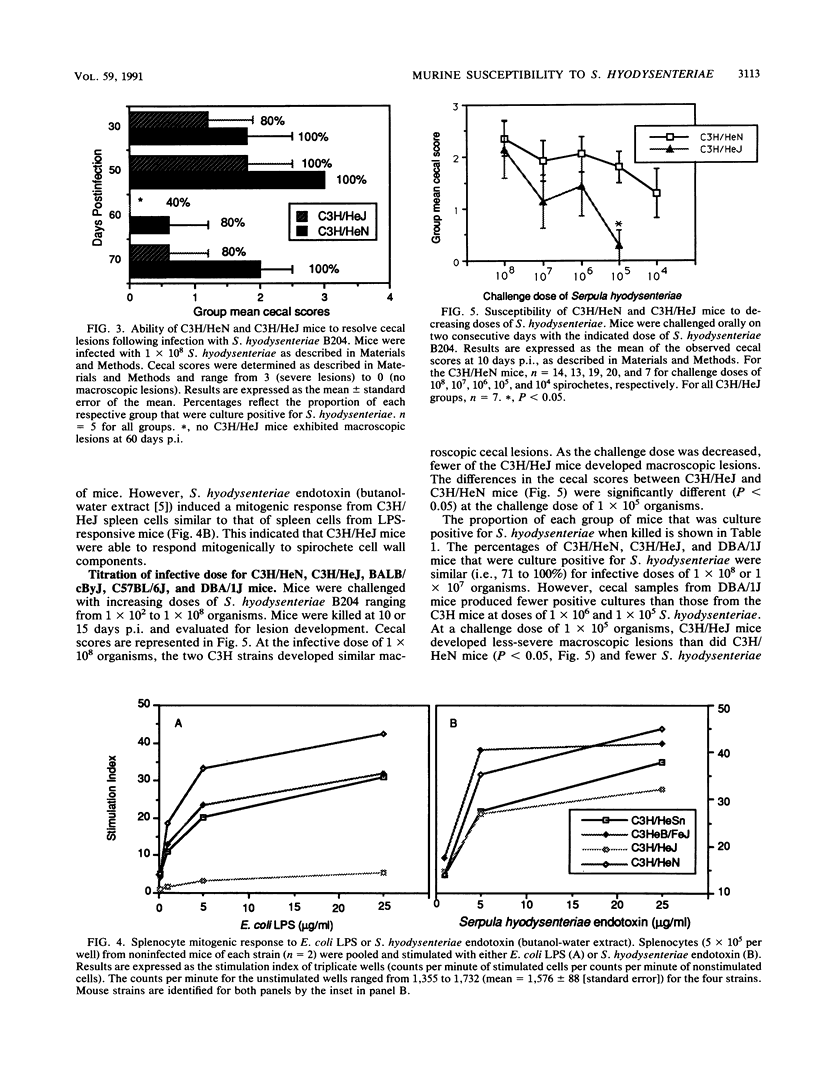
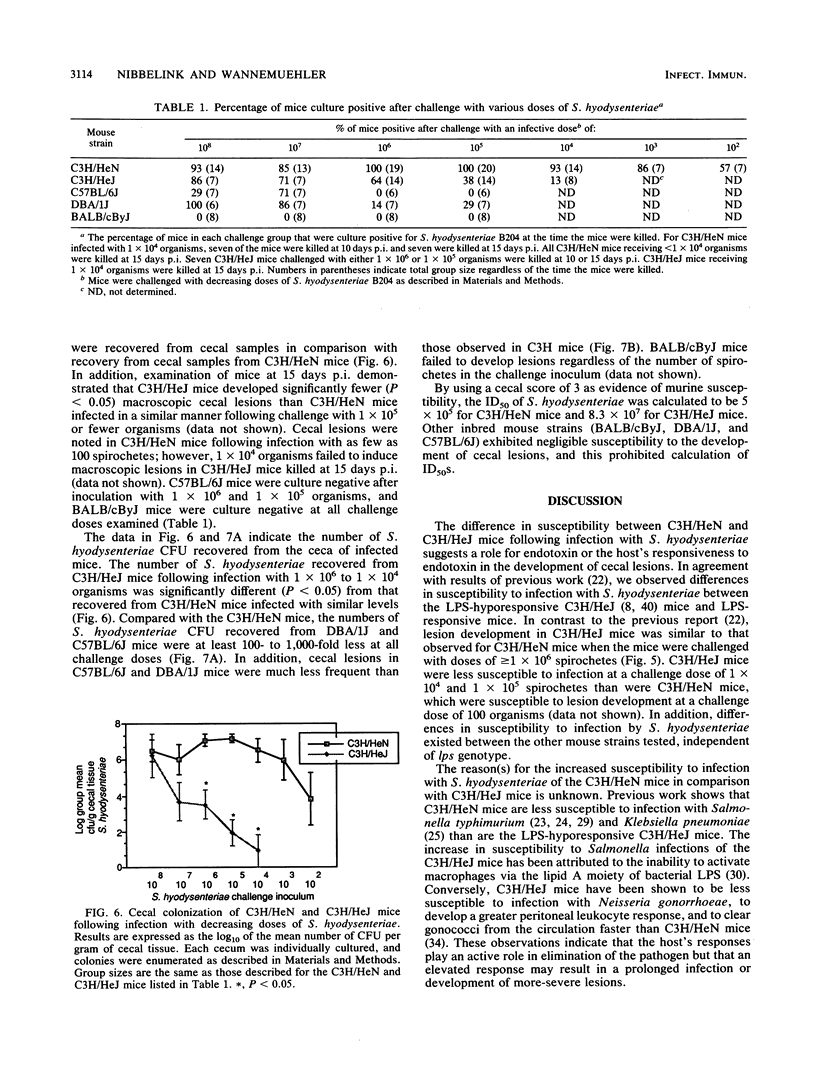
Several inbred strains of mice were inoculated with Serpula (Treponema) hyodysenteriae B204 to determine susceptibility to infection. Challenge doses of 10(7) or 10(8) spirochetes induced cecal lesions in C3H/HeJ mice and other C3H strains of mice. However, more than a 100-fold difference existed between the dose required to induce lesions in 50% of the infected C3H/HeJ mice (8.3 x 10(7)) and that required to induce them in 50% of the infected C3H/HeN mice (5 x 10(5)). C3H/HeJ mice lack a splenocyte mitogenic response to Escherichia coli lipopolysaccharide but exhibited a mitogenic response comparable to those of other C3H strains of mice when stimulated with S. hyodysenteriae endotoxin (butanol-water extract). Different inbred strains exhibited different susceptibilities to infection, with the strain C3H/HeN being the most susceptible on the basis of colonization and development of macroscopic cecal lesions. The ity gene had no apparent effect on susceptibility of mice challenged with S. hyodysenteriae. The involvement of the H-2 haplotype with susceptibility is unclear, but the mice bearing H-2k were more susceptible than mice with the H-2b, H-2d, or H-2q haplotype. These data support the hypothesis that the host's responsiveness to lipopolysaccharide influences the susceptibility to infection with S. hyodysenteriae. However, differences in susceptibility between inbred mice exist independent of the lps locus, suggesting that there are other inherent differences between mouse strains that affect susceptibility to infection by S. hyodysenteriae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albassam M. A., Olander H. J., Thacker H. L., Turek J. J. Ultrastructural characterization of colonic lesions in pigs inoculated with Treponema hyodysenteriae. Can J Comp Med. 1985 Oct;49(4):384–390. [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Greer J. M., Wannemuehler M. J. Comparison of the biological responses induced by lipopolysaccharide and endotoxin of Treponema hyodysenteriae and Treponema innocens. Infect Immun. 1989 Mar;57(3):717–723. doi: 10.1128/iai.57.3.717-723.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer J. M., Wannemuehler M. J. Pathogenesis of Treponema hyodysenteriae: induction of interleukin-1 and tumor necrosis factor by a treponemal butanol/water extract (endotoxin). Microb Pathog. 1989 Oct;7(4):279–288. doi: 10.1016/0882-4010(89)90046-6. [DOI] [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Heppner G., Weiss D. W. High Susceptibility of Strain A Mice to Endotoxin and Endotoxin-Red Blood Cell Mixtures. J Bacteriol. 1965 Sep;90(3):696–703. doi: 10.1128/jb.90.3.696-703.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M. M., Vogel S. N. Lipid A-associated proteins provide an alternate "second signal" in the activation of recombinant interferon-gamma-primed, C3H/HeJ macrophages to a fully tumoricidal state. J Immunol. 1987 Dec 1;139(11):3697–3702. [PubMed] [Google Scholar]
- Hughes R., Olander H. J., Williams C. B. Swine dysentery: pathogenicity of Treponema hyodysenteriae. Am J Vet Res. 1975 Jul;36(7):971–977. [PubMed] [Google Scholar]
- Itoh K., Mitsuoka T., Sudo K., Suzuki K. Comparison of fecal flora of mice based upon different strains and different housing conditions. Z Versuchstierkd. 1983;25(3):135–146. [PubMed] [Google Scholar]
- Joens L. A., Glock R. D. Experimental infection in mice with Treponema hyodysenteriae. Infect Immun. 1979 Aug;25(2):757–760. doi: 10.1128/iai.25.2.757-760.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Glock R. D., Kinyon J. M. Differentiation of Treponema hyodysenteriae from T innocens by enteropathogenicity testing in the CF1 mouse. Vet Rec. 1980 Dec 6;107(23):527–529. [PubMed] [Google Scholar]
- Joens L. A., Songer J. G., Harris D. L., Glock R. D. Experimental infection with Treponema hyodysenteriae in guinea pigs. Infect Immun. 1978 Oct;22(1):132–135. doi: 10.1128/iai.22.1.132-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Rosnick D. K., Ulrich R. G., Yancey R. J., Jr Association of Treponema hyodysenteriae with porcine intestinal mucosa. J Gen Microbiol. 1988 Jun;134(6):1565–1576. doi: 10.1099/00221287-134-6-1565. [DOI] [PubMed] [Google Scholar]
- Kunkle R. A., Kinyon J. M. Improved selective medium for the isolation of Treponema hyodysenteriae. J Clin Microbiol. 1988 Nov;26(11):2357–2360. doi: 10.1128/jcm.26.11.2357-2360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M., Burrows M. R. Studies on a haemolysin produced by Treponema hyodysenteriae. J Med Microbiol. 1982 May;15(2):205–214. doi: 10.1099/00222615-15-2-205. [DOI] [PubMed] [Google Scholar]
- Nibbelink S. K., Wannemuehler M. J. Effect of Treponema hyodysenteriae infection on mucosal mast cells and T cells in the murine cecum. Infect Immun. 1990 Jan;58(1):88–92. doi: 10.1128/iai.58.1.88-92.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuessen M. E., Birmingham J. R., Joens L. A. Biological activity of a lipopolysaccharide extracted from Treponema hyodysenteriae. Infect Immun. 1982 Jul;37(1):138–142. doi: 10.1128/iai.37.1.138-142.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuessen M. E., Joens L. A., Glock R. D. Involvement of lipopolysaccharide in the pathogenicity of Treponema hyodysenteriae. J Immunol. 1983 Aug;131(2):997–999. [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S., Rosenstreich D. L. Defect in macrophage effector function confers Salmonella typhimurium susceptibility on C3H/HeJ mice. Cell Immunol. 1982 Mar 1;67(2):325–333. doi: 10.1016/0008-8749(82)90224-6. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L. Inheritance of lipopolysaccharide-enhanced nonspecific resistance to infection and of susceptibility to endotoxic shock in lipopolysaccharide low-responder mice. Infect Immun. 1977 May;16(2):432–438. doi: 10.1128/iai.16.2.432-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Saheb S. A., Massicotte L., Picard B. Purification and characterization of Treponema hyodysenteriae hemolysin. Biochimie. 1980;62(11-12):779–785. doi: 10.1016/s0300-9084(80)80133-7. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S., Casey T. A., Tordoff L. A., Dewhirst F. E., Paster B. J. Reclassification of Treponema hyodysenteriae and Treponema innocens in a new genus, Serpula gen. nov., as Serpula hyodysenteriae comb. nov. and Serpula innocens comb. nov. Int J Syst Bacteriol. 1991 Jan;41(1):50–58. doi: 10.1099/00207713-41-1-50. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Corbeil L. B. Gonococcal infection in endotoxin-resistant and endotoxin-susceptible mice. Infect Immun. 1981 Apr;32(1):105–110. doi: 10.1128/iai.32.1.105-110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga I., Yamazaki T. Experimental Treponema hyodysenteriae infection of mice. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Aug;257(3):348–356. [PubMed] [Google Scholar]
- Sueyoshi M., Adachi Y., Shoya S. Enteropathogenicity of Treponema hyodysenteriae in young chicks. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):469–477. doi: 10.1016/s0176-6724(87)80229-8. [DOI] [PubMed] [Google Scholar]
- Wannemuehler M. J., Hubbard R. D., Greer J. M. Characterization of the major outer membrane antigens of Treponema hyodysenteriae. Infect Immun. 1988 Dec;56(12):3032–3039. doi: 10.1128/iai.56.12.3032-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemuehler M. J., Michalek S. M., Jirillo E., Williamson S. I., Hirasawa M., McGhee J. R. LPS regulation of the immune response: Bacteroides endotoxin induces mitogenic, polyclonal, and antibody responses in classical LPS responsive but not C3H/HeJ mice. J Immunol. 1984 Jul;133(1):299–305. [PubMed] [Google Scholar]
- Watson J., Riblet R., Taylor B. A. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977 Jun;118(6):2088–2093. [PubMed] [Google Scholar]
- de Man P., van Kooten C., Aarden L., Engberg I., Linder H., Svanborg Edén C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infect Immun. 1989 Nov;57(11):3383–3388. doi: 10.1128/iai.57.11.3383-3388.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]