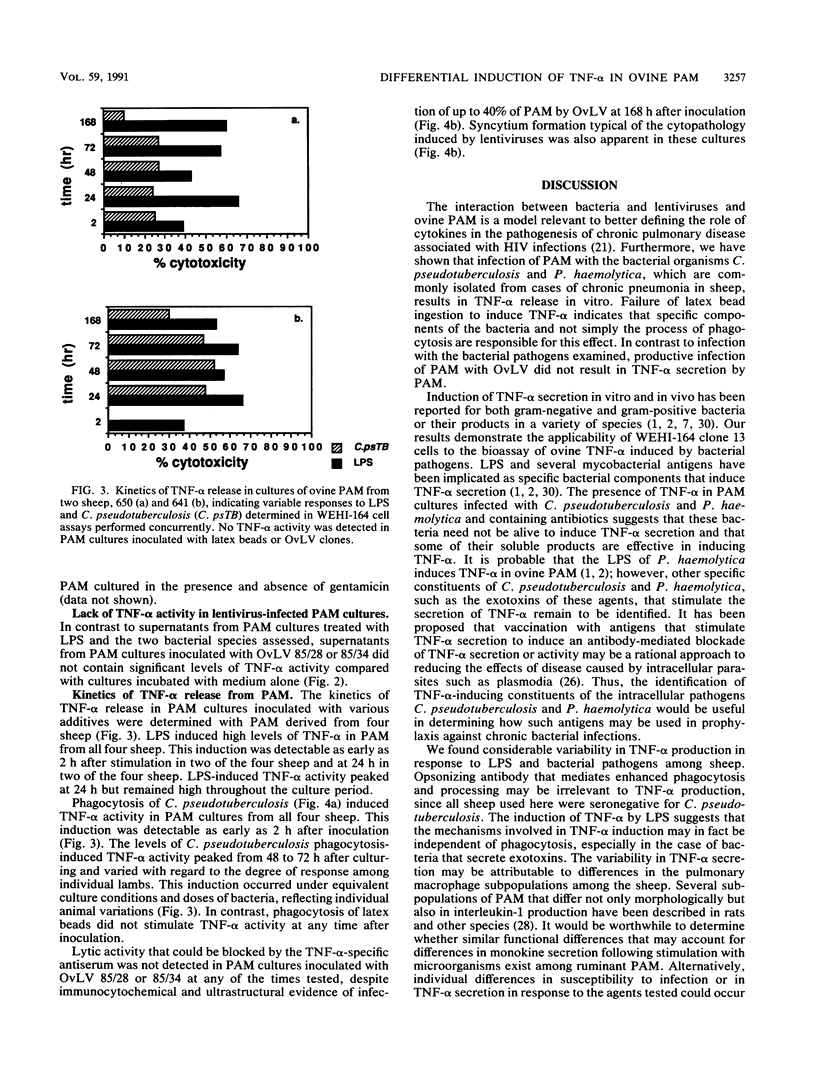
Abstract
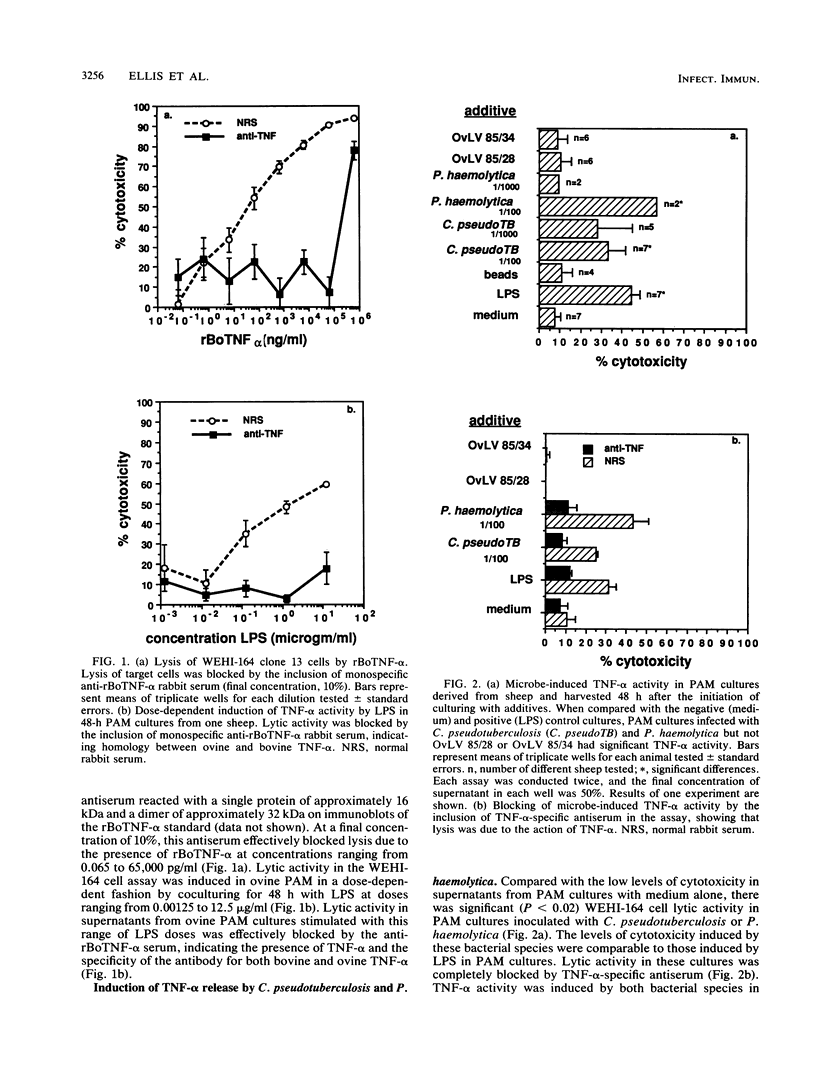
Soluble mediators such as tumor necrosis factor alpha (TNF-alpha) may be important in the pathogenesis of many chronic pulmonary infections. We examined the ability of Corynebacterium pseudotuberculosis, Pasteurella haemolytica, and ovine lentiviruses (OvLV) to induce TNF-alpha secretion by pulmonary alveolar macrophages (PAM). Bronchoalveolar lavage cells, composed of greater than 90% PAM, were obtained from normal sheep. Bronchoalveolar lavage cells were cultured for 2, 24, 48, 72, or 168 h in endotoxin-free RPMI medium (with 10% autologous serum) or in medium containing one of the following additives: lipopolysaccharide, 1-micron polystyrene beads, C. pseudotuberculosis, P. haemolytica, or one of two plaque-cloned OvLV, 85/28 or 85/34. Lipopolysaccharide, C. pseudotuberculosis, and P. haemolytica induced TNF-alpha activity in PAM cultures as early as 2 h after inoculation, as assessed by a colorimetric cytotoxicity assay. This activity could be blocked by rabbit anti-recombinant bovine TNF-alpha serum. In contrast, medium alone, polystyrene beads, and productive infection by OvLV did not induce TNF-alpha activity in PAM cultures. Bacterial pathogens which infect pulmonary macrophages may elicit the secretion of TNF-alpha within the lungs and lead to the cachectic state associated with chronic pneumonia.
Full text
PDF

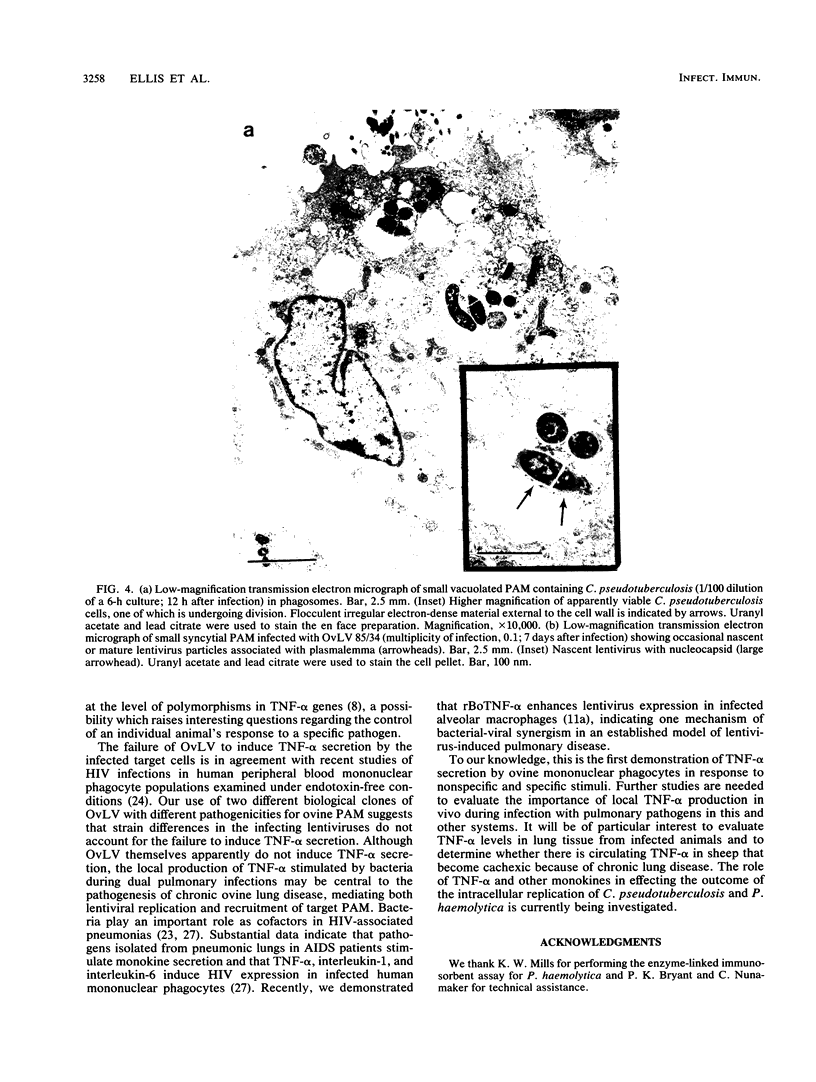
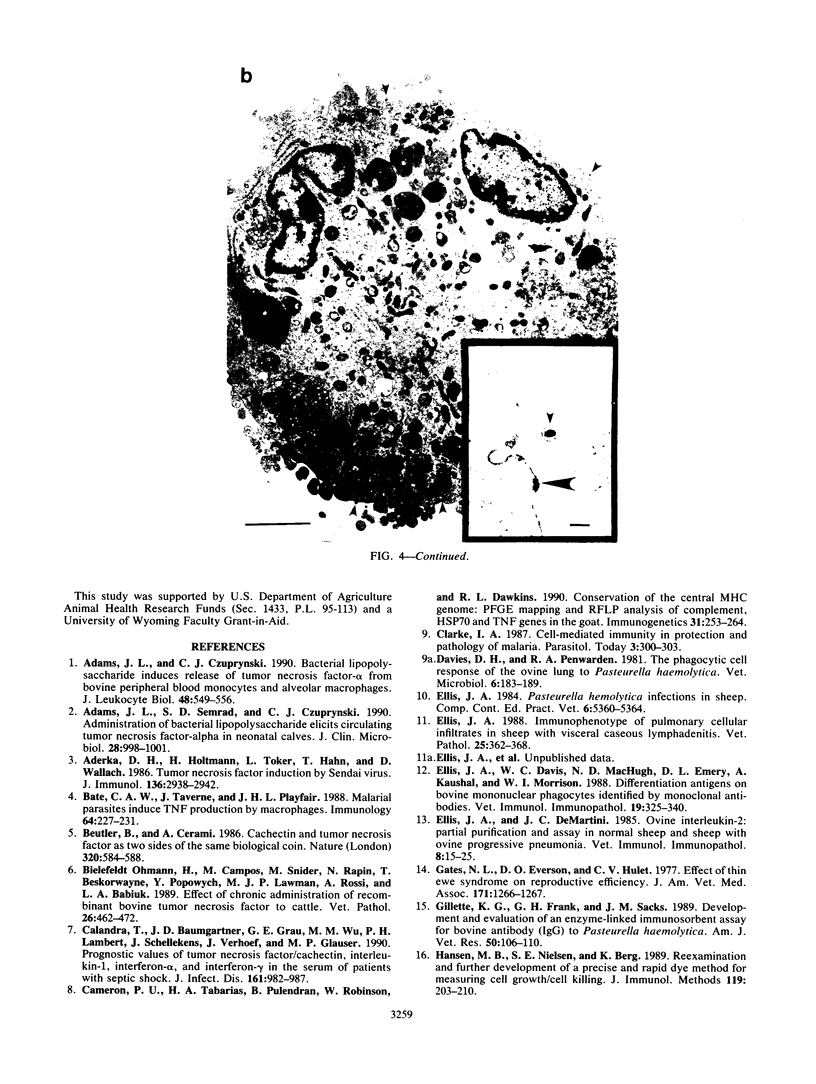

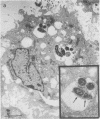
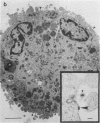
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. L., Czuprynski C. J. Bacterial lipopolysaccharide induces release of tumor necrosis factor-alpha from bovine peripheral blood monocytes and alveolar macrophages in vitro. J Leukoc Biol. 1990 Dec;48(6):549–556. doi: 10.1002/jlb.48.6.549. [DOI] [PubMed] [Google Scholar]
- Adams J. L., Semrad S. D., Czuprynski C. J. Administration of bacterial lipopolysaccharide elicits circulating tumor necrosis factor-alpha in neonatal calves. J Clin Microbiol. 1990 May;28(5):998–1001. doi: 10.1128/jcm.28.5.998-1001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Campos M., Snider M., Rapin N., Beskorwayne T., Popowych Y., Lawman M. J., Rossi A., Babiuk L. A. Effect of chronic administration of recombinant bovine tumor necrosis factor to cattle. Vet Pathol. 1989 Nov;26(6):462–472. doi: 10.1177/030098588902600602. [DOI] [PubMed] [Google Scholar]
- Calandra T., Baumgartner J. D., Grau G. E., Wu M. M., Lambert P. H., Schellekens J., Verhoef J., Glauser M. P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990 May;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- Cameron P. U., Tabarias H. A., Pulendran B., Robinson W., Dawkins R. L. Conservation of the central MHC genome: PFGE mapping and RFLP analysis of complement, HSP70, and TNF genes in the goat. Immunogenetics. 1990;31(4):253–264. doi: 10.1007/BF00204897. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Cell-mediated immunity in protection and pathology of malaria. Parasitol Today. 1987 Oct;3(10):300–305. doi: 10.1016/0169-4758(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Dakkak A., Daoudi A., Ruckebusch Y. Haemonchus contortus and Ostertagia circumcincta: fenbendazole treatment and abomasal permeability changes in sheep. Am J Vet Res. 1985 Jan;46(1):209–211. [PubMed] [Google Scholar]
- Ellis J. A., Davis W. C., MacHugh N. D., Emery D. L., Kaushal A., Morrison W. I. Differentiation antigens on bovine mononuclear phagocytes identified by monoclonal antibodies. Vet Immunol Immunopathol. 1988 Oct;19(3-4):325–340. doi: 10.1016/0165-2427(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Ellis J. A., DeMartini J. C. Ovine interleukin-2: partial purification and assay in normal sheep and sheep with ovine progressive pneumonia. Vet Immunol Immunopathol. 1985 Jan;8(1-2):15–25. doi: 10.1016/0165-2427(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Ellis J. A. Immunophenotype of pulmonary cellular infiltrates in sheep with visceral caseous lymphadenitis. Vet Pathol. 1988 Sep;25(5):362–368. doi: 10.1177/030098588802500505. [DOI] [PubMed] [Google Scholar]
- Gates N. L., Everson D. O., Hulet C. V. Effects of thin ewe syndrome on reproductive efficiency. J Am Vet Med Assoc. 1977 Dec 15;171(12):1266–1267. [PubMed] [Google Scholar]
- Gillette K. G., Frank G. H., Sacks J. M. Development and evaluation of an enzyme-linked immunosorbent assay for bovine antibody (IgG) to Pasteurella haemolytica. Am J Vet Res. 1989 Jan;50(1):106–110. [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Joshi V. V., Oleske J. M. Pulmonary lesions in children with the acquired immunodeficiency syndrome: a reappraisal based on data in additional cases and follow-up study of previously reported cases. Hum Pathol. 1986 Jun;17(6):641–642. doi: 10.1016/s0046-8177(86)80143-5. [DOI] [PubMed] [Google Scholar]
- Krishnan V. L., Meager A., Mitchell D. M., Pinching A. J. Alveolar macrophages in AIDS patients: increased spontaneous tumour necrosis factor-alpha production in Pneumocystis carinii pneumonia. Clin Exp Immunol. 1990 May;80(2):156–160. doi: 10.1111/j.1365-2249.1990.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Akita G. Y., Russell H. I., DeMartini J. C. Replication and cytopathic effects of ovine lentivirus strains in alveolar macrophages correlate with in vivo pathogenicity. J Virol. 1987 Dec;61(12):4038–4042. doi: 10.1128/jvi.61.12.4038-4042.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Poulson J. M., Adducci T. A., DeMartini J. C. Lentivirus-induced lymphoproliferative disease. Comparative pathogenicity of phenotypically distinct ovine lentivirus strains. Am J Pathol. 1988 Jan;130(1):80–90. [PMC free article] [PubMed] [Google Scholar]
- Marchevsky A., Rosen M. J., Chrystal G., Kleinerman J. Pulmonary complications of the acquired immunodeficiency syndrome: a clinicopathologic study of 70 cases. Hum Pathol. 1985 Jul;16(7):659–670. doi: 10.1016/s0046-8177(85)80148-9. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Scadden D. T., Amirault C., Woon A., Vannier E., Dinarello C. A., Groopman J. E. Human immunodeficiency virus does not induce interleukin-1, interleukin-6, or tumor necrosis factor in mononuclear cells. J Virol. 1990 Jun;64(6):2901–2906. doi: 10.1128/jvi.64.6.2901-2906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Taverne J., Bate C. A., de Souza J. B. The malaria vaccine: anti-parasite or anti-disease? Immunol Today. 1990 Jan;11(1):25–27. doi: 10.1016/0167-5699(90)90007-v. [DOI] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenic mechanisms of HIV infection: cytokine induction of HIV expression. Immunol Today. 1990 May;11(5):176–180. doi: 10.1016/0167-5699(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. Am Rev Respir Dis. 1984 May;129(5):747–753. doi: 10.1164/arrd.1984.129.5.747. [DOI] [PubMed] [Google Scholar]
- Valone S. E., Rich E. A., Wallis R. S., Ellner J. J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988 Dec;56(12):3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward L. D., Leendertsen L., Shen D. T. Microimmunodiffusion test for diagnosis of ovine progressive pneumonia. Am J Vet Res. 1979 Apr;40(4):564–566. [PubMed] [Google Scholar]
- Yang T. J., Jantzen P. A., Williams L. F. Acid alpha-naphthyl acetate esterase: presence of activity in bovine and human T and B lymphocytes. Immunology. 1979 Sep;38(1):85–93. [PMC free article] [PubMed] [Google Scholar]