Summary
Intracellular mRNA localization directs protein synthesis to particular subcellular domains to establish embryonic polarity in a wide range of vertebrates and invertebrates. In Drosophila, bicoid (bcd) mRNA is pre-localized at the anterior of the oocyte. After fertilization, this RNA is translated to produce a Bcd protein gradient that determines anterior cell fates [1, 2]. Recent analysis of bcd mRNA during late stages of oogenesis led to a model for steady-state localization of bcd by continual active transport [3]. Here, we elucidate the path and mechanism of sustained bcd mRNA transport by direct observation of bcd RNA particle translocation in living oocytes. However, this mechanism cannot explain maintenance of bcd localization throughout the end of oogenesis, when microtubules disassemble in preparation for embryogenesis [4, 5] or retention of bcd at the anterior in mature oocytes, which can remain dormant, but developmentally competent, for weeks prior to fertilization [6]. Through temporal analysis of bcd RNA particle dynamics, we show that bcd mRNA shifts from continuous active transport to stable actin-dependent anchoring at the end of oogenesis, ensuring the developmental integrity of the oocyte during dormancy. Egg activation triggers release of bcd from the anterior cortex for proper deployment in the fertilized egg, probably through reorganization of the actin cytoskeleton. These findings uncover a surprising parallel between flies and frogs, as cortically tethered Xenopus Vg1mRNA undergoes a similar redistribution during oocyte maturation [7]. Our results highlight a conserved mechanism used by invertebrates and vertebrates to regulate mRNA anchoring and redeployment during the oocyte-to-embryo transition.
Results and Discussion
Using live cell imaging of mRNA labeled in vivo with GFP or RFP, we previously determined that bcd mRNA accumulates at the anterior cortex primarily late in oogenesis, as the ovarian nurse cells extrude their cytoplasm into the oocyte, a process known as nurse cell dumping [3]. This localization requires a special population of microtubules that are anchored to the anterior cortical actin cytoskeleton. Concurrently, nanos (nos) mRNA accumulates at the oocyte posterior, but unlike bcd, nos localization is microtubule-independent [8]. Fluorescence recovery after photobleaching (FRAP) analysis showed that, at steady state, the population of bcd mRNA at the anterior cortex remains in flux. In contrast, the population of nanos (nos) mRNA localized at the posterior pole of the oocyte is static [3]. To elucidate the mechanistic basis for this difference between bcd and nos, we visualized RNA particles in oocytes expressing GFP-labeled bcd or nos mRNA (bcd*GFP or nos*GFP) [3, 8] using widefield imaging methods that allow increased sensitivity and temporal resolution.
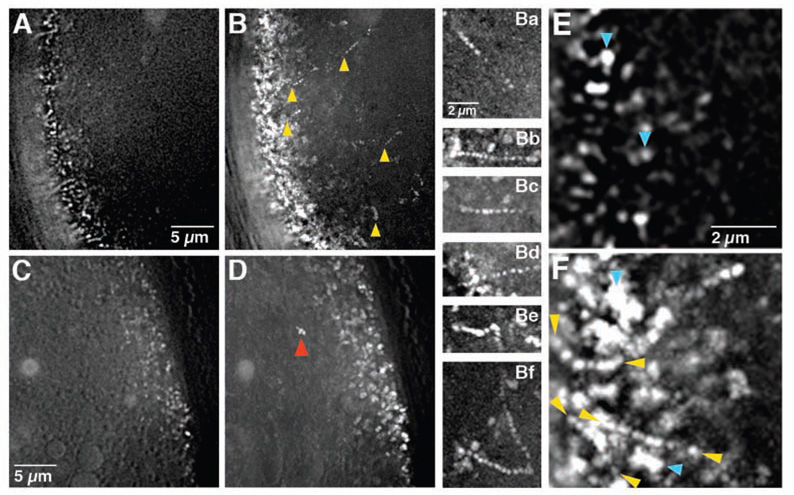
Drosophila oogenesis progresses through 14 morphologically defined stages. Dumping, which transfers bcd and nos to the oocyte, initiates at stage 10b, and is accompanied by vigorous movement of the oocyte cytoplasm (ooplasmic streaming) that mixes the oocyte and incoming nurse cell cytoplasm. Particles of bcd*GFP are highly dynamic during stages 10b-13, even after dumping and ooplasmic streaming have ceased (Figure 1A, 1B, 1Ba–Bf, 1E, 1F; Movie S1; data not shown). In contrast, little movement was detected among nos*GFP particles visualized at the oocyte posterior (Fig 1C, D; Movie S1).
Figure 1. bcd and nos mRNA particle dynamics in late oocytes.
(A–D) Time lapse imaging of bcd*GFP (A–B) and nos*GFP (C–D) in stage 12 oocytes. (A, C) Starting point (t = 0 sec) of 100 frame time series (Movie S1) taken at the oocyte anterior (A) and posterior (C). (B, D) Trail images in which 100 sequential frames (from t = 0 to t = 29 sec) from the anterior (B) or posterior (D) time series are superimposed. Moving particles appear as sets of dots. Yellow arrowheads in (B) indicate bcd RNA particles undergoing directed runs and are positioned at the first point visible in this time sequence. Red arrowhead in (D) indicates a nos particle traveling with the ooplasm, which is still streaming in this oocyte. (Ba–Bf) Examples of individual bcd RNA particle runs (see Movie S2, Movie S4). (E–F) Higher power time-lapse images of bcd*GFP in a stage 12 oocyte. (E) Starting point (t = 0 sec). (F) Trail image compiled from 100 successive frames (from t = 0 to t = 29 sec). Yellow arrowheads indicate directed runs, blue arrowheads indicate jiggling. All images were collected using a DeltaVision microscope and deconvolved (see Experimental Procedures).
To establish whether bcd mRNA particles move in a directed manner, we monitored all particles within representative regions of the anterior or posterior oocyte cortex. Particles were scored as static (no significant detectable movement), jiggling (movement within one particle length in any direction), or directed (sustained runs of more than 1 µm in a single direction) (Fig 1E, F). Among 11 stage 10b-13 oocytes, we observed movement by 85% (n = 283/332) of bcd*GFP particles (Table 1). Nearly 25% of these show directed runs, but this value is likely an underestimate since we were unable to track particles that moved rapidly in or out of the plane of focus. While the majority of particles were detected within 5 µm of the cortex, bcd*GFP particles undergoing directed movement could be detected as far as 20 µm from the anterior.
Table 1.
Temporal analysis of RNA particle dynamics
| Stage | Static | Jiggling | Directed | Total |
|---|---|---|---|---|
|
bicoid RNA | ||||
| 10b-12 | 25 (11%) | 151 (66%) | 52 (23%) | 228 |
| 13 | 22 (21%) | 63 (63%) | 17 (16%) | 104 |
| 14 | 39 (51%) | 29 (38%) | 8 (11%) | 78 |
|
nanos RNA | ||||
| 10b-12 | 73 (86%) | 12 (14%) | 0 | 85 |
| 13 | 83 (93%) | 6 (7%) | 0 | 89 |
| 14 | 40 (91%) | 4 (9%) | 0 | 44 |
Tracking of individual particles showed that 70% travel toward or laterally along the anterior cortex and 30% travel away from the anterior, with average instantaneous velocities of 0.36–2.15 µm/sec (Fig 1E, F). Occasionally, bcd*GFP particles exhibit pauses during runs and, more rarely, direction reversals (Movie S2, S3). In addition, complex trajectories were observed that suggest translocation of particles between microtubules (Movie S4). In contrast to bcd, only 12% (n = 21/174) of nos*GFP particles at the posterior cortex exhibit any detectable movement and all of this movement is due to jiggling (Table 1). We also detected flow of nos*GFP particles in concert with yolk granules during ooplasmic streaming, consistent with passive movement (Fig 1D; Movie S1).
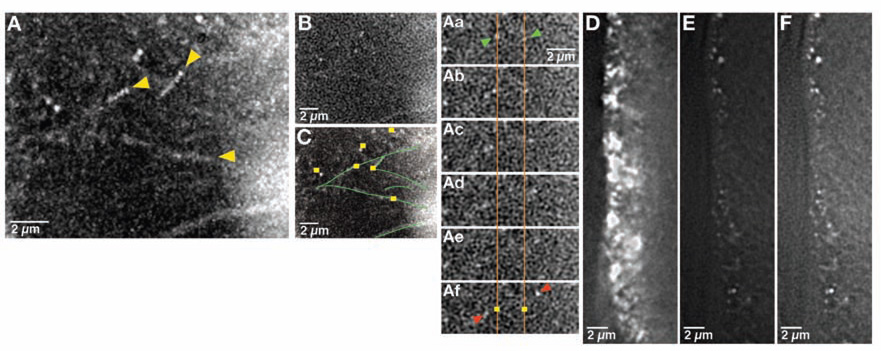
The directed runs of bcd particles we observed suggest translocation on microtubules. We tested this hypothesis by covisualizing microtubules labeled with tau-GFP together with bcd*GFP in stage 12 oocytes. Our results show individual bcd*GFP particles moving along microtubule tracks, providing direct in vivo evidence for microtubule-based RNA transport (Figure 2A–2C; Movie S5). Previous work is consistent with transport of bcd mRNA in late oocytes being Dynein-dependent [3]. To test this hypothesis directly, we analyzed the effect of hypomorphic mutations in Dynein heavy chain (Dhc), the force generating motor subunit, on bcd particle movement. We found that all movement of bcd*GFP particles is completely eliminated in Dhc mutant oocytes, including both unidirectional and bidirectional motility (Movie S6). These observations are consistent with recent evidence that Dynein can mediate both minus- and plus-end directed transport [9–11]. The minimal amount of bcd detected at the anterior cortex could be due to residual Dynein function of the hypomorphic alleles used, which is sufficient at least to permit oogenesis (Figure 2D–2F). Alternatively, this RNA may have been localized during mid-oogenesis, when anchoring is microtubule-independent [3], a notion that is supported by the fact that in contrast to bcd*GFP particles at the anterior of wild-type oocytes, residual bcd*GFP particles in Dhc mutant oocytes are stationary. Taken together, these results provide direct visual evidence that bcd is transported to the anterior on microtubules by dynein, and that sustained transport achieves steady-state anterior localization.
Figure 2. bcd RNA particles are transported on microtubules by dynein.
(A–C) Region near the anterior cortex of a stage 12 egg oocyte expressing bcd*GFP and tau-GFP. Particles can be detected traveling on labeled microtubules at velocities that are indistinguishable from velocities of particles traveling on unlabeled microtubules (see Movie S5). (A) Trail image showing particles traveling on microtubules (yellow arrowheads indicate starting point for each run). (Aa–Af) High power images tracking two representative particles. Orange lines and yellow squares show original starting positions of particles of interest (indicated by green arrowheads in Aa). Red arrows in Af indicate the positions of these particles after 3.5 seconds. (B, C) Single frame at t = 0 for the trail image shown in (A). (C) Schematic based on (A) indicating bcd RNA particles (yellow squares) and microtubules (green lines). (D–F) Anterior cortex of a stage 13 oocyte expressing bcd*GFP in wild type (D) and Dhc6-6/ Dhc6–12 (E) females. (D) and (E) are displayed at comparable settings, while (F) has been enhanced so that residual bcdRNA particles are visible. Particles of bcd RNA, visualized using the enhanced setting, show no movement at stage 13 in Dhc6-6/ Dhc6–12 oocytes (see Movie S6). All images were collected using a DeltaVision microscope and deconvolved (see Experimental Procedures).
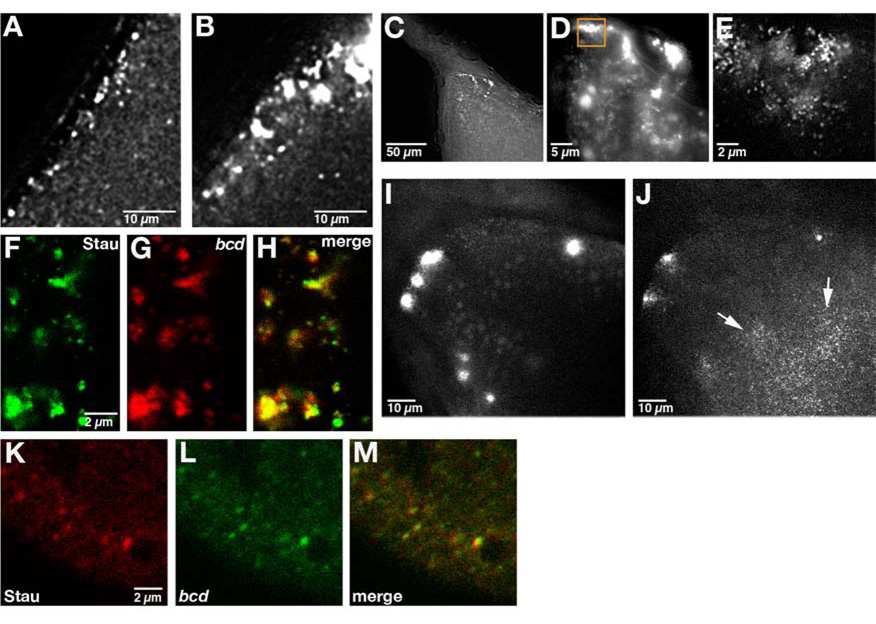
Following dumping, oogenesis proceeds very rapidly to produce a mature oocyte in approximately 8 hours. In contrast, mature (stage 14) oocytes are long lived and can be stored for weeks by the female prior to fertilization. These stored oocytes appear to be physiologically stable as they give rise to viable progeny[6]. It remained unclear whether bcd mRNA transport is sustained during stage 14 for long periods by continuous active transport or, alternatively, whether bcd mRNA transitions to a stably anchored state to ensure maintenance over long time periods. We distinguished between these two possibilities by analyzing the dynamics of bcd RNA particles at successive stages of oogenesis after the onset of dumping. We found a marked decrease in the frequency of directed runs as oogenesis progresses in laying females (Table 1). By stage 14, all mobility declines as particles coalesce to form large, stationary foci at the anterior cortex (Figure 3A–3E, Movie S7). These foci remain associated with the anterior cortex through stage 14 and appear to be composed of many individual bcd particles (Figure 3F; Movie S8). Consistent with their ability to support normal development, bcd remains localized in stage 14 oocytes held for several days by unfertilized females (data not shown).bcd RNA particles contain Staufen (Stau), a double-stranded RNA binding protein required for bcd localization from stage 10b onward [3, 12], and this association is maintained when particles coalesce into foci (Figure 3F–3H).nos particles, which are anchored to the actin cytoskeleton, do not change their distribution at the posterior cortex during this time (data not shown). These results are consistent with the acquisition of a stable cortical anchoring mechanism for bcd toward the end of oogenesis.
Figure 3. Accumulation and actin-dependent anchoring of bcd foci at stage 14.
(A, B) Section of a small region at the anterior of (A) stage 13 and (B) stage 14 oocytes expressing bcd*GFP (see Movie S7). bcd RNA particles coalesce to form large foci at stage 14. (C–E) Section of the anterior of a stage 14 oocyte expressing bcd*GFP. (C) 20x magnification; (D) 63x magnification; (E) 100x magnification of region indicated in (D). High magnification shows that these foci are composed of many small particles (see Movie S8). Images in A–E were collected using a DeltaVision microscope and deconvolved (see Experimental Procedures). (F–H) Confocal section of the anterior of a stage 14 oocyte expressing GFP-Stau and bcd*RFP. Stau and bcd RNA colocalize in large foci at the anterior (H). (I, J) Projections of the anterior of a cultured stage 14 egg chamber expressing bcd*GFP imaged (B) prior to and (C) 30 minutes after addition of 10 µg/ml of cytoD to the culture medium. bcd RNA foci disperse into small particles (arrows). (K–M) Confocal section of the anterior region of an early embryo expressing bcd*GFP and immunostained for Stau. Stau and bcd RNA remain colocalized at the anterior in the early embryo as shown in the merged image (K).
Maintenance of bcd at the anterior cortex during stages 10b-13 is both microtubule and actin-dependent. The actin-dependence at these stages is indirect, however, through a requirement for actin in anchoring microtubules to the anterior cortex [3]. To test the role of the cytoskeleton in stable bcd mRNA anchoring at stage 14, we studied the effects of actin and microtubule depolymerizing drugs on bcd mRNA localization at the final stage of oogenesis. Treatment of stage 14 oocytes with the microtubule depolymerizing drug colcemid, either in culture or through ingestion by females, did not affect maintenance of bcd*GFP foci at the anterior (data not shown). In contrast, treatment of cultured stage 14 oocytes with cytochalasin D (cyto D) to disrupt the actin cytoskeleton resulted in dissociation of bcd*GFP foci from the anterior and their dispersion into individual particles (Figure 3I, 3J). We conclude that maintenance of bcd by continual transport is replaced by an actin-based anchoring mechanism by the end of oogenesis, ensuring the developmental integrity of the mature oocyte.
Embryogenesis marks a new phase, in which pre-localized bcd mRNA is translated for the first time to produce the morphogenetic Bcd gradient. Since Bcd protein concentration is linearly related to the amount of bcd mRNA [13], cortically anchored bcd foci could provide concentrated point sources in the fertilized embryo to generate the peak Bcd concentration. However, through visualization of bcd*GFP, we found that bcd undergoes a final transition from its localization in cortical foci at stage 14, becoming dispersed as fine particles throughout the anterior region of the newly fertilized embryo (Figure 4A–4C). These particles retain Stau, suggesting that the RNA particle composition is not grossly altered during this reorganization (Figure 3K–3M). Time lapse imaging showed that bcd*GFP particles do not exhibit directed movement in the embryo but remain fixed in place as though tethered (data not shown).
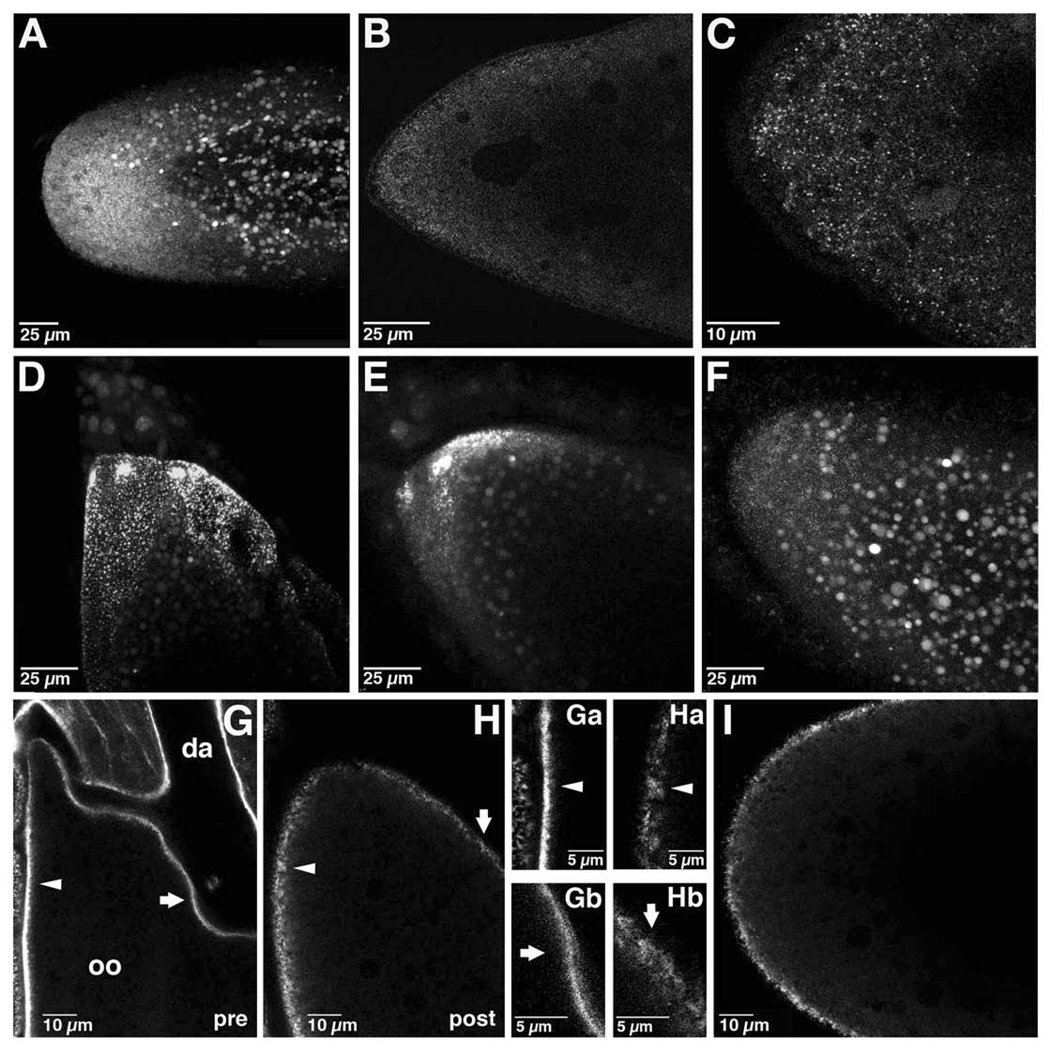
Figure 4. Redistribution of bcd and cortical actin during the transition from oogenesis to embryogenesis.
(A–C) Anterior region of early embryos expressing bcd*GFP. (A) Projection of approximately 52 µm through the anterior half of a newly fertilized embryo. (B) Single confocal section of the anterior region of an early embryo. (C) Confocal section from embryo in (B) taken at higher power. bcd mRNA is dispersed in small particles at the anterior in the early embryo. (D–F) In vitro activation of a stage 14 oocyte expressing bcd*GFP. Projections of confocal images taken (D) prior to addition of activation buffer, (E) 5 minutes after addition, and (F) 25 minutes after addition. bcd mRNA foci disperse into fine particles rapidly upon activation. (G–H) Single confocal sections of oocytes expressing GFP-moe. (G) Stage 14 oocyte, prior to in vitro activation (pre). GFP-moe also decorates the overlying follicle cells. Oo: oocyte; da: dorsal appendage. (H) The same oocyte, post-activation in vitro. The arrowhead and arrow indicate corresponding regions of the cortex pre- and post-activation.(Ga–Hb) Higher power images of the cortical actin cytoskeleton pre- (Ga, Gb) and post- (Ha, Hb) activation in the regions corresponding to the arrowheads and arrows in (G) and (H). (I) Confocal section of a newly fertilized embryo expressing GFP-moe.
The transition from oogenesis to embryogenesis requires calcium dependent events, collectively termed "egg activation", that occur inside the female to allow the newly fertilized egg to complete meiosis and initiate embryonic development [14–17]. We tested whether egg activation triggers the redistribution of bcd RNA, using stage 14 oocytes activated in vitro. We found that egg activation eliminates or severely reduces foci of bcd*GFP; instead, bcd*GFP particles become dispersed within the anterior region, resembling their distribution in the embryo (Figure 4D–4F). Time lapse imaging showed that foci begin to dissipate within 3–5 minutes of activation (Figure S1A–S1C). The requirement for actin in bcd anchoring at stage 14 prompted us to investigate whether egg activation might affect bcd localization by regulating the actin cytoskeleton. The actin cytoskeleton was monitored using a GFP fusion to the F-actin binding tail of moesin (GFP-moe), which decorates cortical actin without disrupting actin function [18]. When visualized at stage 14, the actin cytoskeleton appears dense and tightly apposed to the oocyte cortex (Figure 4G, 4Ga, 4Gb). In vitro egg activation causes a rapid alteration in actin organization, producing a diffuse actin network that extends into the interior of the egg (Figure 4H, 4Ha, 4Hb). A similar distribution of actin is observed in the newly fertilized embryo (Figure 4I). Moreover, this rearrangement of the actin cytoskeleton can be detected within 3 minutes of activation, corresponding to the onset of bcd dispersal (Figure S1D–S1F).
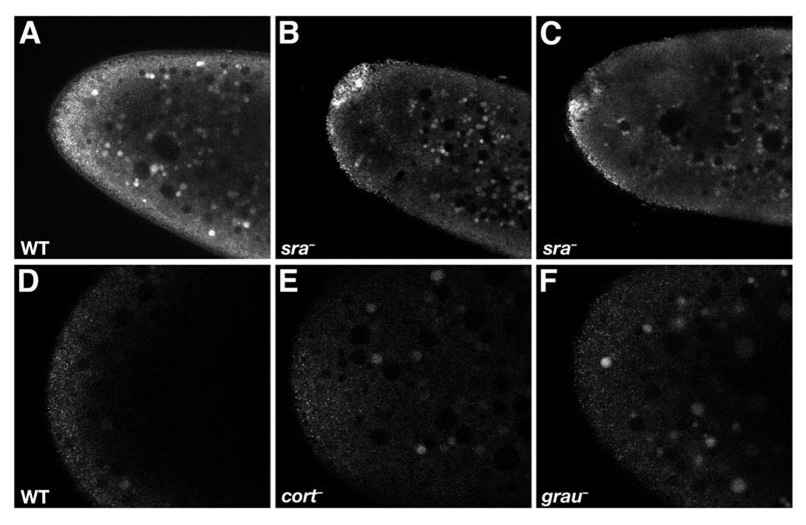
To confirm that the change in bcd localization is indeed a physiological consequence of egg activation, we examined the effect of mutations that disrupt egg activation on bcd localization in the early embryo. sarah(sra) encodes calcipressin, an inhibitor of the calcium dependent phosphatase calcineurin, and acts early in the egg activation pathway [16, 17]. In contrast to wild-type embryos, bcd*GFP remains cortically associated in sra mutant embryos and forms foci characteristic of stage 14 oocytes (Figure 5A–5C). However, mutations in two other genes required during egg activation, cortex (cort), which encodes a Cdc20 family member [5, 19] and grauzone (grau), which activates cort expression [20], do not prevent redistribution of bcd*GFP (Figure 5D–5F). The timing of bcd dispersal and its dependence on sra, but not cort or grau, lead us to conclude that an early, sarah-dependent event during egg activation triggers redistribution of bcd RNA for embryogenesis. Given Sra's function as a calcium regulator [16, 17], we propose that this event is likely to involve calcium signalling.
Figure 5. Effect of mutations that disrupt egg activation on bcd localization in the early embryo.
Single confocal sections of the anterior of early embryos expressing bcd*GFP in (A,D) wild type, (B,C) sraA108/sra687, (E) cortQW55/cortRH65, and (F) grau mutant embryos. bcd mRNA remains associated with the anterior cortex in large foci in sra mutant embryos but is disperse in cort and grau mutant embryos.
Together, our studies show that as oogenesis proceeds toward formation of a developmentally competent egg, bcd mRNA transitions from being continuously transported to a stably anchored state. By providing a mechanism to ensure bcd localization for long periods of time in dormant stage 14 oocytes, stable anchoring may play an important role in buffering reproduction against environmental challenges. The delay in stable anchoring of bcd mRNA at the anterior may be caused by the rapid influx of nurse cell cytoplasm occurring at the oocyte anterior during dumping. Alternatively, deployment of this anchoring mechanism may require factors provided to the oocyte during dumping. In either case, continual transport provides a mechanism to retain bcd at the anterior until other developmental processes are complete. Previous work has shown that cortically associated microtubules are replaced by short, randomly oriented cytoplasmic microtubules as the oocyte matures during stages 13–14 [4]. These cytoplasmic microtubules disappear by fertilization [5], when microtubules are nucleated by centrosomes for nuclear divisions and migration [21]. Although we have not been able to determine if and when the specific anterior population of microtubules that transport bcd is disassembled, it is likely that disassembly of oocyte microtubules in preparation for embryogenesis is coordinated with, or required for bcd mRNA anchoring.
A surprising finding is the release of bcd from its anterior cortical tether during egg activation. In the early embryo, bcd mRNA particles contain Stau, indicating release from the anterior cortex does not result from disassembly of bcd/Stau complexes (Figure 3K–M). Rather, our results suggest a mechanism for release of bcd from the cortex by an activation-dependent re-structuring of the actin cytoskeleton. Interestingly, a similar mechanism may be used in Xenopus oocytes for the disassociation of localized Vg1 mRNA from the vegetal cortex. Like bcd, release of Vg1 mRNA is triggered by egg activation and can be mimicked by pharmacological disruption of the actin cytoskeleton [7]. Dispersal of Vg1 mRNA prior to cleavage divisions presumably promotes its inheritance by vegetal cells, where production of Vg1 protein is required for mesoderm induction and embryonic patterning [22]. We propose that, in addition to cell cycle progression, a conserved feature of egg activation is the redeployment of mRNAs localized during oogenesis for their embryonic functions.
Conclusion
Although the molecular determinants of embryonic patterning may differ between invertebrates and vertebrates, mRNA localization plays a fundamental role in the deployment of key patterning molecules in both Drosophila and Xenopus. It is well recognized that while RNA cargos may not be conserved, the machinery for delivering these cargos to the correct destinations is [23, 24]. Our findings suggest that these parallels go even deeper to include mRNA anchoring and redeployment mechanisms that act during the transition from oogenesis to embryogenesis to ensure proper patterning.
Experimental Procedures
Fly strains
The following mutants and transgenic lines were used: Dhc6-6, Dhc6-12(ref [25]); sraA108, sra687 (ref [16]); cortQW55, cortRH65, grauQQ36, grauRG1 (refs [5, 26]); hsp83-MCP-GFP, hsp83-MCP-RFP, bcd-(ms2)6(ref [3]); nos-(ms2)18(ref [27]), tau-GFP(ref [28]); GFP-Stau(ref [29]); GFP-moe (sGMCA; ref [18]). The bcd-(ms2)6 and nos-(ms2)18 transgenes completely rescue the bcd and nos mutant patterning defects, respectively [3, 27].
Manipulation and confocal imaging of oocytes and embryos
Oocytes were cultured and treated with cytochalasin D (Sigma; 10 µg/ml final concentration) or colcemid (Sigma; final concentration 50 µg/ml) as previously described [3]. For colcemid feeding, fresh females were starved for 2–3 days, then for 4 hours with fed yeast paste supplemented to 50 µg/ml colcemid. In vitro activation of stage 14 oocytes was carried out according to Page and Orr-Weaver [30] with modification: individual stage 14 oocytes from well fed females were dissected in Schneider’s insect culture medium (GIBCO-BRL) and individual egg chambers were transferred to uncoated #1.5 glass bottom culture dishes (MatTek) and covered with a 1 mm2 coverslip cut from a #1.5 glass coverslip (Corning). Schneider’s medium was then removed and replaced by activation buffer [30]. Embryos were collected for 0–2 hour periods on yeasted apple juice agar plates, dechorionated for 1 minute in 50% bleach, then mounted on No. 1 coverslips and covered with Series 95 halocarbon oil. Time-lapse and Z-series imaging were carried out using a Leica TCS SP5 confocal microscope, 63x/1.3 NA objective.
For co-visualization of bcd and Stau, ovaries expressing bcd*RFP and GFP-Stau were dissected into Schneider's medium and isolated egg chambers were imaged directly. Embryos from females expressing bcd*GFP were fixed in 1:5 4% paraformaldehyde/PBS:heptane for 20 minutes, washed with PBS, and vitelline membranes were removed manually. Anti-Stau immunostaining was performed using 1:2000 rabbit anti-Stau (gift of D. St Johnston) and 1:1000 Alexa Fluor 568 goat anti-rabbit (Invitrogen/Molecular Probes). Embryos were mounted in Aqua PolyMount (Polysciences, Inc) and imaged with a Zeiss LSM510 confocal microscope.
Live imaging and analysis of RNA particles
Ovaries from well fed females were dissected in Series 95 halocarbon oil (KMZ Chemicals) onto No. 1 coverslips (Corning) and separated from ovarioles using tungsten needles. Time lapse imaging was performed using a wide-field DeltaVision microscope (Applied Precision, Olympus IX70 microscope, Coolsnap HQ [Roper] camera) with 100x/1.35NA objective. Images were deconvolved using Sedat/Agard algorithms with Applied Precision software [31]. Particle movement was analyzed and tracked semi-manually, using Image-Pro Plus (Leeds) and Metamorph (Universal Imaging Corporation) software packages.
Supplementary Material
(A–C) Confocal Z-series projections of the anterior of oocyte expressing bcd*GFP collected prior to activation in vitro (A), 3.5 minutes after the onset of activation in vitro (B), and 7 minutes after the onset of activation in vitro. (D–F) Single confocal sections of the anterior of an oocyte expressing GFP-moe collected prior to activation (D), 4.5 minutes after the onset of activation in vitro (E), and 6.5 minutes after the onset of activation in vitro (F).
Time lapse movies of bcd*GFP (left) and nos*GFP (right) in stage 12 oocytes. Images were collected at approximately 3.5 frames per second and 50 frames are displayed at 15 frames per second.
Trail movie showing a directed movement of a single particle of bcd*GFP at the the anterior of a stage 12/13 oocyte. Images were collected at approximately 3.5 frames per second and 24 frames are displayed at 15 frames per second.
Single particle of bcd*GFP at the the anterior of a stage 12/13 oocyte showing directional reversal. Images were collected at approximately 3.5 frames per second and 24 frames are displayed at 15 frames per second.
Single particle of bcd* GFP showing directed movement at the the anterior of a stage 12/13 oocyte: trail movie (left) and original collection (right). Images were collected at approximately 3.5 frames per second and 42 frames are displayed at 15 frames per second.
Simultaneous imaging of bcd*GFP and tau-GFP near the anterior cortex of a stage 12 oocyte. Images were collected at approximately 3.5 frames per second and 36 frames are displayed at 25 frames per second.
bcd*GFP particles at the anterior cortex of a stage 13 Dhc6-6/ Dhc6–12 oocyte. Images were collected at approximately 3.5 frames per second and 120 frames are displayed at 25 frames per second.
Time-lapse movies of bcd*GFP in a small region at the anterior of stage 13 (left) and stage 14 (right) oocytes. Both movies are displayed using the same parameters. Images were collected at approximately 3.5 frames per second and 50 frames are displayed at 15 frames per second.
High magnification, three dimensional projection of a Z-series showing a focus of bcd*GFP at the anterior of a stage 14 egg chamber.
Acknowledgements
We thank T. Hays, D. Kiehart, A. Martin, T. Schüpbach, M. Welte, and M. Wolfner for fly stocks, D. St Johnston for fly stocks and anti-Stau antibody, J. Goodhouse for assistance with confocal microscopy, and T. Schüpbach and E. Wieschaus for comments on the manuscript. This work was supported by a grant from the National Institutes of Health (GM067758) to E.R.G. and Senior Fellowship from the Wellcome Trust to I.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Driever W, Nüsslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 2.Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weil TT, Forrest KM, Gavis ER. Localization of bicoid mRNA in late oocytes is maintained by continual active transport. Dev. Cell. 2006;11:251–262. doi: 10.1016/j.devcel.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Theurkauf WE, Smiley S, Wong ML, Alberts BM. Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development. 1992;115:923–936. doi: 10.1242/dev.115.4.923. [DOI] [PubMed] [Google Scholar]
- 5.Page AW, Orr-Weaver TL. The Drosophila genes grauzone and cortex are necessary for proper female meiosis. J. Cell Sci. 1996;109:1707–1715. doi: 10.1242/jcs.109.7.1707. [DOI] [PubMed] [Google Scholar]
- 6.Wyman R. The temporal stability of the Drosophila oocyte. J. Embryol. Exp. Morphol. 1979;50:137–144. [PubMed] [Google Scholar]
- 7.Yisraeli JK, Sokol S, Melton DA. A two-step model for the localization of maternal mRNA in Xenopus oocytes: Involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990;108:289–298. doi: 10.1242/dev.108.2.289. [DOI] [PubMed] [Google Scholar]
- 8.Forrest KM, Gavis ER. Live imaging of endogenous mRNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr. Biol. 2003;13:1159–1168. doi: 10.1016/s0960-9822(03)00451-2. [DOI] [PubMed] [Google Scholar]
- 9.Ross JL, Wallace K, Shuman H, Goldman YE, Holzbaur EL. Processive bidirectional motion of dynein-dynactin complexes in vitro. Nat. Cell Biol. 2006;8:562–570. doi: 10.1038/ncb1421. [DOI] [PubMed] [Google Scholar]
- 10.Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Force-induced bidirectional stepping of cytoplasmic dynein. Cell. 2007;131:952–965. doi: 10.1016/j.cell.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vendra G, Hamilton RS, Davis I. Dynactin suppresses the retrograde movement of apically localized mRNA in Drosophila blastoderm embryos. RNA. 2007;13:1860–1867. doi: 10.1261/rna.509007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Johnston D, Driever W, Berleth T, Richstein S, Nüsslein-Volhard C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development Suppl. 1989;107:13–19. doi: 10.1242/dev.107.Supplement.13. [DOI] [PubMed] [Google Scholar]
- 13.Gregor T, Tank DW, Wieschaus EF, Bialek W. Probing the limits to positional information. Cell. 2007;130:153–164. doi: 10.1016/j.cell.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahowald AP, Goralski TJ, Caulton JH. In vitro activation of Drosophila eggs. Dev. Biol. 1983;98:437–445. doi: 10.1016/0012-1606(83)90373-1. [DOI] [PubMed] [Google Scholar]
- 15.Heifetz Y, Yu J, Wolfner MF. Ovulation triggers activation of Drosophila oocytes. Dev. Biol. 2001;234:416–424. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- 16.Horner VL, Czank A, Jang JK, Singh N, Williams BC, Puro J, Kubli E, Hanes SD, McKim KS, Wolfner MF, et al. The Drosophila calcipressin Sarah is required for several aspects of egg activation. Curr. Biol. 2006;16:1441–1446. doi: 10.1016/j.cub.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Takeo S, Tsuda M, Akahori S, Matsuo T, Aigaki T. The calcineurin regulator Sra plays an essential role in female meiosis in Drosophila. Curr. Biol. 2006;16:1435–1440. doi: 10.1016/j.cub.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 18.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu T, Henrion G, Haegeli V, Strickland S. Cortex, a Drosophila gene required to complete oocyte meiosis, is a member of the Cdc20/fizzy protein family. Genesis. 2001;29:141–152. doi: 10.1002/gene.1017. [DOI] [PubMed] [Google Scholar]
- 20.Harms E, Chu T, Henrion G, Strickland S. The only function of Grauzone required for Drosophila oocyte meiosis is transcriptional activation of the cortex gene. Genetics. 2000;155:1831–1839. doi: 10.1093/genetics/155.4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan W, Theurkauf WE. The cytoskeleton and morphogenesis of the early Drosophila embryo. Curr. Opin. Cell Biol. 1995;7:18–22. doi: 10.1016/0955-0674(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 22.Birsoy B, Kofron M, Schaible K, Wylie C, Heasman J. Vg 1 is an essential signaling molecule in Xenopus development. Development. 2006;133:15–20. doi: 10.1242/dev.02144. [DOI] [PubMed] [Google Scholar]
- 23.Oleynikov Y, Singer RH. RNA localization: different zipcodes, same postman? Trends Cell. Biol. 1998;8:381–383. doi: 10.1016/s0962-8924(98)01348-8. [DOI] [PubMed] [Google Scholar]
- 24.Gavis ER, Singer RH, Hüttelmaier S. Localized translation through messenger RNA localization. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational Control. Cold Spring Harbor, NY: Cold Spring Harbor Laborator Press; 2007. pp. 687–717. [Google Scholar]
- 25.Gepner J, Li M, Ludmann S, Kortas C, Boylan K, Iyadurai SJ, McGrail M, Hays TS. Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics. 1996;142:865–878. doi: 10.1093/genetics/142.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brechbiel JL, Gavis ER. Spatial regulation of nanos is required for its function in dendrite morphogenesis. Curr. Biol. 2008;18:745–750. doi: 10.1016/j.cub.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micklem DR, Dasgupta R, Elliot H, Gergely F, Davidson C, Brand A, González-Reyes A, St Johnston D. The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr. Biol. 1997;7:468–478. doi: 10.1016/s0960-9822(06)00218-1. [DOI] [PubMed] [Google Scholar]
- 29.Schuldt AJ, Adams JH, Davidson CM, Micklem DR, Haseloff J, St Johnston D, Brand AH. Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev. 1998;12:1847–1857. doi: 10.1101/gad.12.12.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page AW, Orr-Weaver TL. Activation of the meiotic divisions in Drosophila oocytes. Dev. Biol. 1997;183:195–207. doi: 10.1006/dbio.1997.8506. [DOI] [PubMed] [Google Scholar]
- 31.Clark A, Meignin C, Davis I. A Dynein-dependent shortcut rapidly delivers axis determination transcripts into the Drosophila oocyte. Development. 2007;134:1955–1965. doi: 10.1242/dev.02832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–C) Confocal Z-series projections of the anterior of oocyte expressing bcd*GFP collected prior to activation in vitro (A), 3.5 minutes after the onset of activation in vitro (B), and 7 minutes after the onset of activation in vitro. (D–F) Single confocal sections of the anterior of an oocyte expressing GFP-moe collected prior to activation (D), 4.5 minutes after the onset of activation in vitro (E), and 6.5 minutes after the onset of activation in vitro (F).
Time lapse movies of bcd*GFP (left) and nos*GFP (right) in stage 12 oocytes. Images were collected at approximately 3.5 frames per second and 50 frames are displayed at 15 frames per second.
Trail movie showing a directed movement of a single particle of bcd*GFP at the the anterior of a stage 12/13 oocyte. Images were collected at approximately 3.5 frames per second and 24 frames are displayed at 15 frames per second.
Single particle of bcd*GFP at the the anterior of a stage 12/13 oocyte showing directional reversal. Images were collected at approximately 3.5 frames per second and 24 frames are displayed at 15 frames per second.
Single particle of bcd* GFP showing directed movement at the the anterior of a stage 12/13 oocyte: trail movie (left) and original collection (right). Images were collected at approximately 3.5 frames per second and 42 frames are displayed at 15 frames per second.
Simultaneous imaging of bcd*GFP and tau-GFP near the anterior cortex of a stage 12 oocyte. Images were collected at approximately 3.5 frames per second and 36 frames are displayed at 25 frames per second.
bcd*GFP particles at the anterior cortex of a stage 13 Dhc6-6/ Dhc6–12 oocyte. Images were collected at approximately 3.5 frames per second and 120 frames are displayed at 25 frames per second.
Time-lapse movies of bcd*GFP in a small region at the anterior of stage 13 (left) and stage 14 (right) oocytes. Both movies are displayed using the same parameters. Images were collected at approximately 3.5 frames per second and 50 frames are displayed at 15 frames per second.
High magnification, three dimensional projection of a Z-series showing a focus of bcd*GFP at the anterior of a stage 14 egg chamber.