Abstract
Dopaminergic innervation of the frontal cortex in adults is important for a variety of cognitive functions and behavioral control. However, the role of frontal cortical dopaminergic innervation for neurobehavioral development has received little attention. In the current study, rats were given dopaminergic lesions in the frontal cortex with local micro-infusions of 6-hydroxydopamine (6-OHDA) at one week of age. The long-term behavioral effects of neonatal frontal cortical 6-OHDA lesions were assessed in a series of tests of locomotor activity, spatial learning and memory, and intravenous (IV) nicotine self-administration. In addition, neurochemical indices were assessed with tissue homogenization and HPLC in the frontal cortex, striatum, and nucleus accumbens of neonatal and adult rats after neonatal 6-OHDA lesions. In neonatal rats, frontal 6-OHDA lesions as intended caused a significant reduction in frontal cortical dopamine without effects on frontal cortical serotonin and norepinephrine. The frontal cortical dopamine depletion increased serotonin and norepinephrine levels in the nucleus accumbens. Locomotor activity assessment during adulthood in the Figure-8 maze showed that lesioned male rats were hyperactive relative to sham lesioned males. Locomotor activity of female rats was not significantly affected by the neonatal frontal 6-OHDA lesion. Learning and memory in the radial-arm maze was also affected by neonatal frontal 6-OHDA lesions. There was a general trend toward impaired performance in early maze acquisition and a paradoxical improvement at the end of cognitive testing. Nicotine self-administration showed significant lesion × sex interactions. The sex difference in nicotine self-administration with females self-administering significantly more nicotine than males was reversed by neonatal 6-OHDA frontal cortical lesions. Neurochemical studies in adult rats showed that frontal cortical dopamine and DOPAC levels significantly correlated with nicotine self-administration in the 6-OHDA lesioned animals but not in the controls. Frontal cortical serotonin and 5HIAA showed inverse correlations with nicotine self-administration in the 6-OHDA lesioned animals but not in the controls. These results show that interfering with normal dopamine innervation of the frontal cortex during early postnatal development has persisting behavioral effects, which are sex-specific.
Keywords: 6-OHDA, radial-arm maze, self-administration, dopamine, serotonin, nucleus accumbens, striatum
Introduction
Nicotine addiction varies considerably across population subgroups. Nicotine addiction rate in patients with psychiatric illness and concurrent addiction to other drugs are of particular interest. Schizophrenics and alcoholics have smoking rates triple that of the general population (Hughes et al., 1986, Hughes, 1995). Not only do these subpopulations have higher rates of nicotine addiction, but they also have a heightened inability to achieve nicotine cessation. This raises the possibility that patients in these psychiatric subpopulations have different neurobehavioral mechanisms underlying their nicotine addiction, which are recalcitrant to standard smoking cessation methods. Defects in both dopaminergic and serotonergic systems have been implicated in these clinical populations. Both dopaminergic (DA) and serotonergic innervations of the nucleus accumbens have been shown to be important in the process of nicotine addiction. However, DA also functions in a variety of other brain areas that are involved in nicotine addiction. Especially important are those regions involved in the processes of learning and memory (Robbins and Everitt, 2002). In particular, DA innervation of the frontal cortex and limbic system appears to be important for the associative and learning aspects of addiction (Volkow et al., 2002a). These areas are also important for behavioral inhibition, which may be critical for preventing relapse after cessation.
Frontal cortical systems have descending glutamatergic projections with nicotinic α7 receptors on their endings (Schilstrom et al., 2000). These neurons provide feedback control over ventral tegmental area (VTA), which signal reward (Kalivas, 1993). Frontal cortical feedback to the VTA is a critical mechanism in providing control over the aversive vs. rewarding effects of nicotine (Laviolette and van der Kooy, 2004). The current study was conducted to determine the persisting impact of neonatal lesions of frontal cortical dopamine innervation on locomotor activity, learning and memory, and nicotine reinforcement. The dopaminergic innervation of the frontal cortex originates from cell bodies of A9 and A10 mesencephalaic dopaminergic neurons and influences cognition. We hypothesize that neonatal lesions of frontal cortical dopamine would cause long-term disruptions in frontal dopamine activity which may lead to persisting cognitive defects and make rats more susceptible to nicotine addiction. Cognitive functions (learning and memory) will be determined by spatial discrimination in the 8 arm radial maze. Susceptibility to nicotine addiction will be determined with the duel lever self-administration task. The dependence producing effects of nicotine have been successfully modeled in this rat self-administration procedure (Corrigall and Coen, 1989, Corrigall, 1991, Corrigall, 1992, Corrigall et al., 1994, Corrigall et al., 2000; Donny et al., 1995, Shoaib et al., 1997, Donny et al., 1998, Levin et al., 2003). Additionally, tissue homogenization and HPLC will be used to determine neurochemical changes and underlying mechanisms that result from neonatal 6-OHDA lesioning in both neonatal and adult rats.
Materials and Methods
Subjects
The procedures used in this study were approved by the Duke University Animal Care and Use Committee. Male and female Sprague-Dawley albino rats (Taconic Farms, Gemantown, NY, USA) were housed in groups of three under approved standard laboratory protocols. To minimize stress induced by transportation, the rats were housed in a vivarium facility near the behavioral testing rooms. The day-night cycle was reversed cycle (lights on 18:00-6:00) so that the rats were in their active phase during behavioral testing. All rats had ad lib access to water and were fed the same rodent chow once daily.
Drug Preparation
Solutions of 6-OHDA was prepared fresh 1 min before the infusion in artificial cerebral spinal fluid (ACSF) containing 0.2 % ascorbic acid. An ACSF solution containing 0.2 % ascorbic acid was used as a control solution for the sham group. Solutions of desipramine and citalopram were prepared in sterilized isotonic saline and injected in a volume of 2 ml/kg. Solutions of nicotine bitartrate for nicotine IV self-administration were prepared weekly in pyrogen-free glassware in sterilized isotonic saline. All drugs and chemicals were purchased from Sigma, St Louis, MO, USA. The doses of nicotine used were calculated as a function of the nicotine base weight. The pH of the nicotine solution was adjusted to 7.0 using NaOH and then the solution was passed through a 0.22 µm Nalgene filter (Nalgene Nunc International, Rochester, NY) for sterilization. Nicotine solutions were kept refrigerated in the dark between experiments.
Neonatal 6-OHDA Lesions
Newborn pups derived from seven litters were randomly assigned to treatment or sham groups. Pups from any given litter were assigned to both sham and lesioned treatment groups. Toe clipping technique was used to identify rats during adulthood. Seven days after birth, the pups were anesthetized via hypothermia by laying the pups on an ice-cold plate covered with cloth. Once anesthetized, the pups were given bilateral microinjection of 6-OHDA (4 µg base/side) into the frontal cortex (1 µl/side over a 2 min period) using a 10 µl Hamilton syringe with a 30-gague needle. The sham group received equal volumes of ACSF into the frontal cortex. The coordinates for 6-OHDA infusions into the frontal cortex were A/P: 1 mm, M/L: 1 mm, D/V: 1 mm from the skull (Paxinox et al., 1994). To prevent the degeneration of noradrenergic and serotonergic neurons, pups were pretreated (SC) with 25 mg/kg desipramine and 5 mg/kg citalopram (IP). Desipramine and citalopram were injected 30 and 20 min before the surgery, respectively (Yoshimoto et al., 1999, Rex and Fink, 2004). After the surgery, pups were placed on a heating pad until they resumed their normal activity. After recovery the pups were returned to their home cage with their littermates and moms. At 25 days of age, the rats (n=36) were weaned and housed 3 rats per cage under standard lab conditions. When the rats were adults, their locomotor activity, learning and memory and their propensity for nicotine self-administration were assessed.
Verification of the Infusion Site
A small sample of pups (n=4) were infused with toluidine dye to verify the location of the infusion site. Seven days after birth the pups were anesthetized via hypothermia by laying the pups on an ice-cold plate covered with cloth. Once anesthetized the pups were given unilateral infusion of toluidine dye (1 µl) using a 10 ml Hamilton syringe with a 30-gague needle. The coordinated were +1.0 mm A/P, ±1.0 mm M/L, and −1.0 mm D/V.
Locomotor Activity
Locomotion and its habituation were assessed when rats were approximately 2 months old. Measurements of locomotor activity were assessed in an automated Figure-8 apparatus for a period of 60 min. The rat’s locomotor activity was detected by motion detectors located inside the Figure 8 apparatus and transmitted to a computer for analysis. This test has been widely shown to be sensitive to the behavioral effects of environmental toxicants and psychoactive drugs. The linear and quadratic trends across twelve 5-min blocks in the 1-hour test session were used. The average activity counts across the 1-hour session served as a measure of locomotor activity.
Figure 8.
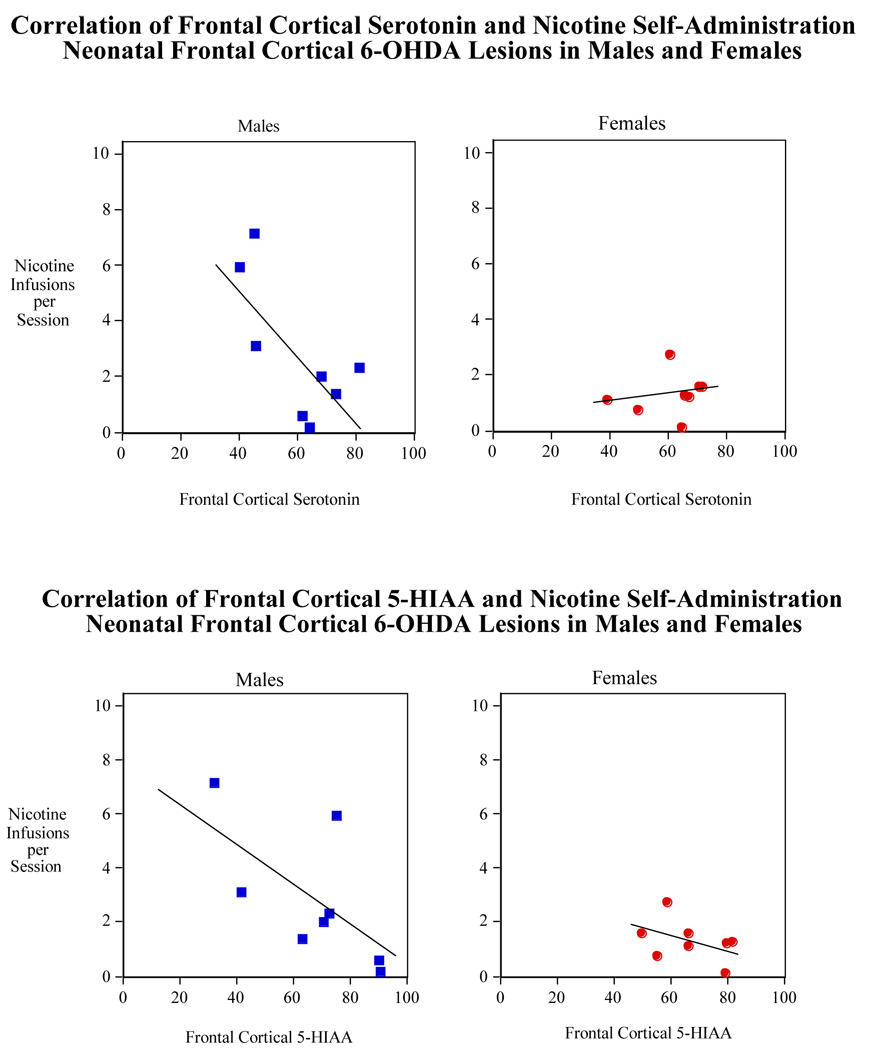
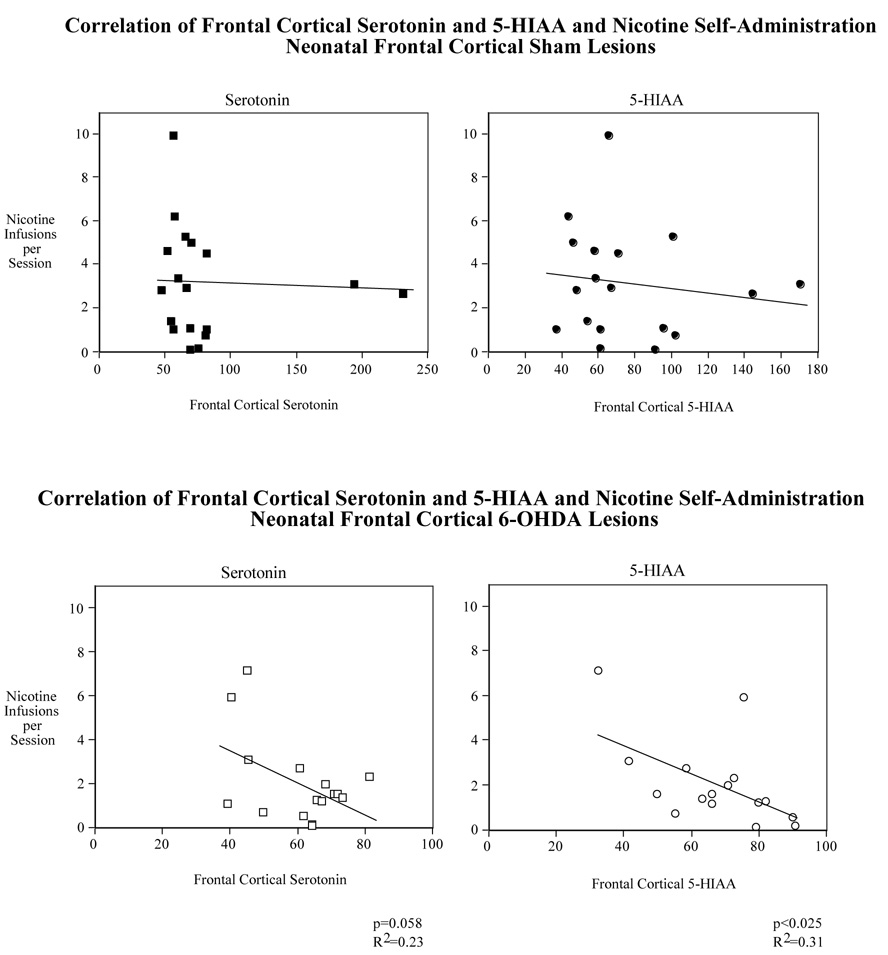
Correlation of nicotine self-administration (sessions 1–20) with frontal cortical serotonin (upper panel) and 5-HIAA (lower panel) in male and female rats with neonatal 6-OHDA lesion. N=8 for all transmitters except for cortical serotonin in males which is 9.
Spatial Learning and Memory
Spatial learning and memory was assessed in an 8-arm radial maze. Training began at about 80 days of age. The black-painted wood maze is at a height of 30 cm from the floor with a central platform of diameter of 50 cm and eight arms, each 10 × 60 cm, projecting radially outward from the central platform; each arm contains a food cup 2 cm from the distal end. The test area was soundproof and contained numerous visual cues. To begin the test, rats were first placed in a large, opaque cylinder on the platform of the maze, given food reinforcement (halves of a sugar-coated cereal, Froot Loops®), and allowed 5 min to eat.
All eight arms were baited once at the beginning of every session to test working memory. Rats were placed in an opaque cylinder on the start platform for 10 sec to allow for orientation and thus to avoid unbiased arm entry. The rats were then allowed to enter any arm for up to 5 min or until all 8 baited arms were entered. Arms were baited only once and a repeated entry into a formerly baited arm was counted as a working memory error. Latency (seconds per arm entry) was recorded as the total number of arms entered, divided by total session time in seconds. Choice accuracy was recorded as the number of errors. The animals were trained for 18 sessions on the maze, approximately twice per week. Rats were on restricted diet during the radial-arm maze test.
Nicotine Self-Administration
Rats were hand-trained to press the levers for food pellet reinforcers. They were given two 15-min sessions daily, for four days. During these tutor sessions, correct responses were rewarded by the trainer pressing a button, which caused immediate delivery of one 45-mg food pellet and activation of the feedback tone for 0.5 sec. Half the animals were reinforced for responding on the right lever, and half for responding on the left. The cue light over the correct lever was illuminated while the light over the incorrect lever was covered (under the tutor program, both lights are turned on, but only one cue light is uncovered, to show which lever is correct.). These tutor sessions were followed by 3 daily pellet sessions on a FR-1 schedule, with feedback tone, lasting 45 minutes. This program activated the correct lever and only the cue light above that lever was illuminated. After three training sessions with food reinforcement, rats had catheters and ports (Instech-Solomon, Plymouth Meeting, PA) surgically implanted into the jugular vein to enable them to receive nicotine infusions., The anesthesia was a mixture of ketamine (60 mg/kg) and domitor (15 mg/kg) injected ip (Levin et al., 2007).
The catheters were connected to the port, which was sutured subcutaneously on their backs for easy access to the tether delivery line. They were flushed daily with a 0.3 ml solution containing 100 units/ml heparinized saline (Baxter Health Corporation, Deerfield, IL) and 8 mg/ml Gentamicin (American Pharmaceutical Partners, Shaumburg, IL) as an antibiotic. Following recovery from the surgery, rats were placed in dual lever test chambers for nicotine self-administration. Each chamber was equipped with a tone generator, house light, cue light above each lever (Med Associates, Vermont, USA), and a stainless steel tether to cover and protect the drug delivery line (Instech-Solomon Plymouth Meeting, PA). A Pentium computer programmed with MED-PC software controlled experimental events and data collection. Each catheter and port was connected to a High Speed Micro-Liter Syringe Pump (MED-Associates, Georgia, VT) with polyethylene tubing for drug delivery. The tubing tether set had a huber needle on one end to access the port (Instech-Solomon, Plymouth Meeting, PA). During each session, the rats wore Covance infusion harnesses connected to the stainless steel tethers. The sequence of testing was as follows: 3 sessions of lever pressing for food reinforcement, and then 20 sessions of nicotine reinforcement alone over a period of 4 weeks. During the nicotine sessions, a lever press on the active side resulted in the activation of the feedback tone for 0.5 sec, and the immediate delivery of one 50-µl infusion of nicotine in less than 1 sec. Each infusion was immediately followed by a one-minute timeout in which the cue lights went out and responses were recorded but not reinforced.
Neurochemical Analysis
The levels of dopamine, serotonin, norepinephrine, and their major metabolites in the frontal cortex, striatum, and nucleus accumbens were assayed using tissue homogenization and standard HPLC-EC methods. For tissue homogenization, rats were euthanized by decapitation and the brains were rapidly excised. For brain dissection, the anterior and posterior boundaries of the frontal cortex were defined as the tissue extending from the frontal pole caudally to the bregma (7 mm in the adults and 3 mm in the pups) (Paxinos et al., 1994). The dorsal and ventral boundaries were the dorsal surface of the cortex ventrally to the dorsal surface of the corpus callosum. The lateral boundaries were determined by a horizontal slice just above the most dorsal surface of the corpus callosum and extended laterally in a planar fashion to the sides of the brain. This region encompassed the area indicated by stained samples.
The adult brains were further dissected into eight regions: left and right (midline being the divider), medial and lateral (lateral boundary starting 2.5 mm from the midline), and anterior and posterior (posterior section beginning 4 mm from the frontal pole). This yielded the following sections: left medial anterior, left medial posterior, left lateral anterior, left lateral posterior, right medial anterior, right medial posterior, right lateral anterior, and right lateral posterior.
Once dissected, the brain regions were homogenized with an ultrasonic tissue homogenizer in a 0.1N Perchloric Acid/100 mM EDTA solution (10X volume/tissue weight). After column purification, to remove solid cellular particulate, the homogenate was diluted 25X with purified water and dopamine and serotonin concentrations were determined with HPLC.
The HPLC system consisted of an isocratic pump (model LC1120, GBC Separations), a Rheodyne injector (model 7725i) with a 20 µl PEEK loop, and an INTRO amperometric detector (Antec Leyden). The electrochemical flow cell (model VT 03, Antec Leyden) has a 3mm glassy carbon working electrode with a 25 µm spacer, and an Ag/AgCl reference electrode. The cell potential was set at 700 mV. The signal was filtered with a low pass in-line noise killer, LINK (Antec Leyden) set at a 14 s peak width and a cut off frequency of 0.086 Hz. The signal was integrated using the EZChrom elite chromatography software (Scientific Software Inc.). The injector, flow cell, and analytical column were placed in the Faraday-shielded compartment of the detector where the temperature was maintained at 30°C. The stationary phase was a reverse phase BDS Hypersil C18 column 100 mm × 2.1 mm, with 5 µm particle size and 120 Å pore size (Keystone Scientific). The mobile phase was 50 mM H3PO4, 50 mM citric acid, 100 mg/L 1-octanesulfonic acid (sodium salt), 40 mg/L EDTA (disodium salt dihydrate), 2mM KCl and 3% methanol, corrected to pH 3.0 with NaOH. The mobile phase was continually degassed with a Degasys Populaire on-line degasser (Sanwa Tsusho Co. Ltd.) and delivered at a flow rate of 0.26 ml/min.
Statistical Analysis
The self-administration data were assessed by analysis of variance. An alpha level of p<0.05 was used as a cutoff for statistical significance. The between subjects factor was 6-OHDA, sex and cohort and the repeated measure was repeated testing. The dependent measures were photobeam breaks for the locomotor activity test, entries to repeat (number of correct entries before the first error) for the spatial learning and memory test and number of infusions per session for the nicotine self-administration test. As recommended by Snedecor and Cochran, (Snedecor and Cochran, 1967) interactions with p-values less than 0.10 were followed-up by tests of the simple main effects. In all cases the final threshold for statistical significance was p<0.05, two-tailed.
Results
Neurochemistry (Rat Pups)
A small sample of rat pups was analyzed for the extent to which the 1 µl infusion would disperse. To accomplish this toluidine blue dye was infused at the exact coordinates as 6-OHDA infusion, the pups were euthanized via cervical dislocation, and the brains were dissected and examined. The site of infusion was confirmed to be in the intended region of the medial frontal cortex.
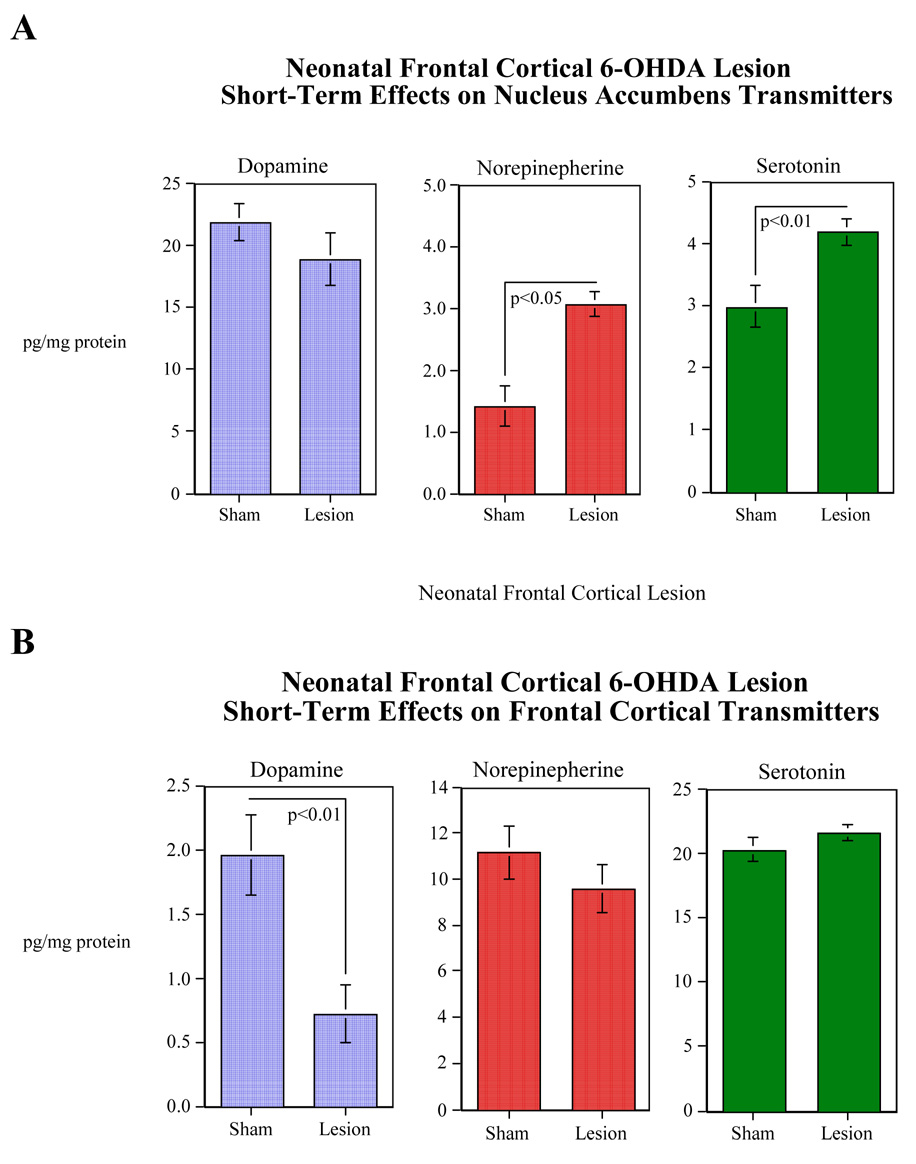
To determine if 6-OHDA infusion successfully depleted DA levels, a subset of rat pups derived from 4 litters were euthanized ten days after 6-OHDA (4 µg of the base weight of 6-OHDA/side) or ACSF infusion into their frontal cortex and neurochemical concentrations were determined in the frontal cortex, dorsal striatum, and nucleus accumbens. Bilateral microinfusion of 6-OHDA significantly (F(1,14)=10.46, p<0.01) reduced the concentration of dopamine in the frontal cortexes of the rat pups. These values were 0.72±0.22 and 1.96±0.31 pg/20µl of the frontal cortex tissue for the 6-OHDA and sham-treated pups, respectively (Fig. 1A). As intended, the other monoamines in the frontal cortex were not significantly affected (Fig. 1A). No significant lesion effects were seen with the monoamines in the dorsal striatum (data not shown). Bilateral 6-OHDA lesioning of dopamine terminals of the frontal cortex caused significant increases in norepinephrine (F(1,14)=5.08, p<0.05) and serotonin (F(1,14)=9.57, p<0.01) levels in the nucleus accumbens (Fig. 1B). The serotonin metabolite 5-HIAA was also significantly (F(1,14)=9.93, p<0.01) increased in the nucleus accumbens (Control=3.06±0/16, Lesioned=4.06±0.28).
Figure 1.
Effects of neonatal 6-OHDA lesion of the frontal cortex on levels of dopamine, norepinepherine and serotonin in the frontal cortex (Panel A), and dopamine, norepinepherine and serotonin in the nucleus accumbens (Panel B) 10 days following lesioning (mean±sem). The figure depicts pooled data from both male and female rats. Control animals were infused with artificial an equal volume of artificial cerebral spinal fluid. N=8 for all neurotransmitters in both treatment conditions except for the cortical norepinephrine in the sham group which is 7.
Locomotion
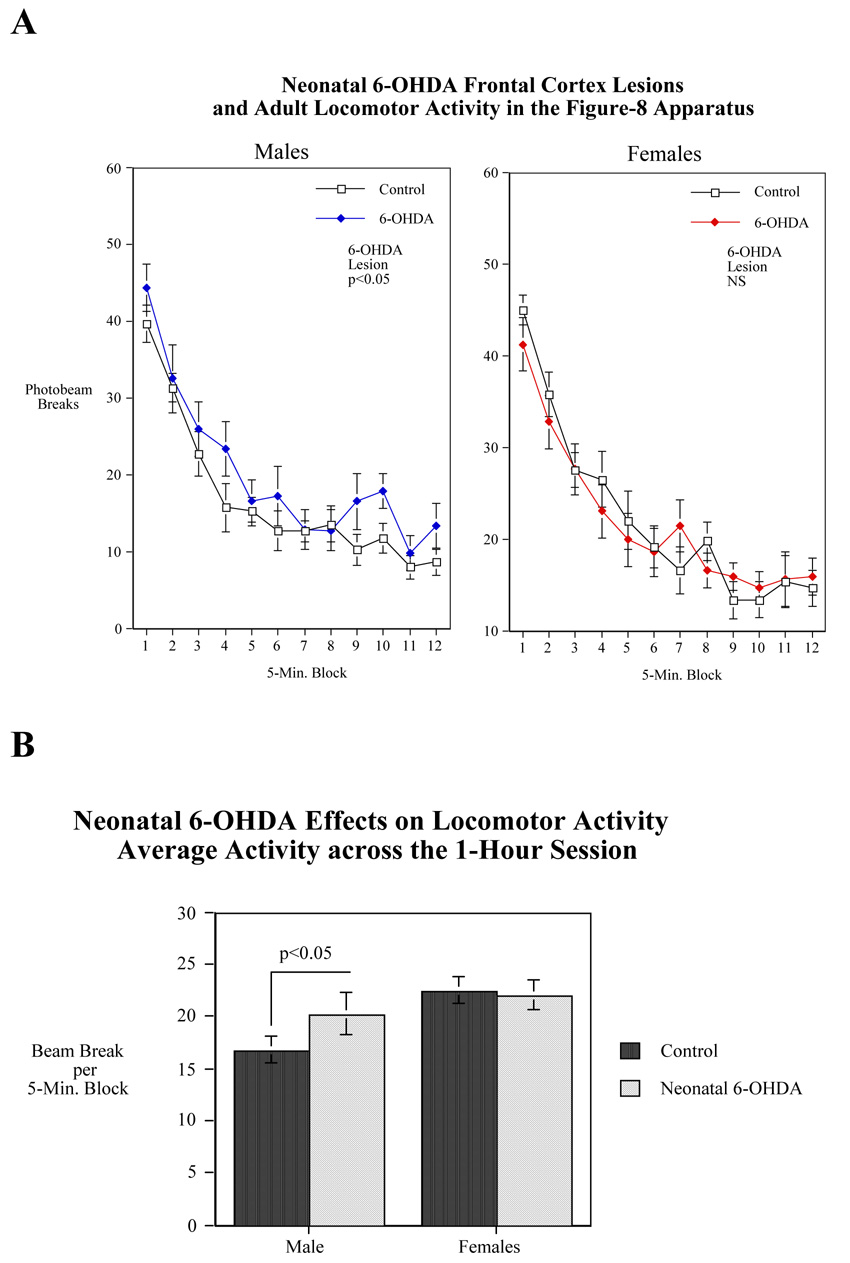
In the Figure-8 locomotor activity apparatus, there was a sex-selective effect of neonatal 6-OHDA treatment. To follow up the treatment × sex interaction (F(1,37)=3.48, p<0.07), 6-OHDA treated male and female rats were compared to same-sexed controls. Males with neonatal 6-OHDA treatment were significantly (p<0.05) hyperactive relative to sham-operated control males (Figs. 2A and 2B). Females were not significantly affected by 6-OHDA in terms of locomotor activity in that there was no significant differences between 6-OHDA and sham lesioned females. There was a significant main effect of sex (F(1,37)=11.13, p<0.005) with females showing the typical more activity than males. The 6-OHDA effect in males made them more like females in this regard (Fig. 2B).
Figure 2.
Effects of neonatal 6-OHDA lesion of the frontal cortex on locomotor activity in the Figure-8 maze during adulthood in male and female rats. (A) Figure 2A shows photobeam breaks per five-minute time block over the course of a 1-hour session (mean±sem). (B) Figure 2B shows average photobeam breaks per five-minute time block over the course of a 1-hour session (mean±sem). N=12 for control males and 11 for lesioned males. For control females N=13 and for lesioned females N=9.
Learning and Memory
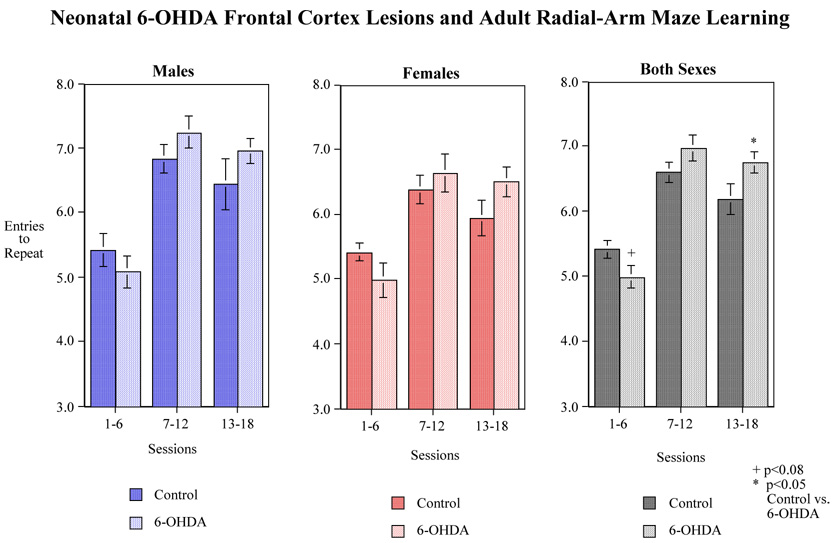
There was a significant 6-OHDA×session block interaction (F(2,66)=4.84, p<0.025) in the radial-arm maze. Tests of the simple main effects of neonatal 6-OHDA at each session block showed a nearly significant (p<0.08) 6-OHDA-induced impairment during the first block and a significant (p<0.05) 6-OHDA-induced improvement in accuracy relative to sham-operated controls during the final session block (Fig. 3). No interactions of treatment by sex were observed. Overall, at the end of 18 training sessions, the 6-OHDA-treated animals performed with fewer errors than the sham-operated animal (Fig. 3).
Figure 3.
Effects of neonatal 6-OHDA lesion of the frontal cortex on choice accuracy (spatial learning and memory) during adulthood in the radial-arm maze. Data show the number of correct entries before the first error (i.e. entries to repeat) Data represent mean±sem. N= the same as Fig. 2.
Nicotine Self-Administration
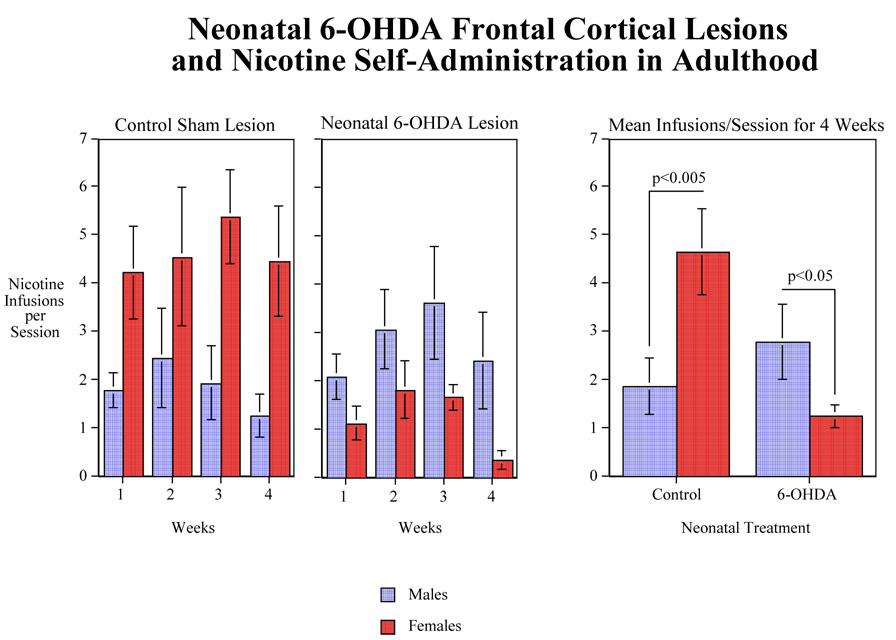
Nicotine self-administration was significantly affected by neonatal 6-OHDA lesions with a significant (F (1,28)=5.67, p<0.025) main effect of 6-OHDA lesion decreasing nicotine self-administration (Fig. 4). The 6-OHDA lesion × sex interaction was also significant (F(1,28)=16.62, p<0.0005). Tests of the simple main effects showed that in the sham-lesioned rats, there was a significant (F(1,28)=11.66, p<0.005) sex difference with the females self-administering more nicotine than males (Fig. 4). In the 6-OHDA lesioned rats, the opposite was the case with the males self-administering significantly (F(1,28)=5.51, p<0.05) more nicotine than the 6-OHDA lesioned females. Male nicotine self-administration was slightly though not significantly increased by the 6-OHDA lesion. However, nicotine self-administration in the females was significantly (F(1,28)=19.94, p<0.0005) decreased by the 6-OHDA lesion (Fig. 4). There was a significant (F(3, 84)=3.57, p<0.025) main effect of week of self-administration. A trend analysis of the change in nicotine self-administration over four weeks showed an initial increase in self-administration followed by a plateau in performance and a tapering off during the final week with no significant interaction with sex of the subjects. This was evidenced by a significant (F(1,84)=10.53, p<0.005) quadratic trend over the weeks.
Figure 4.
Effects of neonatal 6-OHDA lesion of the frontal cortex on nicotine IV self-administration (0.03 mg/kg/infusion) during adulthood. Data show numbers of infusion/session during 4 weeks trial (mean±sem). N=10 for control males and 9 for lesioned males. For control females N=8 and for lesioned females N=9.
To determine if nicotine self-administration in the female rats was dependent on the estrous cycle, the nicotine injections per session were determined with respect to the stage in the estrous cycle. The estrus cycle stage in the female rats did not significantly affect the rate of nicotine self-administration. The nicotine self-administration rates in the different stages of the estrus cycle were: metestrus 3.2±1.3; diestrus 3.0±0.6; proestrus 2.3±0.4; and estrus 2.8±0.7 infusions/session.
Neurochemistry (Adult Rats)
After completion of the behavioral tasks the adult rats were euthanized and neurochemical levels were determined in the frontal cortex, striatum, and nucleus accumbens. The frontal cortex was divided into eight sections to analyze the long-term effects of 6-OHDA on DA levels at the site of infusion and to examine the possibility that local infusion into the anterior frontal cortex could have effects on adjoining regions such the sensory motor cortex in more posterior portions of our dissection. There were no significant differences in neurochemical levels detected in the frontal cortex in any of the regions that were sampled (data not shown). Additionally, there were no significant differences of neurochemical levels in the striatum, or nucleus accumbens of 6-OHDA or sham lesioned rats (data not shown).
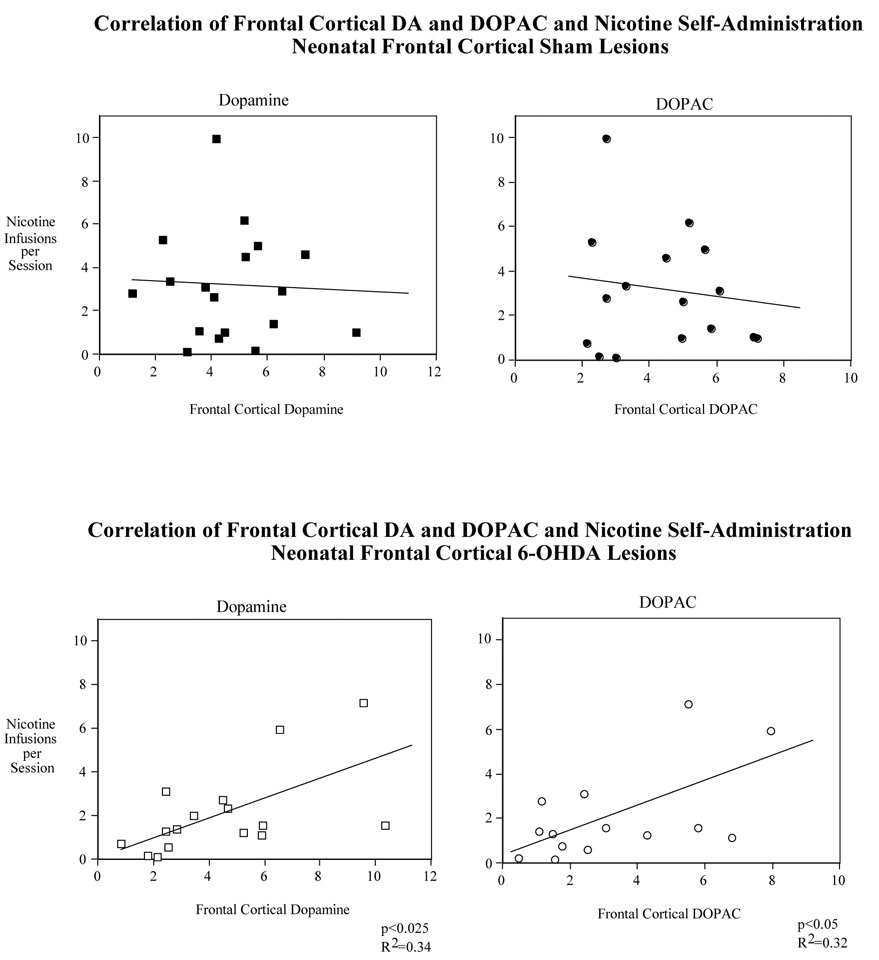
However, frontal cortical dopamine levels significantly correlated (p<0.025, R2=0.34) with the average nicotine self-administration for all the sessions (1–20) for the rats given the neonatal frontal cortical 6-OHDA lesions (Fig. 5 lower panel). This relationship was reinforced by the further finding of a significant correlation (p<0.05, R2=0.32) of frontal cortical levels of the dopamine metabolite DOPAC with average nicotine self-administration (Fig, 5 lower panel). In contrast, neither DA (p=0.77, R2=0.005) nor DOPAC (p=0.54, R2=0.03) had any hint of correlation with average nicotine self-administration in sham-operated rats (Fig. 5 upper panel). Thus, both frontal cortical dopamine and its metabolite DOPAC showed significantly increased levels with increased nicotine self-administration in lesioned but not sham-operated rats.
Figure 5.
Correlation of nicotine self-administration (sessions 1–20) with frontal cortical dopamine and DOPAC levels in rats with neonatal 6-OHDA and sham operated rats. For the sham group N=19 for cortical dopamine and 17 for cortical DOPAC. For the lesioned group N=17 for dopamine and 15 for DOPAC.
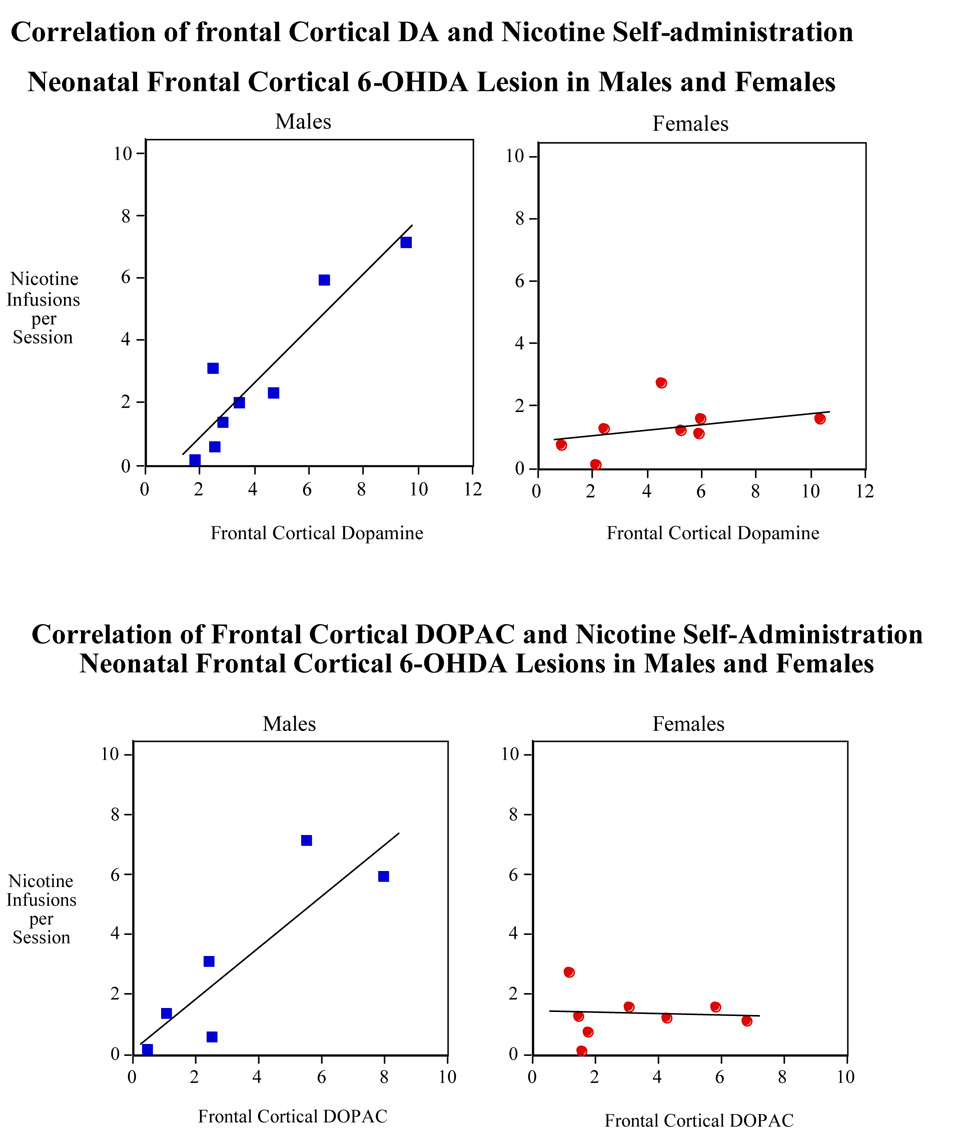
As shown in Figure 6, the correlation between nicotine self-administration and cortical DA (r2 =0.61) and DOPAC (r2 =0.65) in lesioned male rats was significant (p<0.025 for DA and p<0.05 for DOPAC). No correlation was found for these variables in the frontal cortex of lesioned female rats.
Figure 6.
Correlation of nicotine self-administration (sessions 1–20) with frontal cortical dopamine (upper panel) and DOPAC (lower panel) in male and female rats with neonatal 6-OHDA lesion. N=8 for frontal cortical dopamine and DOPAC in females and 9 and 7 for frontal cortical dopamine and DOPAC in males, respectively.
The correlation of frontal cortical serotonin levels and the average nicotine self-administration for all the sessions (1–20) for the rats given the neonatal frontal cortical 6-OHDA lesions were nearly inversely significant (p=0.058, R2=0.23) (Fig. 7 lower panel). A stronger relationship was seen with a significant correlation (p<0.025, R2=0.31) of frontal cortical levels of the serotonergic metabolite 5-HIAA with average nicotine self-administration (Fig, 7 lower panel). In contrast, neither serotonin (p=0.54, R2=0.02) nor 5-HIAA (p=0.47, R2=0.03) had any hint of correlation with average nicotine self-administration in sham operated rats (Fig. 7 upper panel). Thus, frontal cortical 5-HIAA showed significantly decreased levels with increased nicotine self-administration in lesioned, but not control rats. Frontal cortical serotonin levels showed a nearly significant trend in the same inverse direction in the lesioned animals with no hint of a relationship in controls.
Figure 7.
Correlation of nicotine self-administration (sessions 1–20) with frontal cortical serotonin and 5-HIAA levels in rats with neonatal 6-OHDA and sham operated rats. For the sham group N=19 for cortical serotonin and 18 for cortical 5-HIAA. For the lesioned group N= 17 for serotonin and 16 for 5-HIAA.
As illustrated in Figure 8, although there was a trend for an inverse correlation between nicotine self administration and cortical serotonin and 5-HIAA in male rats, it did not reach to the level statistical significance. No correlation was found for these variables in the frontal cortex of lesioned female rats.
Discussion
Our results demonstrated that neonatal 6-OHDA lesioning of the frontal cortex significantly reduced dopamine levels in the frontal cortex while significantly increasing serotonin, 5-HIAA and norepinephrine concentrations in the nucleus accumbens. This neurochemical effect of neonatal 6-OHDA lesioning was absent in the adult rats indicating a recovery of the initial neurochemical defect. Furthermore, our findings showed that neonatal lesions of frontal cortical dopamine innervation by 6-OHDA had lasting impacts on neurobehavioral function. The effects were seen in locomotor activity, learning and memory, and nicotine self-administration during adulthood.
Our findings showed that male rats treated with neonatal 6-OHDA were significantly hyperactive compared to sham lesioned males. Dopaminergic lesioning via 6-OHDA treatment have been shown to cause locomotor defects in a number of animal models. Neonatal ICV 6-OHDA infusion causes considerable and long-lasting locomotor activity in juvenile and adult rats (Shaywitz et al., 1976, Miller et al., 1981, Archer et al., 1988). Lesioning of dopaminergic systems by ICV injection of 6-OHDA or MPTP also causes both catalepsia and bradykinesia in adult rats and monkeys (Zigmond and Stricker, 1973, Deumens et al., 2002). These studies along with the current data suggest an essential role for dopaminergic innervation in locomotor activity (Zigmond and Stricker, 1973, Archer et al., 1988, Deumens et al., 2002). Serotonin also has been implicated in locomotion (Geyer, 1996, Jacobs and Fornal, 1997). Increased levels of serotonin in the brain have been associated with hyperactivity in mice (Chia et al., 1999, Brocco et al., 2002). Recently, it has been shown that increased striatal 5-HT that follows neonatal DA depletion is involved in hyperlocomotor behavior in mice (Avale et al., 2004). In the current study we also observed increased ventral striatal 5-HT and increased hyperlocomotion. These studies highlight the importance of frontal DA and striatal 5-HT systems in locomotion.
In the present study only male rats exhibited hyperactivity after 6-OHDA lesioning. It is possible that male sex hormones exacerbate the effect of 6-OHDA lesioning. The lack of effect of 6-OHDA lesion on the locomotor activity in female rats may be the result of sex-specific effects of 6-OHDA lesioning. However, it is important to note that we did not observe significant differences in frontal DA levels between neonatal male and female rats treated with 6-OHDA.
Neonatal 6-OHDA lesioning of the frontal cortex cause a moderate but significant improvement in 8-arm radial maze performance. This effect was not sex specific in that both adult male and female rats showed increased maze acquisition in the later sessions. This finding contradicts data from other studies. Other investigators have shown that 6-OHDA-treated rats exhibit a retarded acquisition of running responses to acquire food pellets in a modified version of the Olton radial arm maze (Archer et al., 1988). Furthermore, 6-OHDA-treated rats also have been shown to be drastically impaired in the swim maze task compared with controls (Archer et al., 1988). There might be several methodological reasons for the discrepancy between our findings and these results. First, we used local infusions, which were limited to the frontal cortex while Archer’s group used an anatomically-nonspecific bilateral ICV infusion. ICV infusions are likely to have pronounced effects on dopaminergic functioning in several regions of the brain including the frontal cortex, nucleus accumbens, septum, striatum and the amygdala. Secondly, our rats were treated once on Day 7 while Archer et al. treated their animals twice, on Day 3 and Day 6. Lastly, we used a standard 8-arm radial maze task with 18 sessions of training while Archer’s group used a modified version of the Olton radial arm maze which required one session to acquire food pellets. We did observed a general trend toward retarded maze acquisition in the early training sessions which may coincide with the impairments observed in other studies. It is also plausible that decreases in frontal cortical dopamine activity actually improve memory related processes. Some antipsychotics, which block DA receptors and lower dopaminergic activity, actually improve deficits in cognition and executive functioning (Harvey et al., 2005, Wagner et al., 2005).
Neonatal 6-OHDA lesions of the frontal cortex significantly affected nicotine self-administration during adulthood. The 6-OHDA lesion × sex interaction was also significant. There was a significant sex difference in the sham lesioned rats, with the female self-administering more nicotine than their male counterparts. Neonatal 6-OHDA lesioned rats exhibited the opposite effect with lesioned male rats self-administering significantly more nicotine than lesioned female rats. It is difficult speculate on mechanisms that account for the sex-difference in nicotine self-administration and the possible role of estrogen in mediating these effects in that there were no significant differences in nicotine self-administration and estrous cycle. We can only hypothesize that the 6-OHDA lesioning has sex-specific effects in the rodent brain and that female rat brain may be is protected from the 6-OHDA insult (Dluzen, 1996, Dluzen et al., 1996b, Dluzen et al., 1996a, Disshon and Dluzen, 2000, Dluzen and Horstink, 2003).
The data showed a direct link between the rate of nicotine self-administration and dopamine and DOPAC in 6-OHDA treated rats. This effect was absent in sham lesioned rats. Opposite to cortical DA, frontal serotonin levels were nearly significantly correlated with the rate of nicotine self-administration in 6-OHDA lesioned rats. Serotonin’s primary metabolite, 5-HIAA, was found to be significantly correlated with nicotine self-administration in 6-OHDA lesioned rats. This finding may suggest a role for the cortical serotonin in nicotine addiction. Even though 6-OHDA rats were not more prone to increased nicotine self-administration, there was still a significant interaction between increased dopamine levels in the frontal cortex and the number of nicotine infusion per session. It is possible that the lesion disrupted the reward and reinforcing behavior of nicotine in the animals but the underlying mechanism is preserved.
It is also possible that the rat frontal cortex is not as important for mediating addictive behavior. There was no significant interaction between frontal DA levels and nicotine self-administration in the control animals. However, it is important to note that we are sampling transit levels of DA and not actual levels at the time of nicotine self-administration. Determining DA levels in response to nicotine exposure would give us a much better indication of nicotine responsiveness and the contribution of frontal DA to nicotine seeking behavior in control and 6-OHDA lesioned animals.
Evidence from both laboratory animals and human studies implicates dopamine as one of the major neurotransmitter being involved in drug self-administration and addiction (Piazza et al., 1991, Rezvani et al., 1992, Mason et al., 1997, Di Chiara, 1999, Volkow et al., 1999, Rezvani et al., 2000, Goldstein and Volkow, 2002, Volkow et al., 2002b, Kalivas and Volkow, 2005, Nader and Czoty, 2005). In addition to the mesolimbic dopaminergic system, several other neurotransmitter systems including the serotonergic system have been suggested to be involved in drug self-administration (Rezvani et al., 1990, Rezvani and Grady, 1994, Volkow and Li, 2004, Koob, 2000). It appears that lesioning of the neonatal cortical dopamine innervation makes nicotine more aversive and/or less rewarding during adulthood in female rats. The important role of serotonin within the limbic system and its functional interaction with dopaminergic systems in the brain should also be considered in interpretation of these data. It has been shown previously that neonatal DA depletion in the rat is followed by an increase of striatal serotonin (Snyder et al., 1986) that appears as a consequence of an extensive serotonergic hyperinnervation in this area (Descarries et al., 1992, Molina-Holgado et al., 1994, Zhang et al., 2002). Our data demonstrate an increase in serotonin levels in the nucleus accumbens in neonatal rats following frontal 6-OHDA lesioning. The nucleus accumbens plays a paramount role in limbic neuronal circuits that are responsible for behaviors such as compulsive drug seeking (Morgane et al., 2005). Thus, it is possible that increase in serotonin in this part of the reward pathway may lead to a decline in nicotine self-administration. However, functionally, there is considerable overlap within and interactions between dopamine, serotonin, glutamate, GABA and other neurotransmitters in relation to cognition and drug seeking behavior. Considering the complexity of the system and the fact that drug seeking behavior and cognition are not unitary phenomena more extensive research is needed to better understand these functions.
In the present study, we observed behavioral defect in adult rats that received 6-OHDA lesions as neonates. Several mechanisms could explain these long-term behavioral changes. One explanation could be that neonatal lesioning has profound effects on receptor profiles in the frontal cortex. Studies have shown the one consequence of neuronal lesioning is the expression of supersensitive receptors. Depleting dopamine levels in the striatum leads to the expression of supersensitive D1 dopamine receptors (Gerfen et al., 2002). The presence of supersensitive D1 receptors in adult rats could explain long-term behavioral defect even though there was a complete recovery of cortical dopamine levels. If this recovery of dopaminergic innervation happened in the presence of the expression of hypersensitive receptors, then behavioral changes may be expected.
Alternatively, in addition to depleting dopamine levels, 6-OHDA lesions have been shown to effect the expression of genes that are important for proper neuron structure and morphogenesis. Most important was the up-regulation of genes involved in the repulsion of axon guidance and genes that inhibit the growth of dendrites and microtubule formation (Krasnova et al 2007). In addition, genes necessary for tubulin and actin folding were found to be down-regulated (Krasnova et al. 2007). In the context of this model, it is possible that while there is a full recovery of relative dopamine levels, there are altered morphological and structural defects in adulthood. It is possible that while there are relatively the same number of dopaminergic neurons in control and 6-OHDA-treated rats, 6-OHDA-treated rats do not have the proper synapse structure for normal functioning. This is supported by anatomical changes observed in the adult mouse brain after neonatal 6-OHDA lesioning (Krasnova et al. 2007). Lastly, a combination of these two models could account for the long-term effects of 6-OHDA lesioning. However, much more research is needed to distinguish between the two possibilities.
In summary, the neonatal 6-OHDA lesion of the frontal cortex caused a long-lasting neurobehavioral effects during adulthood in rats suggesting the critical role of the neonatal cortical dopamine in locomotor, learning and memory and nicotine addiction. Some of these effects are sex-specific. These data suggest that neonatal frontal cortical dopamine lesion can cause overstimulation of limbic sites and cause long lasting impairments in neurobehavioral functioning.
Acknowledgements
This research was supported by NIH grant DA015756 and an unrestricted grant from Philip Morris USA Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer T, Danysz W, Fredriksson A, Jonsson G, Luthman J, Sundstrom E, Teiling A. Neonatal 6-hydroxydopamine-induced dopamine depletions: motor activity and performance in maze learning. Pharmacol Biochem Behav. 1988;31:357–364. doi: 10.1016/0091-3057(88)90358-9. [DOI] [PubMed] [Google Scholar]
- Avale ME, Nemirovsky SI, Raisman-Vozari R, Rubinstein M. Elevated serotonin is involved in hyperactivity but not in the paradoxical effect of amphetamine in mice neonatally lesioned with 6-hydroxydopamine. J Neurosci Res. 2004;78:289–296. doi: 10.1002/jnr.20245. [DOI] [PubMed] [Google Scholar]
- Brocco M, Dekeyne A, Veiga S, Girardon S, Millan MJ. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A pharmacological characterization of diverse classes of antidepressant agents. Pharmacol Biochem Behav. 2002;71:667–680. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- Chia LG, Ni DR, Cheng FC, Ho YP, Kuo JS. Intrastriatal injection of 5,7-dihydroxytryptamine decreased 5-HT levels in the striatum and suppressed locomotor activity in C57BL/6 mice. Neurochem Res. 1999;24:719–722. doi: 10.1023/a:1020771211305. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Understanding brain mechanisms in nicotine reinforcement. Br J Addict. 1991;86:507–510. doi: 10.1111/j.1360-0443.1991.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. A rodent model for nicotine self-administration. In: Boulton AA, et al., editors. Animal Models of Drug Addiction. vol.24. Totowa, NJ: Humana Press Inc; 1992. pp. 315–344. [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Research. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BLC. Response of nicotine self-administration to manipulations of mu-opioid and GABA receptors in the ventral tegmental area. Psychopharmacology. 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Descarries L, Soghomonian JJ, Garcia S, Doucet G, Bruno JP. Ultrastructural analysis of the serotonin hyperinnervation in adult rat neostriatum following neonatal dopamine denervation with 6-hydroxydopamine. Brain Res. 1992;569:1–13. doi: 10.1016/0006-8993(92)90363-e. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Dluzen DE. Estrogen reduces acute striatal dopamine responses in vivo to the neurotoxin MPP+ in female, but not male rats. Brain Res. 2000;868:95–104. doi: 10.1016/s0006-8993(00)02329-5. [DOI] [PubMed] [Google Scholar]
- Dluzen D, Horstink M. Estrogen as neuroprotectant of nigrostriatal dopaminergic system: laboratory and clinical studies. Endocrine. 2003;21:67–75. doi: 10.1385/endo:21:1:67. [DOI] [PubMed] [Google Scholar]
- Dluzen DE. Effects of testosterone upon MPTP-induced neurotoxicity of the nigrostriatal dopaminergic system of C57/B1 mice. Brain Res. 1996;715:113–118. doi: 10.1016/0006-8993(95)01566-3. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL, Liu B. Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release. J Neurochem. 1996a;66:658–666. doi: 10.1046/j.1471-4159.1996.66020658.x. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL, Liu B. Estrogen as a neuroprotectant against MPTP-induced neurotoxicity in C57/B1 mice. Neurotoxicol Teratol. 1996b;18:603–606. doi: 10.1016/0892-0362(96)00086-4. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Shupenko CD, Mielke MM, Booth S, Hoffman AS, Imerito CA, Rose C, McCallum SE. Nicotine self-administration in male and female rats on fixed and progressive ratio schedules of reinforcement. Society for Neuroscience Abstracts. 1998 [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Rabinowitz J, Eerdekens M, Davidson M. Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry. 2005;162:1888–1895. doi: 10.1176/appi.ajp.162.10.1888. [DOI] [PubMed] [Google Scholar]
- Hughes J. Clinical implications of the association between smoking and alcoholism. In: Fertig J, Allen J, editors. Alcohol and tobacco: From Basic Science to Clinical Practice. NIAAA Research Monograph No. 3 NIH Pub. No 95-39-31. Washington, DC: 1995. pp. 171–185. [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. American Journal of Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction: towards the development of new therapies. Ann. N. Y. Acad. Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Betts ES, Dada A, Jefferson A, Ladenheim B, Becker KG, Cadet JL, Hohmann CF. Neonatal dopamine depletion induces changes in morphogenesis and gene expression in the developing cortex. Neurotox Res. 2007;11:107–130. doi: 10.1007/BF03033390. [DOI] [PubMed] [Google Scholar]
- Laviolette S, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nature Reviews Neuroscience. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicology and Teratology. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Mason GA, Rezvani AH, Overstreet DH, Hamedi M, Walker CH, Yang Y, Garbutt JC. Involvement of dopamine D2 receptors in suppressive effect of the TRH analog TA-0910 on alcohol intake in P rats. Alcohol Clin Exp Res. 1997;21:1623–1629. [PubMed] [Google Scholar]
- Miller FE, Heffner TG, Kotake C, Seiden LS. Magnitude and duration of hyperactivity following neonatal 6-hydroxydopamine is related to the extent of brain dopamine depletion. Brain Res. 1981;229:123–132. doi: 10.1016/0006-8993(81)90750-2. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Dewar KM, Descarries L, Reader TA. Altered dopamine and serotonin metabolism in the dopamine-denervated and serotonin-hyperinnervated neostriatum of adult rat after neonatal 6-hydroxydopamine. J Pharmacol Exp Ther. 1994;270:713–721. [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. un Neurobiology. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW. PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am J Psychiatry. 2005;162:1473–1482. doi: 10.1176/appi.ajp.162.8.1473. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Rex A, Fink H. Cholecystokinin tetrapeptide improves water maze performance of neonatally 6-hydroxydopamine-lesioned young rats. Pharmacology, Biochemistry and Behavior. 2004;79:109–117. doi: 10.1016/j.pbb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Garbutt JC, Shimoda K, Garges PL, Janowsky DS, Mason GA. Attenuation of alcohol preference in alcohol preferring rats by a novel TRH analogue TA-0910. Alcoholism Clin Exp Res. 1992;16:326–330. doi: 10.1111/j.1530-0277.1992.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Grady DR. Suppression of alcohol consumption by fenfluramine in Fawn-Hooded rats with serotonin dysfunction. Pharmacol Biochem Behav. 1994;48:105–110. doi: 10.1016/0091-3057(94)90505-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Janowsky DS. Genetic serotonin deficiency and alcohol preference in the fawn hooded rats. Alcohol and Alcoholism. 1990;25:573–575. [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Mason GA, Janowsky DS, Hamedi M, Clark JE, Ying Y. Combination pharmacotherapy: A mixture of small doses of naltrexone, fluoxetine, and a TRH analog reduces alcohol intake in three strains of alcohol preferring rats. Alcohol & Alcoholism. 2000;35:76–83. doi: 10.1093/alcalc/35.1.76. [DOI] [PubMed] [Google Scholar]
- Robbins T, Everitt B. Limbic–striatal memory systems and drug addiction. Neurobiology of Learning and Memory. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Fagerquist M, Zhang X, Hertel P, Panagis G, Nomikos G, Svensson T. Putative role of presynaptic a7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–383. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Yager RD, Klopper JH. Selective brain dopamine depletion in developing rats: an experimental model of minimal brain dysfunction. Science. 1976;191:305–308. doi: 10.1126/science.942800. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: Strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa: Iowa State University Press; 1967. [Google Scholar]
- Snyder AM, Zigmond MJ, Lund RD. Sprouting of serotoninergic afferents into striatum after dopamine-depleting lesions in infant rats: a retrograde transport and immunocytochemical study. J Comp Neurol. 1986;245:274–281. doi: 10.1002/cne.902450209. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of Learning & Memory. 2002a;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002b;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Wagner M, Quednow BB, Westheide J, Schlaepfer TE, Maier W, Kuhn KU. Cognitive improvement in schizophrenic patients does not require a serotonergic mechanism: randomized controlled trial of olanzapine vs amisulpride. Neuropsychopharmacology. 2005;30:381–390. doi: 10.1038/sj.npp.1300626. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Kaneda S, Kawai Y, Ueda S, Takeuchi Y, Matsushita H, Yuri K, Yasuhara M. Treating neonatal rats with 6-hydroxydopamine induced an increase in voluntary alcohol consumption. Alcoholism: Clinical and Experimental Research. 1999;23:2S–6S. doi: 10.1111/j.1530-0277.1999.tb04523.x. [DOI] [PubMed] [Google Scholar]
- Zhang K, Davids E, Tarazi FI, Baldessarini RJ. Serotonin transporter binding increases in caudate-putamen and nucleus accumbens after neonatal 6-hydroxydopamine lesions in rats: implications for motor hyperactivity. Brain Res Dev Brain Res. 2002;137:135–138. doi: 10.1016/s0165-3806(02)00436-4. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Stricker EM. Recovery of feeding and drinking by rats after intraventricular 6-hydroxydopamine or lateral hypothalamic lesions. Science. 1973;182:717–720. doi: 10.1126/science.182.4113.717. [DOI] [PubMed] [Google Scholar]