Abstract
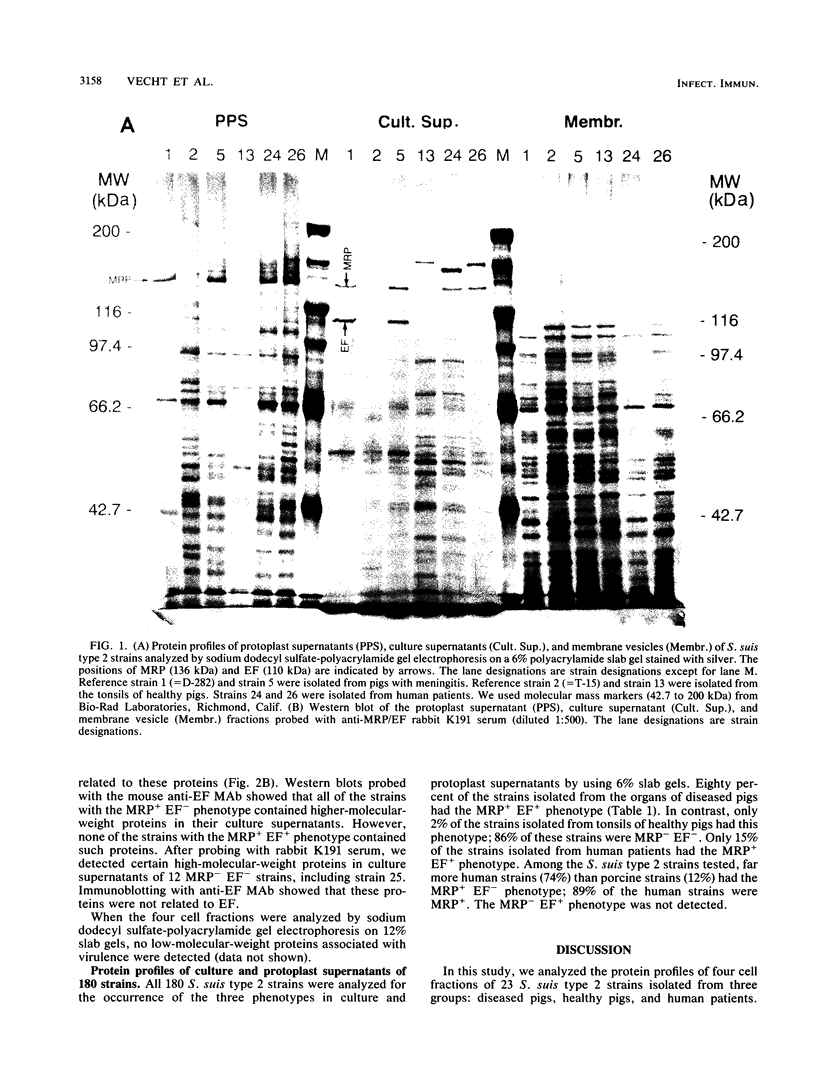
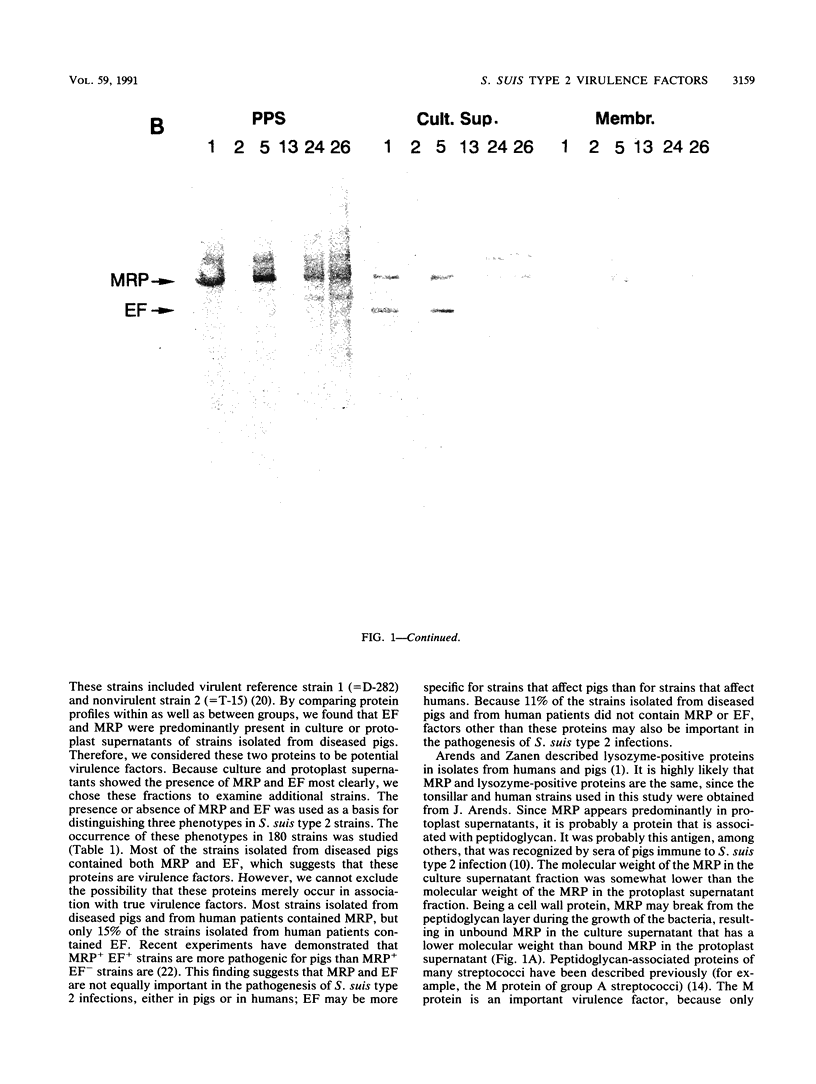
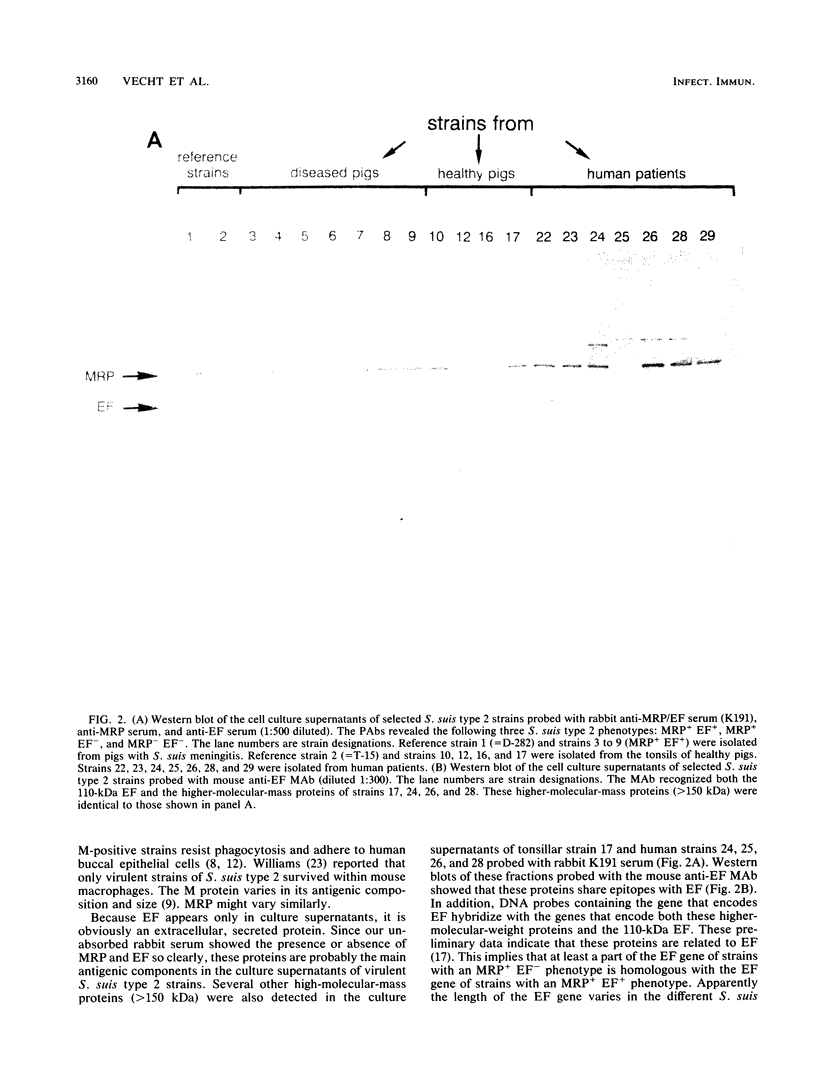
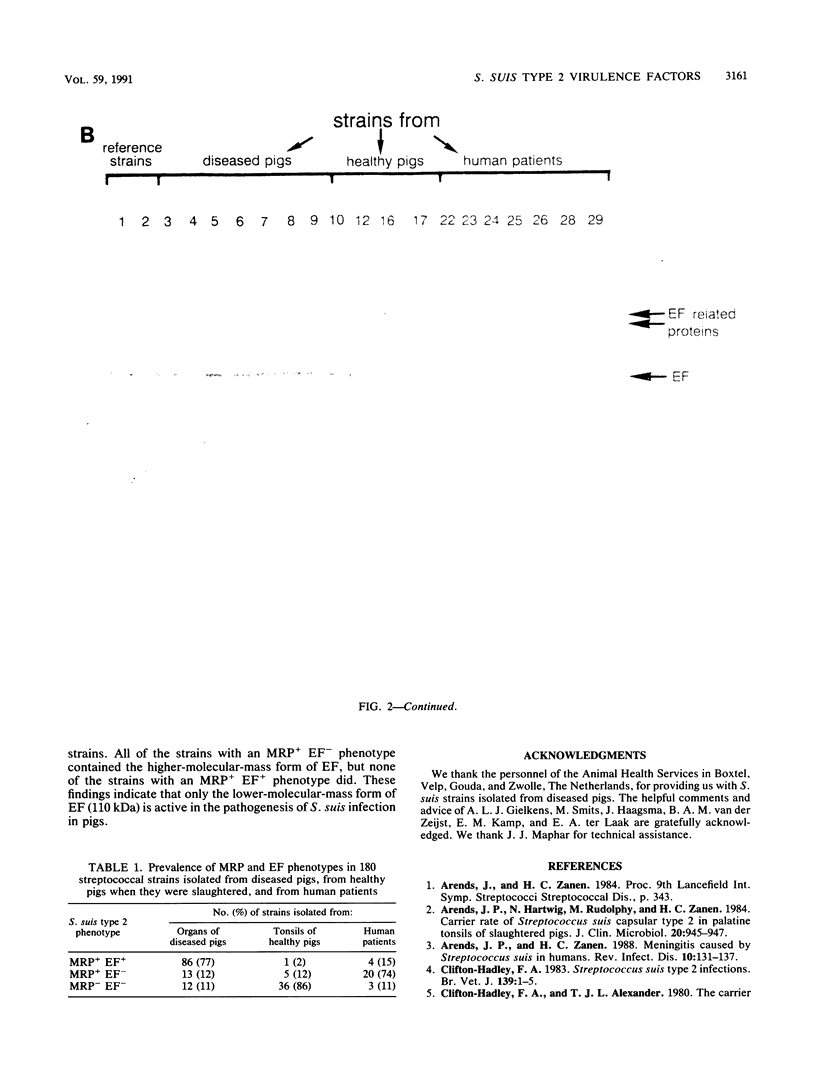
The protein profiles of various cell fractions of 180 strains of Streptococcus suis type 2, which were isolated from diseased pigs, from healthy pigs when they were slaughtered, and from human patients, were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. The isolates from diseased pigs contained two proteins that were absent in most of the isolates from healthy pigs. One of these proteins was a 136-kDa protein that was previously identified as the muramidase-released protein (MRP). This protein was predominantly detected in protoplast supernatants and culture supernatants. The second protein was a 110-kDa protein that was detected only in culture supernatants and therefore was provisionally called extracellular factor (EF). Three phenotypes of S. suis type 2 strains were recognized. Isolates from organs of diseased pigs mainly belonged to the MRP+ EF+ phenotype (77%), while isolates from tonsils of healthy pigs mainly had the MRP- EF- phenotype (86%). Most of the isolates from human patients contained MRP (89%); 74% had the MRP+ EF- phenotype. These findings confirm the results of previous investigations which demonstrated that S. suis type 2 strains differ in virulence. Monoclonal antibodies raised against the 110-kDa EF recognized proteins with higher molecular weights in culture supernatants of all of the strains with the MRP+ EF- phenotype. However, none of the strains with the MRP+ EF+ phenotype produced these high-molecular-weight proteins. Our results demonstrate that MRP and EF are associated with virulence. This suggests that one or both of these proteins are virulence factors that play a role in the pathogenesis of S. suis type 2 infections in pigs and human patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends J. P., Hartwig N., Rudolphy M., Zanen H. C. Carrier rate of Streptococcus suis capsular type 2 in palatine tonsils of slaughtered pigs. J Clin Microbiol. 1984 Nov;20(5):945–947. doi: 10.1128/jcm.20.5.945-947.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends J. P., Zanen H. C. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988 Jan-Feb;10(1):131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- Clifton-Hadley F. A., Alexander T. J., Enright M. R., Guise J. Monitoring herds for Streptococcus suis type 2 by sampling tonsils of slaughter pigs. Vet Rec. 1984 Dec 1;115(22):562–564. doi: 10.1136/vr.115.22.562. [DOI] [PubMed] [Google Scholar]
- Clifton-Hadley F. A. Streptococcus suis type 2 infections. Br Vet J. 1983 Jan-Feb;139(1):1–5. doi: 10.1016/s0007-1935(17)30581-x. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M. E., Enright M. R., Alexander T. J. Studies of the protective effect of different fractions of sera from pigs immune to Streptococcus suis type 2 infection. J Comp Pathol. 1989 May;100(4):435–442. doi: 10.1016/0021-9975(89)90009-1. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Parker M. T. The pattern of streptococcal disease in man. Soc Appl Bacteriol Symp Ser. 1978;7:71–106. [PubMed] [Google Scholar]
- Perch B., Kristjansen P., Skadhauge K. Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol Microbiol Scand. 1968;74(1):69–76. [PubMed] [Google Scholar]
- Sanford S. E., Tilker M. E. Streptococcus suis type II-associated diseases in swine: observations of a one-year study. J Am Vet Med Assoc. 1982 Oct 1;181(7):673–676. [PubMed] [Google Scholar]
- Vecht U., Arends J. P., van der Molen E. J., van Leengoed L. A. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am J Vet Res. 1989 Jul;50(7):1037–1043. [PubMed] [Google Scholar]
- Vecht U., van Leengoed L. A., Verheijen E. R. Streptococcus suis infections in pigs in the Netherlands (Part I). Vet Q. 1985 Oct;7(4):315–321. doi: 10.1080/01652176.1985.9694005. [DOI] [PubMed] [Google Scholar]
- Williams A. E. Relationship between intracellular survival in macrophages and pathogenicity of Streptococcus suis type 2 isolates. Microb Pathog. 1990 Mar;8(3):189–196. doi: 10.1016/0882-4010(90)90046-s. [DOI] [PubMed] [Google Scholar]
- Windsor R. S., Elliott S. D. Streptococcal infection in young pigs. IV. An outbreak of streptococcal meningitis in weaned pigs. J Hyg (Lond) 1975 Aug;75(1):69–78. doi: 10.1017/s0022172400047070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor R. S. Meningitis in pigs caused by Streptococcus suis type II. Vet Rec. 1977 Nov 5;101(19):378–379. doi: 10.1136/vr.101.19.378. [DOI] [PubMed] [Google Scholar]
- van Zijderveld F. G., Westenbrink F., Anakotta J., Brouwers R. A., van Zijderveld A. M. Characterization of the F41 fimbrial antigen of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect Immun. 1989 Apr;57(4):1192–1199. doi: 10.1128/iai.57.4.1192-1199.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen J. M., Kok J., Venema G. Construction of cloning, promoter-screening, and terminator-screening shuttle vectors for Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1985 Aug;50(2):540–542. doi: 10.1128/aem.50.2.540-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]