Abstract
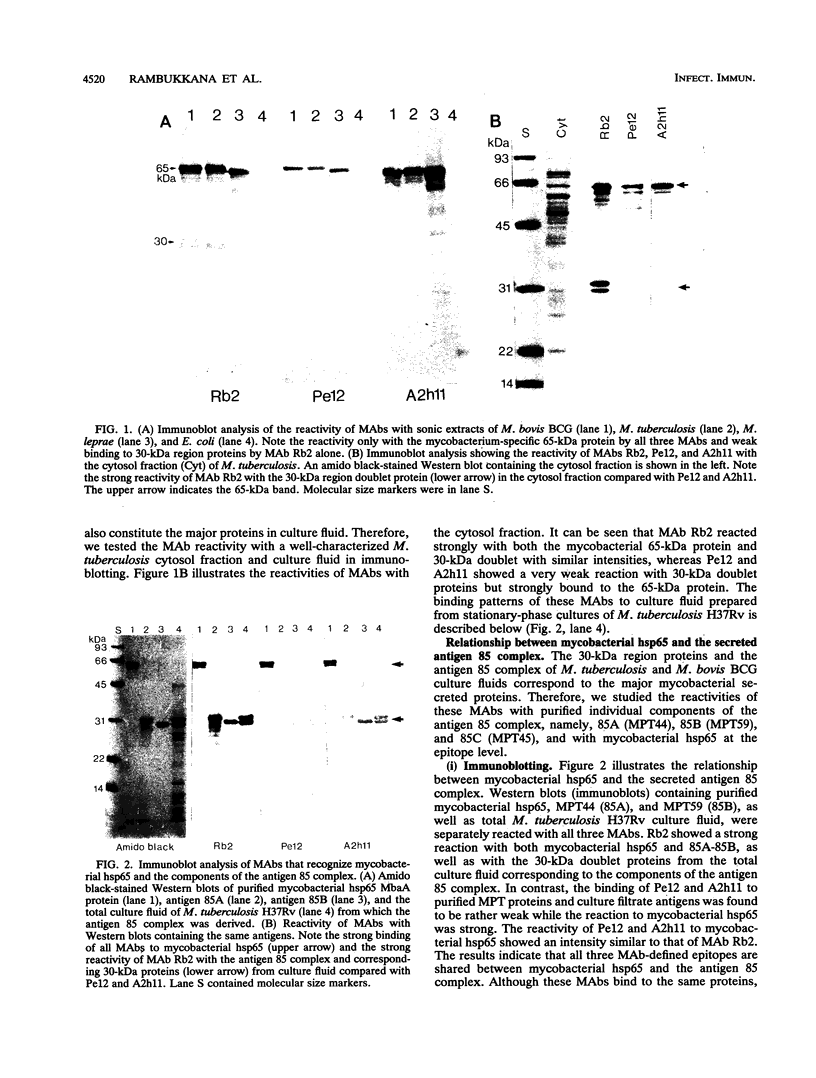
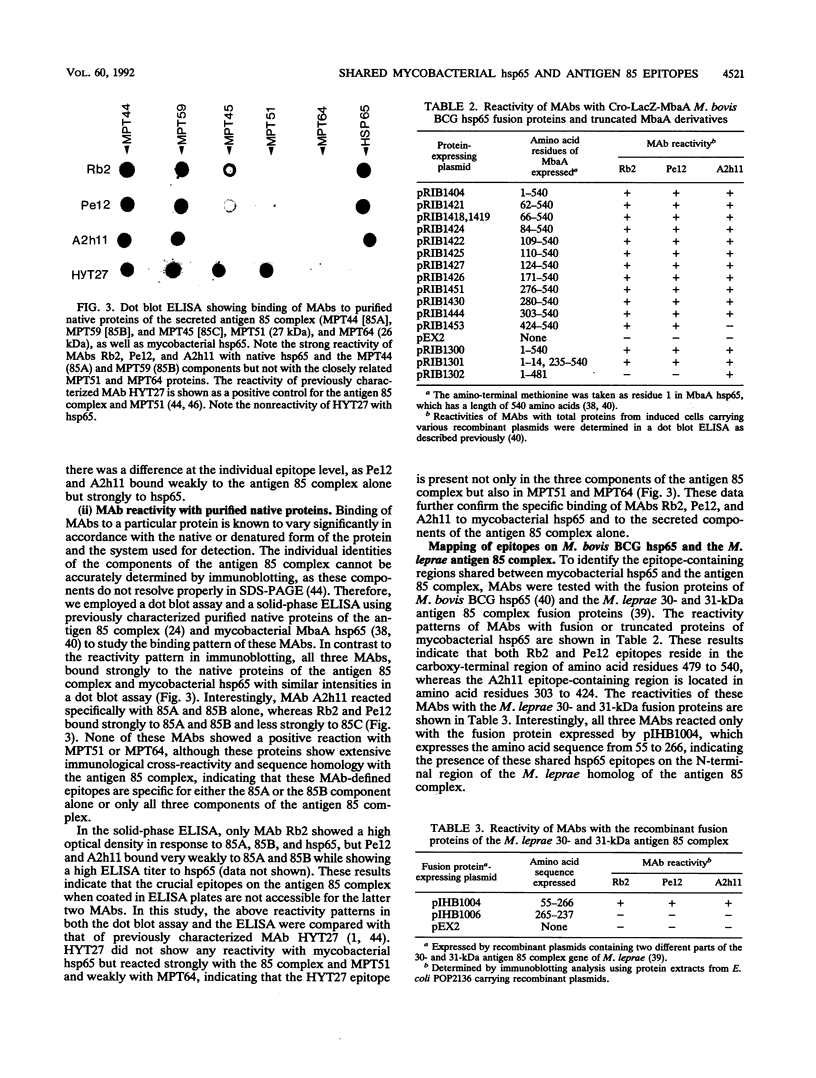
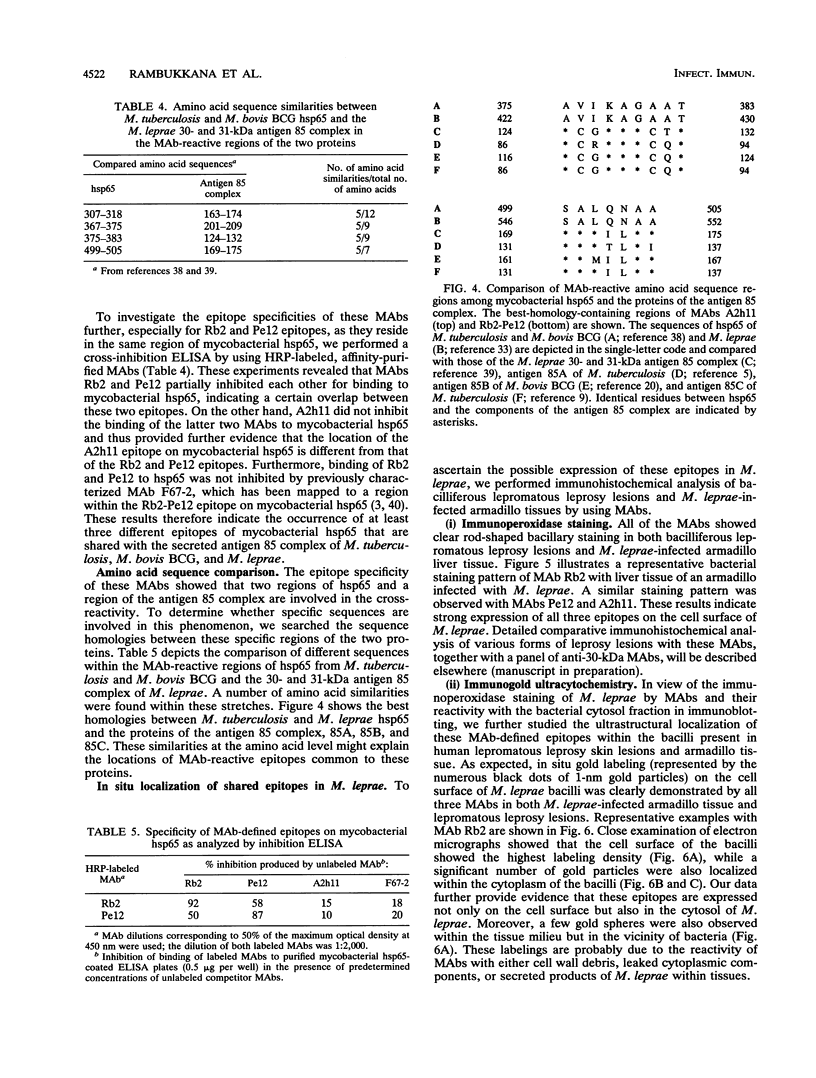
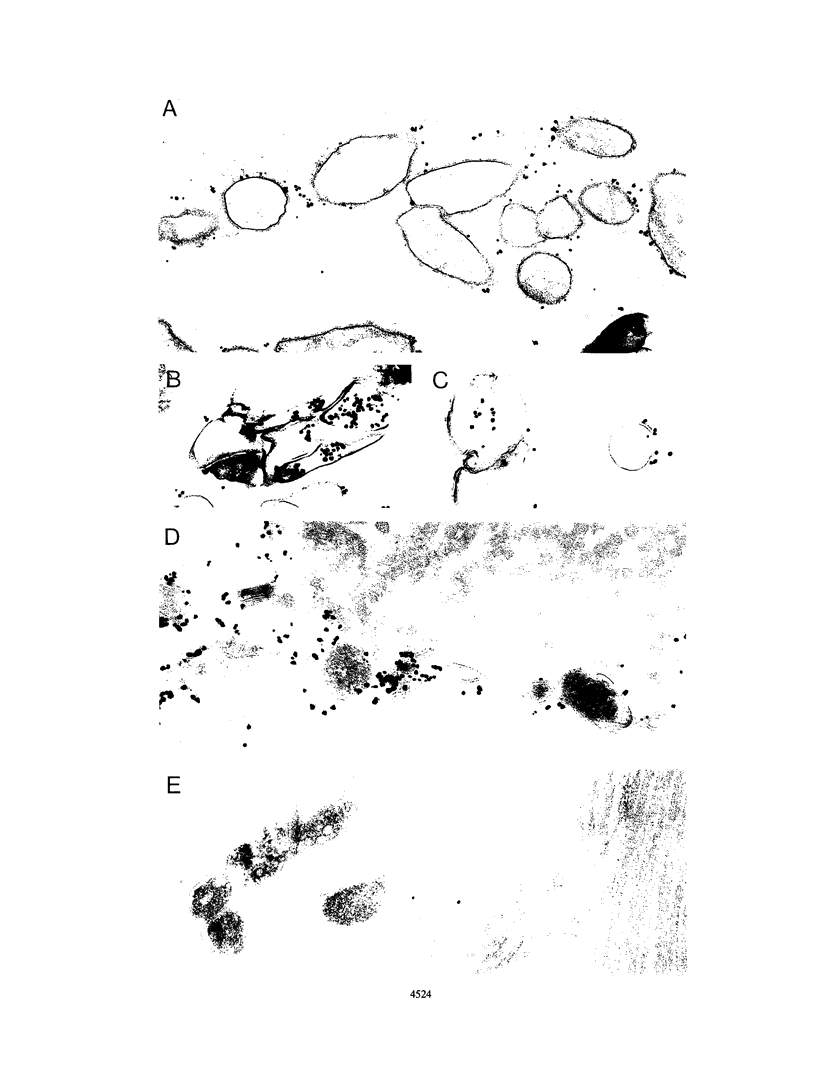
Both mycobacterial hsp65 and the actively secreted antigen 85 complex of 30-kDa region proteins are considered to be major immune targets in mycobacterial diseases. In this study, by using a novel series of monoclonal antibodies (MAbs) directed to these antigens, we identified and partially characterized three unique epitopes (Rb2, Pe12, and A2h11) that are shared between mycobacterial hsp65 and the individual components of the antigen 85 complex. Dot blot assays with native purified proteins revealed that all three MAbs are strongly bound to hsp65 and antigens 85A (MPT44) and 85B (MPT59), while a weak reaction or no reaction was found with antigen 85C (MPT45). Immunoblotting showed that MAb Rb2 reacted strongly with both hsp65 and the antigen 85 complex proteins, whereas MAbs Pe12 and A2h11 reacted strongly with the former but weakly with the latter. Moreover, these MAbs did not react with other closely related MPT51 and MPT64 secreted proteins. Further characterization of these epitopes was performed by using recombinant fusion and truncated proteins of Mycobacterium bovis BCG hsp65 (MbaA) and the M. leprae 30- and 31-kDa antigen 85 complex fusion proteins. In hsp65, Rb2-Pe12- and A2h11-reactive epitopes were found to reside in the C-terminal region of amino acid residues 479 to 540 and 303 to 424, respectively. In the M. leprae 30- and 31-kDa antigen 85 complex, all three epitopes were located in an N-terminal region of amino acid residues 55 to 266, one of the known fibronectin-binding sites of the M. leprae antigen 85 complex. Comparison of these MAb-reactive amino acid sequence regions between mycobacterial hsp65 and the components of the antigen 85 complex revealed that these regions show certain amino acid sequence identities. Furthermore, by immunoperoxidase and immunogold ultracytochemistry, we demonstrated that Rb2-, Pe12-, and A2h11-reactive epitopes are expressed both on the cell wall surface and in the cytosol of M. leprae bacilli within the lesions of lepromatous leprosy patients and in M. leprae-infected armadillo liver tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Barry M. E., Buchanan T. M. Exact definition of species-specific and cross-reactive epitopes of the 65-kilodalton protein of Mycobacterium leprae using synthetic peptides. J Immunol. 1988 Jul 15;141(2):607–613. [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Borremans M., de Wit L., Volckaert G., Ooms J., de Bruyn J., Huygen K., van Vooren J. P., Stelandre M., Verhofstadt R., Content J. Cloning, sequence determination, and expression of a 32-kilodalton-protein gene of Mycobacterium tuberculosis. Infect Immun. 1989 Oct;57(10):3123–3130. doi: 10.1128/iai.57.10.3123-3130.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R., Young D. B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991 Apr;12(4):105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Content J., de la Cuvellerie A., De Wit L., Vincent-Levy-Frébault V., Ooms J., De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991 Sep;59(9):3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit J., Sampson C., Zúiga M., Smith P. G., Plata J., Silva J., Molina J., Pinardi M. E., Bloom B. R., Salgado A. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet. 1992 Feb 22;339(8791):446–450. doi: 10.1016/0140-6736(92)91056-e. [DOI] [PubMed] [Google Scholar]
- Das P. K., Rambukkana A., Baas J. G., Groothuis D. G., Halperin M. Enzyme-linked immunosorbent assay for distinguishing serological responses of lepromatous and tuberculoid leprosies to the 29/33-kilodalton doublet and 64-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Feb;28(2):379–382. doi: 10.1128/jcm.28.2.379-382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Wiker H. G., Duncan J. R., Garcia M. M., Dukes T. W., Brooks B. W., Turcotte C., Nagai S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol. 1990 May;28(5):913–921. doi: 10.1128/jcm.28.5.913-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Wiker H. G., Nagai S. Protein antigens of mycobacteria studied by quantitative immunologic techniques. Clin Infect Dis. 1992 Jan;14(1):313–319. doi: 10.1093/clinids/14.1.313. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Løvik M., Closs O. Induction of immunity against live Mycobacterium lepraemurium: a requirement for viable bacilli? Immunology. 1984 Sep;53(1):165–173. [PMC free article] [PubMed] [Google Scholar]
- Martins-Green M. M., Tokuyasu K. T. A pre-embedding immunolabeling technique for basal lamina and extracellular matrix molecules. J Histochem Cytochem. 1988 Apr;36(4):453–458. doi: 10.1177/36.4.3279113. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Torigian V. K., Mandich D., Reichel M., Young S. M., Salgame P., Convit J., Hunter S. W., McNeil M. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J Immunol. 1989 Apr 15;142(8):2873–2878. [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon-Kaplan J., Hunter S. W., McNeil M., Stewart C., Modlin R. L., Rea T. H., Convit J., Salgame P., Mehra V., Bloom B. R. Immunological significance of Mycobacterium leprae cell walls. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1917–1921. doi: 10.1073/pnas.85.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Pessolani M. C., Rumjanek F. D., Marques M. A., de Melo F. S., Sarno E. N. Serological response of patients with leprosy to a 28- to 30-kilodalton protein doublet from early cultures of Mycobacterium bovis BCG. J Clin Microbiol. 1989 Oct;27(10):2184–2189. doi: 10.1128/jcm.27.10.2184-2189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Chand A., Baas J. G., Groothuis D. G., Kolk A. H. Subcellular distribution of monoclonal antibody defined epitopes on immunodominant Mycobacterium tuberculosis proteins in the 30-kDa region: identification and localization of 29/33-kDa doublet proteins on mycobacterial cell wall. Scand J Immunol. 1991 Jun;33(6):763–775. doi: 10.1111/j.1365-3083.1991.tb02551.x. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Krieg S., Faber W. R. Association of the mycobacterial 30-kDa region proteins with the cutaneous infiltrates of leprosy lesions. Evidence for the involvement of the major mycobacterial secreted proteins in the local immune response of leprosy. Scand J Immunol. 1992 Jul;36(1):35–48. doi: 10.1111/j.1365-3083.1992.tb02938.x. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Krieg S., Young S., Le Poole I. C., Bos J. D. Mycobacterial 65,000 MW heat-shock protein shares a carboxy-terminal epitope with human epidermal cytokeratin 1/2. Immunology. 1992 Oct;77(2):267–276. [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A., Yong S., Das P. K. Identification of a novel B-cell epitope of restricted specificity on the hsp 65-kDa protein of Mycobacterium tuberculosis. FEMS Microbiol Immunol. 1991 Feb;3(1):39–45. doi: 10.1111/j.1574-6968.1991.tb04161.x. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Rumschlag H. S., Shinnick T. M., Cohen M. L. Serological responses of patients with lepromatous and tuberculoid leprosy to 30-, 31-, and 32-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Oct;26(10):2200–2202. doi: 10.1128/jcm.26.10.2200-2202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S. J., Howland C., Stone M. M., Sutherland I. BCG vaccination of children against leprosy in Uganda: final results. J Hyg (Lond) 1981 Oct;87(2):233–248. doi: 10.1017/s002217240006945x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Hindersson P., de Bruyn J., Cremers F., van der Zee J., de Cock H., Tommassen J., van Eden W., van Embden J. D. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathog. 1988 Jan;4(1):71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turneer M., Van Vooren J. P., De Bruyn J., Serruys E., Dierckx P., Yernault J. C. Humoral immune response in human tuberculosis: immunoglobulins G, A, and M directed against the purified P32 protein antigen of Mycobacterium bovis bacillus Calmette-Guérin. J Clin Microbiol. 1988 Sep;26(9):1714–1719. doi: 10.1128/jcm.26.9.1714-1719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Leprosy: understanding protective immunity. Immunol Today. 1989 Jul;10(7):218–221. doi: 10.1016/0167-5699(89)90253-3. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Bennedsen J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):830–838. doi: 10.1164/ajrccm/141.4_Pt_1.830. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Nagai S., Harboe M., Ljungqvist L. A family of cross-reacting proteins secreted by Mycobacterium tuberculosis. Scand J Immunol. 1992 Aug;36(2):307–319. doi: 10.1111/j.1365-3083.1992.tb03104.x. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Sletten K., Nagai S., Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990 Jan;58(1):272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B. Structure of mycobacterial antigens. Br Med Bull. 1988 Jul;44(3):562–583. doi: 10.1093/oxfordjournals.bmb.a072268. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]