Abstract
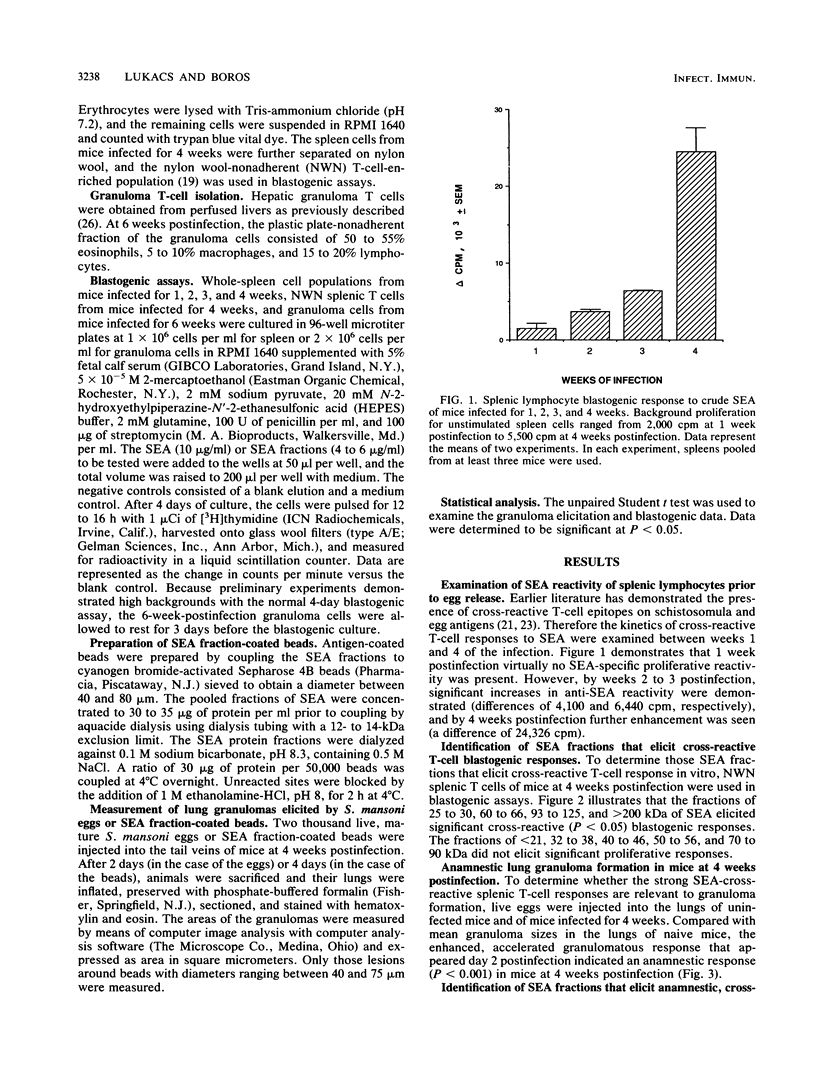
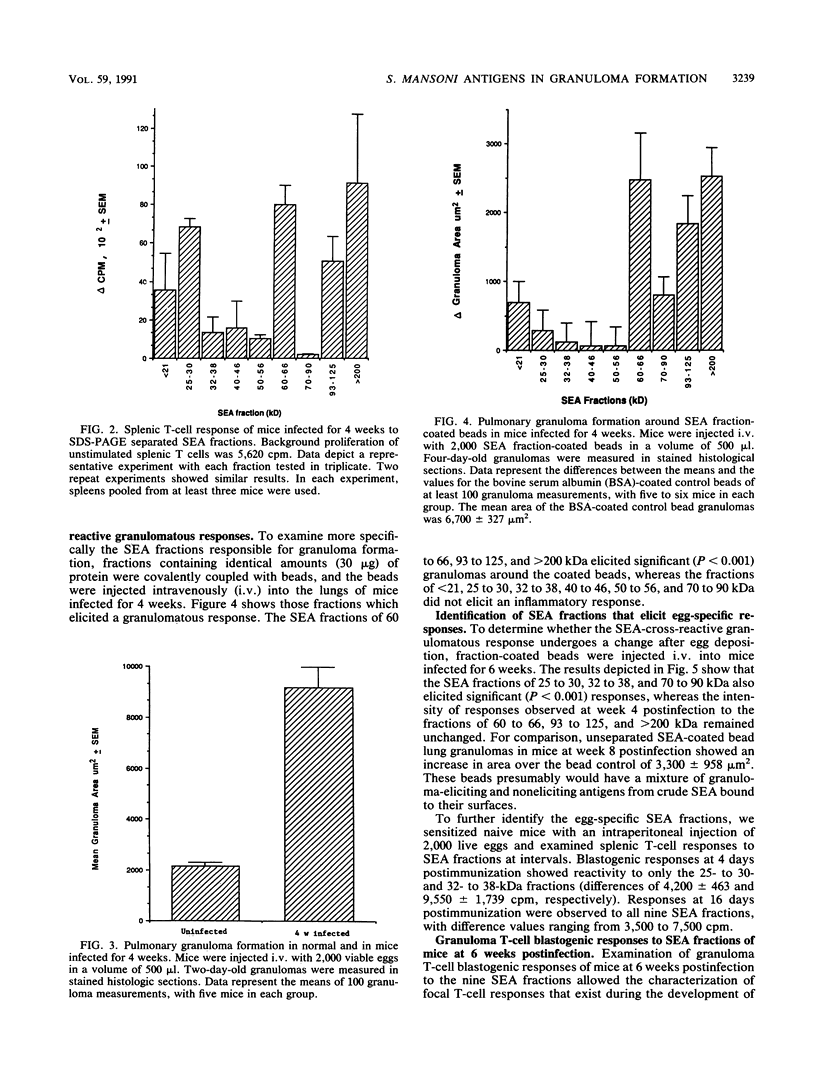
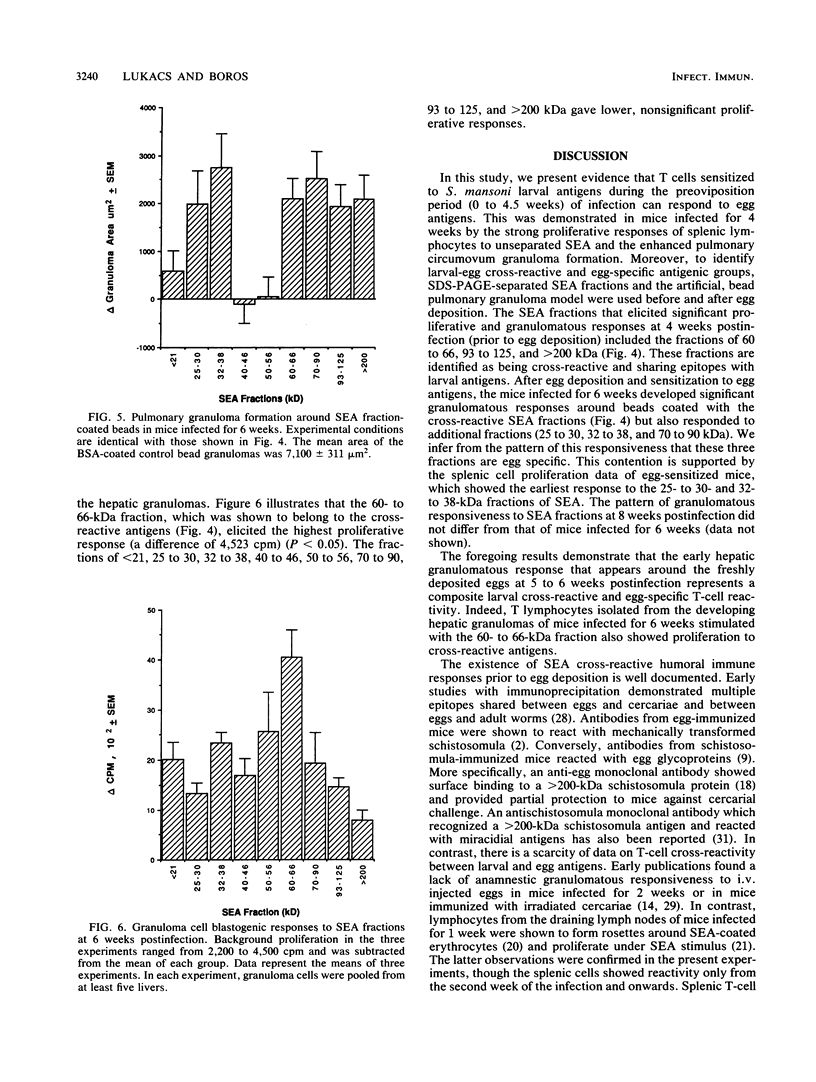
Cross-reactive humoral immune responses between antigens of different developmental stages of the worm Schistosoma mansoni have previously been demonstrated. In contrast, information on antigenic cross-reactivity at the T-cell level is still very sparse. The present study examined the cross-reactive T-cell responses to eggs and crude and fractionated soluble egg antigens (SEA) in infected mice prior to (from 0 to 4 weeks of infection) and after (5 weeks and onwards) egg deposition. Splenic lymphocyte proliferation to unfractionated SEA was detected as early as 2 weeks postinfection and increased rapidly by 4 weeks postinfection. Injections of live eggs into the lungs of infected mice at 4 weeks postinfection demonstrated enhanced granuloma formation, indicating the presence of primed T cells that respond to egg antigens. Further experiments with the artificial granuloma model and polyacrylamide gel electrophoresis-separated SEA fractions demonstrated that in mice infected for 4 weeks the 60- to 66-, 93- to 125-, and greater than 200-kDa SEA fraction-coated beads elicited significant pulmonary granulomas. By 6 weeks postinfection, when eggs are deposited in the livers, in addition to the cross-reactive fractions (60 to 66, 93 to 125, and greater than 200 kDa), beads coated with fractions of 25 to 30, 32 to 38, and 70 to 90 kDa also elicited significant granulomatous reactions. These antigenic fractions are considered to have elicited egg stage-specific T-cell responsiveness. In addition hepatic granuloma T cells from the 6th week of infection demonstrated the strongest blastogenic response to the 60- to 66-kDa cross-reactive fraction. Thus, in vitro and in vivo experiments demonstrated T-cell cross-reactivity between the larval and egg stages of the worm. On the basis of these observations, the appearance of the primary circumovum granulomatous response in infected mice is considered to represent the sum of larval cross-reactive and egg-specific T-cell responsiveness.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Colley D. G. Modulation of Schistosoma mansoni egg-induced granuloma formation. III. Evidence for an anti-idiotypic, I-J-positive, I-J-restricted, soluble T suppressor factor. J Immunol. 1984 Apr;132(4):2084–2088. [PubMed] [Google Scholar]
- Bickle Q. D., Ford M. J., Andrews B. J. Studies on the development of anti-schistosomular surface antibodies by mice exposed to irradiated cercariae, adults and/or eggs of S. mansoni. Parasite Immunol. 1983 Sep;5(5):499–511. doi: 10.1111/j.1365-3024.1983.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Boros D. L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989 Jul;2(3):250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Colley D. G. Immune responses to a soluble schistosomal egg antigen preparation during chronic primary infection with Schistosoma mansoni. J Immunol. 1975 Jul;115(1):150–156. [PubMed] [Google Scholar]
- Colley D. G., Lewis F. A., Todd C. W. Adoptive suppression of granuloma formation by T lymphocytes and by lymphoid cells sensitive to cyclophosphamide. Cell Immunol. 1979 Aug;46(1):192–200. doi: 10.1016/0008-8749(79)90258-2. [DOI] [PubMed] [Google Scholar]
- Dalton J. P., Strand M., Mangold B. L., Dean D. A. Identification of Schistosoma mansoni glycoproteins recognized by protective antibodies from mice immunized with irradiated cercariae. J Immunol. 1986 Jun 15;136(12):4689–4694. [PubMed] [Google Scholar]
- Damian R. T. Immunity in schistosomiasis: a holistic view. Contemp Top Immunobiol. 1984;12:359–420. doi: 10.1007/978-1-4684-4571-8_10. [DOI] [PubMed] [Google Scholar]
- Damian R. T., Roberts M. L., Powell M. R., Clark J. D., Lewis F. A., Stirewalt M. A. Schistosoma mansoni egg granuloma size reduction in challenged baboons after vaccination with irradiated cryopreserved schistosomula. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3552–3556. doi: 10.1073/pnas.81.11.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A., Bukowski M. A., Cheever A. W. Relationship between acquired resistance, portal hypertension, and lung granulomas in ten strains of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1981 Jul;30(4):806–814. doi: 10.4269/ajtmh.1981.30.806. [DOI] [PubMed] [Google Scholar]
- Dean D. A., Minard P., Murrell K. D., Vannier W. E. Resistance of mice to secondary infection with Schistosoma mansoni. II. Evidence for a correlation between egg deposition and work elimination. Am J Trop Med Hyg. 1978 Sep;27(5):957–965. doi: 10.4269/ajtmh.1978.27.957. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. Endogenous desensitization: changing host granulomatou response to schistosome eggs at different stages of infection with schistosoma mansoni. Am J Pathol. 1968 Feb;52(2):369–379. [PMC free article] [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation and modulation around Schistosoma mansoni eggs in vitro. II. Regulatory T cell subsets. J Immunol. 1982 Jan;128(1):37–42. [PubMed] [Google Scholar]
- Fidel P. L., Jr, Boros D. L. Regulation of granulomatous inflammation in murine schistosomiasis. V. Antigen-induced T cell-derived suppressor factors down-regulate proliferation and IL-2, but not IL-4, production by CD4+ effector T cells. J Immunol. 1991 Mar 15;146(6):1941–1948. [PubMed] [Google Scholar]
- Harn D. A., Mitsuyama M., David J. R. Schistosoma mansoni. Anti-egg monoclonal antibodies protect against cercarial challenge in vivo. J Exp Med. 1984 May 1;159(5):1371–1387. doi: 10.1084/jem.159.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Khoury P. B., Phillips S. M. Kinetics and characterization of antigen-binding and antibody-producing cells in the regional draining lymph nodes and spleen during initial murine schistosomiasis. II. Cellular Responses against egg immunogens. Cell Immunol. 1981 Apr;59(2):246–255. doi: 10.1016/0008-8749(81)90406-8. [DOI] [PubMed] [Google Scholar]
- Lewis F. A., Wilson E. M. Regional and splenic lymphocyte proliferative responses of mice exposed to normal or irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):505–513. doi: 10.4269/ajtmh.1982.31.505. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Splenic and granuloma T-lymphocyte responses to fractionated soluble egg antigens of Schistosoma mansoni-infected mice. Infect Immun. 1991 Mar;59(3):941–948. doi: 10.1128/iai.59.3.941-948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak N. K., Sanderson C. J. The T-cell mediated immune response to Schistosoma mansoni. I. Generation of stage-specific, MHC-restricted proliferative T-cell clones to soluble egg antigens. Immunology. 1985 Apr;54(4):625–633. [PMC free article] [PubMed] [Google Scholar]
- Perrin P. J., Phillips S. M. The molecular basis of granuloma formation in schistosomiasis. I. A T cell-derived suppressor effector factor. J Immunol. 1988 Sep 1;141(5):1714–1719. [PubMed] [Google Scholar]
- Phillips S. M., Lammie P. J. Immunopathology of granuloma formation and fibrosis in schistosomiasis. Parasitol Today. 1986 Nov;2(11):296–302. doi: 10.1016/0169-4758(86)90123-7. [DOI] [PubMed] [Google Scholar]
- Ragheb S., Boros D. L. Characterization of granuloma T lymphocyte function from Schistosoma mansoni-infected mice. J Immunol. 1989 May 1;142(9):3239–3246. [PubMed] [Google Scholar]
- Sadun E. H., Schoenbechler M. J., Bentz M. Multiple antibody response in Schistosoma mansoni infections: antigenic constituents in eggs, cercariae, and adults (excretions and secretions) determined by flocculation reactions, cross absorption and double diffusion studies. Am J Trop Med Hyg. 1965 Nov;14(6):977–995. doi: 10.4269/ajtmh.1965.14.977. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O. Schistosoma mansoni: stage specificity of granuloma formation around eggs after exposure to irradiated cercariae, unisexual infections, or dead worms. Exp Parasitol. 1970 Feb;27(1):60–66. doi: 10.1016/s0014-4894(70)80010-8. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The secret of the immunopathogenesis of schistosomiasis: in vivo models. Immunol Rev. 1982;61:189–213. doi: 10.1111/j.1600-065x.1982.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Yi X. Y., Omer-Ali P., Kelly C., Simpson A. J., Smithers S. R. IgM antibodies recognizing carbohydrate epitopes shared between schistosomula and miracidia of Schistosoma mansoni that block in vitro killing. J Immunol. 1986 Dec 15;137(12):3946–3954. [PubMed] [Google Scholar]


