Abstract
Protease-activated receptor 2 (PAR2) is expressed by vascular endothelial cells and other cells in which its function and physiological activator(s) are unknown. Unlike PAR1, PAR3, and PAR4, PAR2 is not activatable by thrombin. Coagulation factors VIIa (FVIIa) and Xa (FXa) are proteases that act upstream of thrombin in the coagulation cascade and require cofactors to interact with their substrates. These proteases elicit cellular responses, but their receptor(s) have not been identified. We asked whether FVIIa and FXa might activate PARs if presented by their cofactors. Co-expression of tissue factor (TF), the cellular cofactor for FVIIa, together with PAR1, PAR2, PAR3, or PAR4 conferred TF-dependent FVIIa activation of PAR2 and, to lesser degree, PAR1. Responses to FXa were also observed but were independent of exogenous cofactor. The TF/FVIIa complex converts the inactive zymogen Factor X (FX) to FXa. Strikingly, when FX was present, low picomolar concentrations of FVIIa caused robust signaling in cells expressing TF and PAR2. Responses in keratinocytes and cytokine-treated endothelial cells suggested that PAR2 may be activated directly by TF/FVIIa and indirectly by TF/FVIIa-generated FXa at naturally occurring expression levels of TF and PAR2. These results suggest that PAR2, although not activatable by thrombin, may nonetheless function as a sensor for coagulation proteases and contribute to endothelial activation in the setting of injury and inflammation. More generally, these findings highlight the potential importance of cofactors in regulating PAR function and specificity.
Proteases trigger cellular responses in part via protease-activated G protein-coupled receptors (PARs). PAR1, the prototype for this receptor subfamily, mediates responses to thrombin in platelets and other cells (1). Thrombin activates PAR1 by cleaving the receptor's amino terminal exodomain between arginine 41 and serine 42. This cleavage event unmasks a new amino terminus beginning with the sequence SFLLRN, which in turn serves as a tethered ligand, binding intramolecularly to the body of the receptor to trigger transmembrane signaling. Functionally, PAR1 is an important mediator of thrombin signaling in human platelets and endothelial cells (1). Three additional PARs have been identified. PAR2 can be activated by trypsin and by other proteases with trypsin-like specificity, but not by thrombin (2, 3). PAR3 and PAR4 can be activated by thrombin, and PAR4 functions at least in part as a second thrombin receptor in human platelets (4–6).
Thus far, PAR activation by proteases has been studied as a simple binary interaction between protease and receptor. However, protease-substrate interactions are often made possible by cofactors that both localize and regulate the activity of the protease. Factor VIIa (FVIIa) and Factor Xa (FXa) are coagulation proteases that function upstream of thrombin in the coagulation cascade, and both proteases form physiologically important complexes with cofactors (7). Factor VIIa binds tissue factor (TF), an integral membrane protein that both localizes Factor VIIa to the surface of cells and markedly enhances its activity. TF is normally expressed on cells extrinsic to the vascular compartment, but its expression can be induced in monocytes and endothelial cells by inflammatory mediators (8). Formation of the TF/FVIIa complex is the major physiological trigger for thrombin generation and blood coagulation (7). The TF/FVIIa complex binds and cleaves the zymogen Factor X to FXa, the active protease. FXa in turn binds Factor Va, a membrane-associated cofactor for FXa-mediated cleavage of prothrombin to active thrombin. Like thrombin, both FVIIa and FXa can trigger signaling events in certain cells (9–18). These responses are independent of thrombin generation, and in most cases require the active site of the protease. In the case of FVIIa, these effects also require TF. The receptors that mediate FVIIa and FXa signaling have not been identified. We have examined the possibility that the known PARs might mediate signaling to FVIIa and/or Xa when appropriate cofactors are present. We report that PAR1 and PAR2 can mediate signaling to FVIIa when TF is co-expressed in the same cell. Indeed, picomolar concentrations of FVIIa elicited signaling when TF, PAR2, and the zymogen factor X were present. These data suggest that TF-dependent generation of FXa can trigger robust signaling events independent of thrombin generation in cells that express PAR2. It is likely that this can occur on the surface of endothelial cells in which TF expression has been induced. Our results emphasize the potential importance of cofactors in orchestrating PAR activation. Moreover, the role of PAR2 expressed in endothelial cells has been obscure, and our results suggest that PAR2 may function as a FVIIa/FXa receptor in certain settings.
Materials and Methods
Materials.
The agonist peptides TFLLRNPNDK (PAR1-specific) and SLIGRL (PAR2-specific) were synthesized as carboxyl amides and were purified by reverse-phase high performance liquid chromatography. Trypsin, hirudin, and the M1 anti-FLAG monoclonal antibody were from Sigma. Recombinant human FVIIa was from American Diagnostica (Greenwich, CT). Human plasma-derived FX, FVIIa, active site-inhibited FVIIa, and an anti-human TF monoclonal antibody were from Enzyme Research Laboratories (South Bend, IN). Preparations of purified FX were immunodepleted of FVII/FVIIa. Human FV, FVa, Gla-domainless FXa, and active site-inhibited FXa were from Hematologic Technologies (Essex Junction, VT). Human FXa was from Enzyme Research Laboratories and Hematologic Technologies. 1 unit/ml FX and FXa corresponds to 174 nM. Human tissue factor pathway inhibitor (TFPI) 1 was a gift from George Broze (Washington University, St. Louis).
Constructs.
All wild-type and mutant human TF and PARs used in this study were epitope-tagged with the FLAG sequence (DYKDDDD) at the amino terminus of the mature receptor protein (19). This did not interfere with receptor cleavage in the case of PARs nor with FVIIa binding in the case of TF. A QuickChange site-directed mutagenesis kit (Stratagene) was used to generate the PAR2S37P mutant in which serine in the P1′ position of the PAR2 cleavage site was replaced by proline. TF/CD8 was generated by fusing cDNA encoding the extracellular domain of TF (amino acids 1–218) and the transmembrane and cytoplasmic domains (162–215) of the CD8 α chain (20) by PCR. The amino acid sequence at the junction (−) was TF extracellular domain… GQEKGEFR-DIYIWAPL… CD8 transmembrane domain. TF devoid of the cytoplasmic domain (TF1–242) was constructed by inserting a stop codon after the sequence coding for amino acid 242 using PCR. Constructs were subcloned into pFROG (21) or pGEM-3Z HE (Emily Liman, Harvard Medical School) for cRNA synthesis and into PBJ (Mark Davis, Stanford University) for mammalian expression. All constructs were sequenced.
Cell Culture.
The KOLF lung fibroblast cell line was derived from PAR1 knockout mice (22). The human keratinocyte line HaCaT (23) was from Norbert Fusenig (German Cancer Research Center, Heidelberg). KOLFs and HaCaT were grown in DMEM with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Human umbilical vein endothelial cells (HUVECs) were grown on fibronectin in endothelial basal medium (Clonetics, San Diego) and were used at passage 2–5. TF expression was induced by treating HUVECs for 6 h with tumor necrosis factor α (10 ng/ml) and vascular endothelial growth factor (1 nM) (24). Cell surface TF expression was confirmed by cell surface ELISA (19) using a TF mAb. KOLF-based cell lines (KOLFTF, KOLFPAR2, and KOLFTF+PAR2) were generated by co-transfecting expression constructs with a hygromycin resistance vector and screening hygromycin B-resistant clones for TF and/or PAR2 expression by cell surface ELISA using FLAG, TF, and PAR2 antibodies. Several clones were tested for each line, and the lines were maintained on 200 μg/ml hygromycin B.
45Ca2+ Efflux in Xenopus Oocytes.
Receptors were expressed by microinjection of Xenopus oocytes with cRNAs as described (21). Receptor expression on the oocyte surface was measured as specific binding of antibody to the FLAG epitope (19). Agonist-stimulated 45Ca2+ efflux was measured as described (21) except that the Ca2+ in the buffer was at 2 mM. Basal 45Ca2+ release was defined as that occurring in the 30-min interval before stimulation, and agonist-induced 45Ca2+ release as that occurring in the subsequent 30 min. Results are expressed as fold increase (stimulated over basal).
Phosphoinositide Hydrolysis in Mammalian Cells.
Cells in 24-well plates were loaded overnight with 2 μCi/ml 3H-myoinositol in DMEM/BSA (0.2%)/Hepes (10 mM) with (HaCaT) or without (KOLF) 10% FBS, or in endothelial growth medium (HUVEC). HUVECs were washed in PBS and were incubated in DMEM/BSA/Hepes with or without cytokines for 6 h before agonist addition. Cells were washed and treated with 20 mM LiCl in DMEM/BSA/Hepes with or without agonist for 90 min and then were extracted with formic acid. Released total 3H-inositol phosphates were quantitated (19).
Cytoplasmic Calcium Transients in Mammalian Cells.
KOLF clones were detached by using Cell Dissociation Buffer (GIBCO/BRL) and were loaded with 4 μg/ml Fura-2/AM (Molecular Probes) for 45 min at 37°C. Agonist-dependent calcium mobilization was measured by using a Hitachi F2000 fluorometer (19).
Results
We first determined whether FVIIa or FXa could activate PARs heterologously expressed in Xenopus oocytes. Agonist-triggered 45Ca2+ release, which reflects phosphoinositide hydrolysis, was used as an endpoint. FVIIa triggered 45Ca2+ release from oocytes that expressed TF together with PAR1 or PAR2 but not PAR3 or PAR4 (Fig. 1A). No responses to FVIIa were detected in the absence of TF expression (not shown). By contrast, FXa triggered signaling in oocytes expressing PAR1, PAR2, or PAR4 with no requirement for TF (Fig. 1A). Indeed, co-expression of TF did not change the concentration response to FXa (not shown). Recombinant FVIIa and FVIIa purified from plasma were equally potent in this system (data not shown). Moreover, the thrombin inhibitor hirudin, at a concentration sufficient to block a thrombin response of greater magnitude, did not substantially inhibit responses to FVIIa or FXa (Fig. 1B). These data and the TF-dependence of FVIIa signaling militate against a contaminating protease or thrombin generated in the oocyte system (perhaps from any remaining Xenopus prothrombin) being responsible for the signaling in Fig. 1, and strongly suggest that FVIIa can activate PAR1 and PAR2 and that FXa can activate PAR1, PAR2, and PAR4.
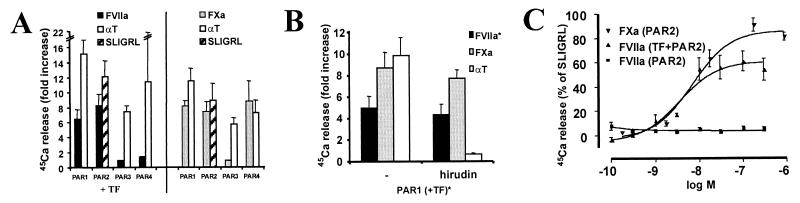
Figure 1.
PAR activation and cofactor dependence. Xenopus oocytes were injected with cRNAs encoding TF (TF) and PAR1, PAR2, PAR3, or PAR4, and agonist-triggered 45Ca2+ release was assessed. Surface expression of TF and PARs was confirmed by anti-FLAG antibody binding (not shown). (A) Activation of PARs by FVIIa (50 nM), FXa (174 nM), SLIGRL (100 μM), or thrombin (αT, 10 nM). TF expression was necessary for FVIIa responses (C), but did not affect responses to FXa (not shown). Data shown are fold increase 45Ca release (mean ± SE of 2–10 independent experiments each done in triplicate). (B) Effect of the thrombin inhibitor hirudin (10 units/ml) on PAR1 activation by other proteases. Protease concentrations were as in A. *, TF cRNA was co-injected with PAR1 cRNA when FVIIa was used as an agonist. Representative experiment (mean ± SD, n = 3). (C) Concentration responses to FVIIa and FXa in oocytes expressing PAR2 with or without TF as indicated. Average of 2–3 independent experiments done in triplicate (mean ± SE).
In most settings in which substantial FVIIa and FXa are generated in vivo, one would expect concomitant thrombin generation. Thrombin can activate PAR1 and PAR4; thus, the relative importance of the observation that FVIIa and FXa can activate these receptors is unknown. However, thrombin cannot activate PAR2. Trypsin and tryptase, the known activators of PAR2, seem unlikely to account for its activation in all cell types, and the notion that PAR2 might sense activation of the coagulation cascade is potentially important. We therefore focused on characterizing the actions of FVIIa and FXa on PAR2. In oocytes expressing only PAR2, even 300 nM FVIIa failed to cause signaling (Fig. 1C). By contrast, when TF and PAR2 were co-expressed, FVIIa caused robust signaling with an EC50 of 3.5 nM. This is similar to the Kd reported for FVII/FVIIa binding to cell surface TF (25) and below the plasma concentration of zymogen FVII (10 nM) but well above estimated plasma concentrations of FVIIa (26). FXa activated PAR2-expressing oocytes independent of cofactor with an EC50 of 7 nM (Fig. 1C), well below the plasma concentration of zymogen FX (174 nM, 1 unit/ml) (7).
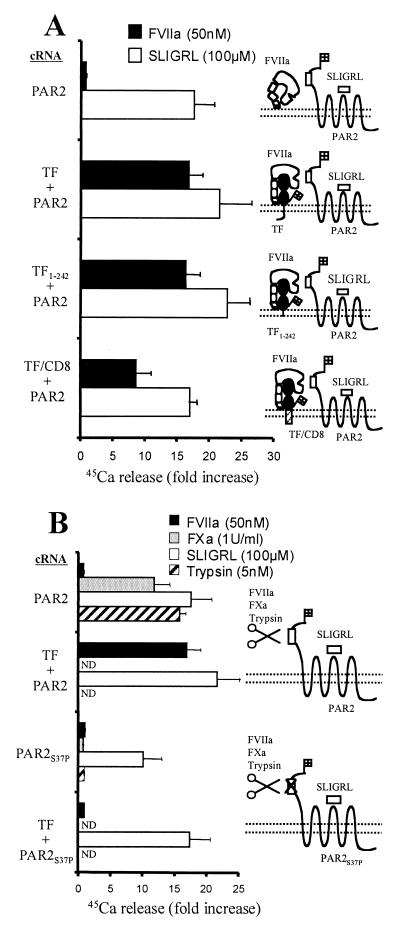
The cytoplasmic carboxyl tail of TF was not required to promote activation of PAR2 by FVIIa. Both the carboxyl tail deletion mutant TF1–242 and TF/CD8, in which the extracellular domain of TF was fused to the transmembrane domain of CD8, displayed cofactor activity (Fig. 2A). TF/CD8 was slightly less effective in promoting PAR2 activation by FVIIa than wild-type TF, and this was not attributable to different levels expressed on the cell surface. Whether TF/CD8 positions FVIIa's active site at a suboptimal distance above the membrane or, more interestingly, has an altered preference for specific domains in the plasma membrane that renders it less effective for presenting FVIIa to PAR2 is unknown.
Figure 2.
Mechanism of PAR2 activation. Xenopus oocytes were injected with cRNAs encoding wild-type or mutant TF and/or PAR2, and agonist-triggered 45Ca2+ mobilization was measured. Representative experiments (mean ± SD, n = 3) are shown. (A) FVIIa-induced responses in oocytes expressing PAR2 with or without wild-type or mutant TF. (B) Responses in oocytes expressing PAR2 or the PAR2 cleavage site mutant (PAR2S37P). Receptors were co-expressed with TF where indicated. ND, not determined.
The PAR2 mutant S37P, in which the cleavage site was rendered uncleavable, failed to mediate responsiveness to FVIIa or FXa or to trypsin. Responsiveness to exogenous tethered ligand peptide SLIGRL, which bypasses the requirement for receptor cleavage, was preserved (Fig. 2B). These data strongly support the model that the TF/FVIIa complex and FXa activate PAR2 by cleavage at the R36/S37 peptide bond.
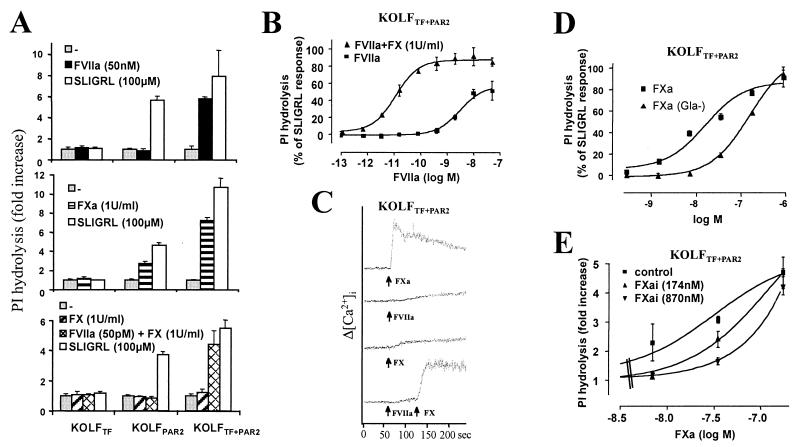
Xenopus oocytes express receptors at extraordinary levels. To determine whether FVIIa and FXa can trigger PAR2 signaling in mammalian cells in which receptors are expressed at more physiological levels, we expressed TF and PAR2 alone and together in KOLFs, a lung fibroblast cell line derived from PAR1 knockout mice. Phosphoinositide hydrolysis was measured as an endpoint for signaling. Untransfected KOLFs lack thrombin receptors (22) and PAR2, and did not respond to thrombin, trypsin, or SLIGRL (data not shown). Cells expressing TF alone (KOLFTF) failed to respond to FVIIa or FXa (Fig. 3A). Cells expressing PAR2 alone (KOLFPAR2) responded to FXa but not FVIIa. Cells expressing both TF and PAR2 (KOLFTF+PAR2) responded to both proteases. Similar results were obtained when signaling was measured as cytoplasmic calcium mobilization (see below) or as rapid induction mRNA for egr-1 (not shown). Thus, in agreement with the oocyte experiments, FVIIa elicited signaling via PAR2 only when co-expressed with TF. These data strongly suggest that TF can function as a cofactor that permits FVIIa to activate PAR2. This result stands in contrast to a previous report in which co-transfection of CHO cells with TF and PAR2 did not confer signaling by FVIIa (18). The discrepancy between this negative result with CHO cells and the positive result in current study is likely attributable to differences in the level of TF and/or PAR2 expression achieved in the CHO vs. KOLF systems. It is also possible that CHO cells lack a factor that is present in KOLFs and important for TF-dependent PAR2 activation.
Figure 3.
Activation of PAR2 in stably transfected fibroblasts by FVIIa and FXa. KOLFs expressing TF, PAR2, or TF + PAR2 were used. Results shown are representative of several lines tested. Figures show representative experiments (mean ± SD; n = 3). (A) PI hydrolysis induced by FVIIa (Top), FXa (Middle), or a combination of picomolar FVIIa and zymogen FX (Bottom), all relative to saturating PAR2 agonist (SLIGRL, 100 μM). The maximal response to SLIGRL varied with PAR2 expression and the lower maximal response in KOLFPAR2 relative to KOLFTF+PAR2 is likely caused by different PAR2 expression levels in the particular clones shown. (B) Dose responses for FVIIa-induced PI hydrolysis in KOLFTF+PAR2 in the presence or absence of plasma concentrations of FX. (C) Calcium transients induced by FXa (1 unit/ml) or FVIIa (100 pM) + FX (1 unit/ml) in Fura-2 loaded KOLFTF+PAR2 cells. 1 unit/ml FXa or FX = 174 nM. Increases in cytosolic calcium were measured fluorometrically. Agonists were added at time indicated by arrows. (D) Dose-response for FXa- or Gla-domainless FXa-induced PI hydrolysis. (E) Inhibition of FXa-induced PAR2 activation by preincubation of cells with the indicated concentrations of active site-inhibited FXa .
Are FVIIa and FXa physiological activators of PAR2? The concentration of FVIIa required for EC50 activation of phosphoinositide hydrolysis in KOLFTF+PAR2 was ≈4 nM, comparable to that of zymogen FVII in plasma and well above the 100 pM estimated plasma concentration of FVIIa. Strikingly, however, 50 pM FVIIa triggered robust signaling in KOLFTF+PAR2 cells when the inactive zymogen FX was present at its estimated plasma concentration (1 unit/ml, 174 nM) (Fig. 3A Bottom). Indeed, the EC50 for responses to FVIIa decreased nearly three logs (4 nM to 8 pM; Fig. 3B) when FX was included. In the absence of FX, 50 pM FVIIa was without effect, and, in the absence FVIIa, FX was without effect. Moreover, even in the presence of FX, 50 pM FVIIa did not elicit cellular responses in cells expressing PAR2 alone; co-expression of TF was required. Similar results were obtained when PAR2 activation was assessed as calcium mobilization measured fluorometrically in Fura-2 loaded KOLFs. 100 pM FVIIa or 1 unit/ml FX alone triggered little or no calcium signaling in KOLFTF+PAR2 cells (Fig. 3C). However, addition of both proteins elicited a rapid and robust increase in cytosolic calcium comparable to that triggered by 1 unit/ml FXa. These data strongly suggest that signaling in response to picomolar FVIIa in the presence of zymogen FX is mediated by FXa generated by the TF/FVIIa complex. Moreover, they show that FVIIa levels below those thought to circulate in plasma can trigger signaling in this system.
The EC50 for activation of phosphoinositide hydrolysis in PAR2-expressing cells by soluble FXa was 27 nM. Thus, the FVIIa signaling described above might require substantial conversion of FX to FXa. Alternatively, because of an increased probability of colliding in two instead of three dimensions and/or favorable localization by other mechanisms, FXa generated locally on the cell surface might have a kinetic advantage over soluble exogenous FXa for activating PAR2 on the same membrane. If so, PAR2 activation might be achieved without generating high levels of FXa in solution. It has been difficult to test this prediction directly because, in the absence of inhibitors, TF/FVIIa converts FX to FXa very efficiently, and substantial levels of FXa were generated even in the 30 seconds required to reach peak cytosolic calcium triggered by addition of 100 pM FVIIa/174 nM FX to KOLFTF+PAR2 cells (not shown; see Discussion).
Several experiments do support the notion that localization of FXa to the plasma membrane makes some contribution to its ability to activate PAR2. FXa lacking the Gla domain activated PAR2 with reduced potency (7-fold average increase in EC50; Fig. 3D), consistent with the notion that Gla domain-mediated localization of FXa to the cell surface contributes to PAR2 activation. Moreover, excess active site-inhibited FXa attenuated PAR2 activation by low concentrations of FXa (Fig. 3E). This was not attributable to contaminating chloromethyl ketone and was specific for FXa; 870 nM active site-inhibited FXa did not inhibit signaling to 50 nM FVIIa in parallel experiments (not shown). Addition of FVa, the known FXa cofactor for prothrombin activation, did not enhance FXa activity against PAR2 in the oocyte or KOLF systems (not shown). Taken together, these data suggest that binding of FXa to some saturable site(s) on the plasma membrane contributes to its activity at PAR2. Whether this site is PAR2 itself, a protein cofactor, or another cell membrane component is unknown. The observation that FXa effectively activated PAR2 expressed in both Xenopus oocytes and mammalian fibroblasts militates against a requirement for a specific protein cofactor unless that cofactor is present in the oocyte system.
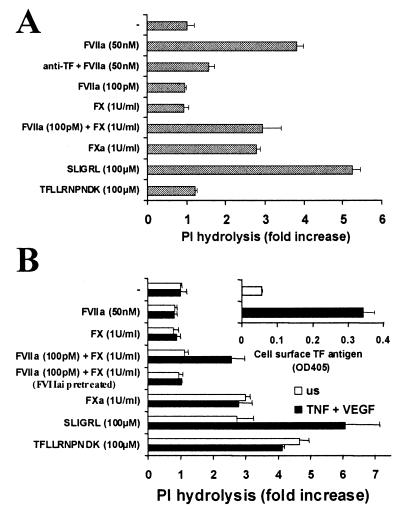
The data presented above show that, in transfected cells, TF and PAR2 expression on the same cell permits both direct activation of PAR2 by TF/FVIIa and indirect activation by TF/VIIa-generated FXa. Do these phenomena occur in untransfected differentiated cells known to respond to FVIIa or FXa? TF and PAR2 are expressed by various epithelial cell types (27, 28), and, provocatively, their expression can be induced (TF) or enhanced (PAR2) in endothelial cells by inflammatory stimuli (8, 29, 30) and in smooth muscle cells by vascular injury (31, 32). Fig. 4 shows signaling induced by FVIIa and FX/FXa in two untransfected cell lines. The human keratinocyte cell line HaCaT expresses PAR2 and TF constitutively, the latter at levels comparable to those in the KOLFTF+PAR2 cells used in these studies (not shown). HaCaT cells indeed behaved much like the KOLFTF+PAR2 cell line; signaling was detected in response to high FVIIa or FXa alone and to the combination of picomolar FVIIa and zymogen FX (Fig. 4A). Unlike HaCaTs, human umbilical venous endothelial cells (HUVECs) express little or no TF unless it is induced by cytokines; even after induction, TF expression is substantially lower than in HaCaTs (not shown). HUVECs do constitutively express PAR2, and PAR2 expression can be increased by cytokines (30). Naïve HUVECs responded to FXa and PAR agonist peptides (Fig. 4B) but not to FVIIa, consistent with the TF-dependence of FVIIa signaling noted in our transfection studies. Cytokine treatment of HUVECs induced TF expression and conferred responsiveness to picomolar FVIIa in combination with FX, but not to FVIIa alone, even when the latter was used at high concentrations (Fig. 4B). This difference between HUVEC vs. HaCaT and KOLFTF+PAR2 is probably explained by the TF expression level in HUVECs being below that required for direct activation of PAR2 by TF/FVIIa, even after cytokine stimulation. Alternatively, tissue factor pathway inhibitor (TFPI) 1 and 2 are expressed by HUVECs (8, 33) and might attenuate the direct TF/FVIIa signaling. Exogenous TFPI did inhibit signaling in KOLFTF+PAR2 cells; 5 nM TFPI-1 inhibited activation of PAR2 by 100 pM FVIIa/1 unit/ml FX, and 125 nM TFPI-1 inhibited activation by 50 nM FVIIa and 35 nM FXa (not shown). The FVIIa and FVIIa/FX signaling effects in both HaCaT and HUVEC systems were substantially reduced by pretreating with active site-inhibited FVIIa or anti-TF antibodies (Fig. 4 A and B; data not shown), again consistent with TF-dependence. Desensitization of PAR2 with SLIGRL virtually abolished FXa signaling in HUVECs (not shown) and HaCaTs (18) whereas thrombin desensitization had little effect. Because SLIGRL also caused some heterologous desensitization of PAR1 signaling in cells that expressed both PAR1 and PAR2, we cannot conclude that all FXa signaling in endothelial cells is PAR2-mediated. Nonetheless, these results suggest that FXa signaling in HUVECs was at least partially PAR2-dependent. Taken together, these data strongly suggest that FVIIa can trigger signaling in human keratinocytes and HUVECs via TF-dependent generation of FXa on the cell surface and consequent PAR2 cleavage and activation.
Figure 4.
Protease responses in keratinocytes and vascular endothelial cells. Data shown are fold increase in 3H-inositol phosphate accumulation (mean ± SD, n = 3) and are representative of at least three separate experiments. (A) PI hydrolysis in response to proteases and peptide agonists in keratinocyte line HaCaT, which expresses TF and PAR2 constitutively. Where indicated, anti-TF mAb was added 1 h before agonists. (B) PI hydrolysis in response to proteases and peptide agonists in naïve HUVEC or HUVEC pretreated for 6 h with tumor necrosis factor α (10 ng/ml) and vascular endothelial growth factor (1 nM). Cytokines induce TF expression and increase PAR2 expression in these cells. Cell surface TF expression as determined by surface ELISA with a TF mAb is shown in the inset. Where indicated, active site-inhibited FVIIa was added 1 h before agonists.
Discussion
We have shown that picomolar FVIIa can activate PAR2 when TF and FX are present. These results imply a cascade of events on the cell surface. FVIIa binds to TF, the TF/FVIIa complex converts inactive zymogen FX to the active protease FXa, then FXa cleaves PAR2 to trigger transmembrane signaling. TF/FVIIa may also directly activate PAR2, especially when TF is expressed at high levels. This model is consistent with previous reports that FVIIa and FXa can elicit cellular responses and likely accounts, at least in part, for many of the active site-dependent cellular effects described (9–18). It also provides a plausible scenario by which PAR2 might function as a receptor for these coagulation proteases in vivo. More generally, these results emphasize a role for cofactors in protease signaling—PAR activation need not be thought of simply in terms of a binary interactions between fluid-phase proteases and the integral membrane PARs. A priori, use of cofactors in PAR activation might serve to promote specificity by demanding that the target cell express both cofactor and PAR to be responsive to a particular protease. In addition, regulation of cofactor expression might also permit cells to vary responsiveness to different proteases while using only one or a few PARs. Our findings with vascular endothelial cells support these notions.
Available data are consistent with the possibility that PAR2 responds to TF/FVIIa and/or FXa in vivo. FVIIa is relatively long-lived in plasma and circulates at ≈100 pM (7, 34). In our studies of FX-dependent PAR2 activation by FVIIa, the EC50 for FVIIa signaling was only ≈8 pM. Zymogen FX was at its plasma concentration. Thus, when TF and FX are present, the level of FVIIa known to circulate in vivo is more than sufficient to initiate FXa generation and signaling. Exactly which protease might dominate in vivo is difficult to predict. Depending on the balance with inhibitors and the level of TF expression, feedback activation of zymogen FVII by FXa might generate sufficient TF/VIIa on the cell surface to directly activate PAR2. Alternatively, FXa generated by TF/VIIa or by VIIIa/IXa might be the dominant actor. Regardless, human keratinocytes naturally express both TF and PAR2 (35), and human endothelial cells naturally express PAR2 and can be induced to express TF by inflammatory cytokines (29). The observation that picomolar FVIIa can trigger signaling in HaCaT cells and in cytokine-induced HUVECs in the presence of FX suggests that such signaling might occur in vivo when plasma proteins contact keratinocytes or TF-expressing endothelial cells at sites of wounding, inflammation, or altered vascular integrity.
Fluid phase FXa is short-lived and susceptible to inhibition by both tissue factor pathway inhibitor and antithrombin III (7). By contrast, FXa generated on the surface of a PAR2-expressing cell might be well positioned to activate nearby receptors before being inactivated by circulating inhibitors. Indeed, the presence of inhibitors such as antithrombin III (36) might allow local cell surface PAR2 activation (and perhaps also local prothrombinase formation) without significant propagation of coagulation.
The function of PAR2 in endothelial cells has been obscure in part because the protease that activates PAR2 in this context has not been identified. Our results suggest that endothelial PAR2 can sense activation of the coagulation cascade, whether triggered by induction of endothelial TF expression by cytokines (29), by monocyte TF in the context of leukocyte-vessel wall interactions, or by vascular injury. Like PAR1 activation, PAR2 activation triggers phosphoinositide hydrolysis and mobilization of intracellular calcium. These signaling events are associated with release of vWF and mobilization of P-selectin to the endothelial surface (37–39). PAR2 activation should thus promote platelet and leukocyte rolling on the vascular endothelium, and pharmacological studies using SLIGRL have indeed implicated PAR2 in this process (40). Thus, PAR2 might participate in the well established link between coagulation and inflammation, and interrupting this positive feedback loop might be beneficial in certain settings (41). This hypothesis is testable by examining the effect of PAR2-deficiency in mouse models of endothelial activation and microvascular thrombosis (42). These results also raise the interesting prospect that PAR2 and PAR1 might serve redundant functions in some settings, a notion that has important implications for interpretation of studies done with PAR1- and PAR2-deficient mice.
A possible role for PAR2 in neurogenic inflammation has recently been proposed (43), but whether the triggering protease in vivo is mast cell tryptase or other protease(s) is unknown. By casting PAR2 as a sensor of coagulation proteases, our results provide a possible link between tissue injury and activation of PAR2 similar to that previously envisioned for PAR1. It is thus possible that TF/VIIa and FXa activation of neuronal PAR2 plays a role in neurogenic inflammation.
Acknowledgments
This work was supported by Grants HL44907, HL59202, and DK50267 from the National Institutes of Health and by the Daiichi Research Center. E.C. was supported by postdoctoral fellowships from The Research Council of Norway and the American Heart Association.
Abbreviations
- FVIIa
factor VIIa
- FXa
factor Xa
- PAR
protease-activated receptor
- TF
tissue factor
- HUVEC
human umbilical vein endothelial cell
- TFPI
tissue factor pathway inhibitor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Coughlin S R. Proc Natl Acad Sci USA. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molino M, Barnathan E S, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie J A, Schechter N, Woolkalis M, Brass L F. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara H, Connolly A J, Zeng D, Kahn M L, Zheng Y W, Timmons C, Tram T, Coughlin S R. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 5.Kahn M L, Zheng Y W, Huang W, Bigornia V, Zeng D, Moff S, Farese R V, Jr, Tam C, Coughlin S R. Nature (London) 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 6.Kahn M L, Nakanishi-Matsui M, Shapiro M J, Ishihara H, Coughlin S R. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mann K G. Thromb Haemostasis. 1999;82:165–174. [PubMed] [Google Scholar]
- 8.Camerer E, Kolstø A B, Prydz H. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 9.Røttingen J A, Enden T, Camerer E, Iversen J G, Prydz H. J Biol Chem. 1995;270:4650–4660. doi: 10.1074/jbc.270.9.4650. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson A C, Nachman R L, Altieri D C, Summers B D, Ruf W, Edgington T S, Hajjar D. J Biol Chem. 1996;271:28407–28413. doi: 10.1074/jbc.271.45.28407. [DOI] [PubMed] [Google Scholar]
- 11.Camerer E, Røttingen J A, Iversen J G, Prydz H. J Biol Chem. 1996;271:29034–29042. doi: 10.1074/jbc.271.46.29034. [DOI] [PubMed] [Google Scholar]
- 12.Poulsen L K, Jacobsen N, Sørensen B B, Bergenhem N C H, Kelly J D, Foster D C, Thastrup O, Ezban M, Petersen L C. J Biol Chem. 1998;273:6228–6232. doi: 10.1074/jbc.273.11.6228. [DOI] [PubMed] [Google Scholar]
- 13.Ollivier V, Bentolila S, Chabbat J, Hakim J, de Prost D. Blood. 1998;91:2698–2703. [PubMed] [Google Scholar]
- 14.Taniguchi T, Kakkar A K, Tuddenham E G D, Williamson R C N, Lemoine N R. Cancer Res. 1998;58:4461–4467. [PubMed] [Google Scholar]
- 15.Senden N H, Jeunhomme T M, Heemskerk J W, Wagenvoord R, van't Veer C, Hemker H C, Buurman W A. J Immunol. 1998;161:4318–4324. [PubMed] [Google Scholar]
- 16.Papapetropoulos A, Piccardoni P, Cirino G, Bucci M, Sorrentino R, Cicala C, Johnson K, Zachariou V, Sessa W C, Altieri D C. Proc Natl Acad Sci USA. 1998;95:4738–4742. doi: 10.1073/pnas.95.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sørensen B B, Freskgård P O, Nielsen L S, Rao L V, Ezban M, Petersen L C. J Biol Chem. 1999;274:21349–21354. doi: 10.1074/jbc.274.30.21349. [DOI] [PubMed] [Google Scholar]
- 18.Camerer E, Røttingen J-A, Gjernes E, Larsen K, Skartlien A H, Iversen J-G, Prydz H. J Biol Chem. 1999;274:32225–32233. doi: 10.1074/jbc.274.45.32225. [DOI] [PubMed] [Google Scholar]
- 19.Ishii K, Hein L, Kobilka B, Coughlin S R. J Biol Chem. 1993;268:9780–9786. [PubMed] [Google Scholar]
- 20.Chen J, Ishii M, Wang L, Ishii K, Coughlin S R. J Biol Chem. 1994;269:16041–16045. [PubMed] [Google Scholar]
- 21.Vu T-K H, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 22.Trejo J, Connolly A J, Coughlin S R. J Biol Chem. 1996;271:21536–21541. doi: 10.1074/jbc.271.35.21536. [DOI] [PubMed] [Google Scholar]
- 23.Boukamp P, Petrusevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camera M, Giesen P L, Fallon J, Aufiero B M, Taubman M, Tremoli E, Nemerson Y. Arterioscler Thromb Vasc Biol. 1999;19:531–537. doi: 10.1161/01.atv.19.3.531. [DOI] [PubMed] [Google Scholar]
- 25.Sakai T, Lund-Hansen T, Paborsky L, Pedersen A H, Kisiel W. J Biol Chem. 1989;264:9980–9988. [PubMed] [Google Scholar]
- 26.Morrissey J H, Macik B G, Neuenschwander P F, Comp P C. Blood. 1993;81:734–744. [PubMed] [Google Scholar]
- 27.Drake T, Morissey J, Edgington T. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 28.D'Andrea M R, Derian C K, Leturcq D, Baker S M, Brunmark A, Ling P, Darrow A L, Santulli R J, Brass L F, Andrade-Gordon P. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 29.Bevilacqua M P, Pober J S, Majeau G R, Fiers W, Cotran R S, Gimbrone M A., Jr Proc Natl Acad Sci USA. 1986;83:4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nystedt S, Ramakrishnan V, Sundelin J. J Biol Chem. 1996;271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 31.Marmur J D, Rossikhina M, Guha A, Fyfe B, Friedrich V, Mendlowitz M, Nemerson Y, Taubman M B. J Clin Invest. 1993;91:2253–2259. doi: 10.1172/JCI116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damiano B P, D'Andrea M R, de Garavilla L, Cheung W M, Andrade-Gordon P. Thromb Haemostasis. 1999;81:808–814. [PubMed] [Google Scholar]
- 33.Sprecher C A, Kisiel W, Mathewes S, Foster D C. Proc Natl Acad Sci USA. 1994;91:3353–3357. doi: 10.1073/pnas.91.8.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemerson Y. In: Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Colman R W, Hirsh J, Marder V J, Salzman E W, editors. Philadelphia: Lippincott; 1994. pp. 81–93. [Google Scholar]
- 35.Santulli R J, Derian C K, Darrow A L, Tomko K A, Eckardt A J, Seiberg M, Scarborough R M, Andrade-Gordon P. Proc Natl Acad Sci USA. 1995;92:9151–9155. doi: 10.1073/pnas.92.20.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Fiehler R, Broze G J., Jr Proc Natl Acad Sci USA. 1998;95:9250–9255. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hattori R, Hamilton K K, Fugate R D, McEver R P, Sims P J. J Biol Chem. 1989;264:7768–7771. [PubMed] [Google Scholar]
- 38.Subramaniam M, Frenette P S, Saffaripour S, Johnson R C, Hynes R O, Wagner D D. Blood. 1996;87:1238–1242. [PubMed] [Google Scholar]
- 39.Denis C, Methia N, Frenette P S, Rayburn H, Ullman-Culleré M, Hynes R O, Wagner D D. Proc Natl Acad Sci USA. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergnolle N. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- 41.Esmon C T. Blood. 2000;95:1113–1116. [PubMed] [Google Scholar]
- 42.Brozna J P. Semin Thromb Hemostasis. 1990;16:326–332. doi: 10.1055/s-2007-1002685. [DOI] [PubMed] [Google Scholar]
- 43.Steinhoff M, Vergnolle N, Young S H, Tognetto M, Amadesi S, Ennes H S, Trevisani M, Hollenberg M D, Wallace J L, Caughey G H, et al. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]