Abstract
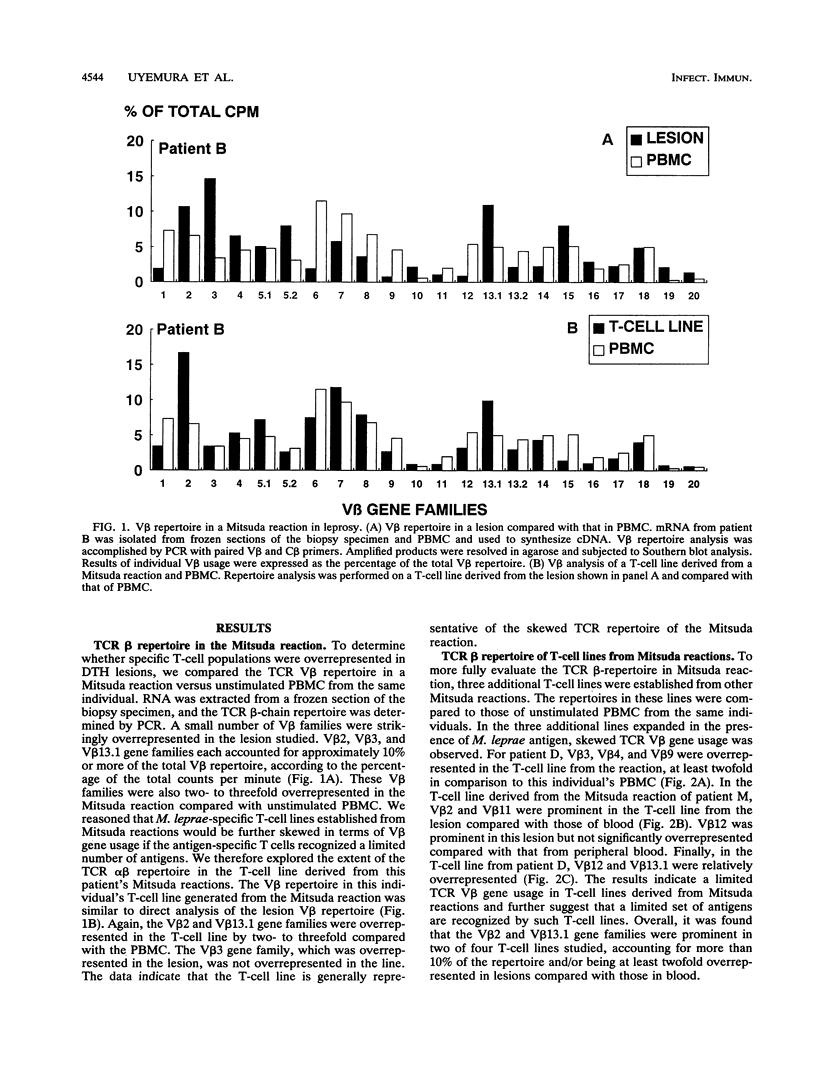
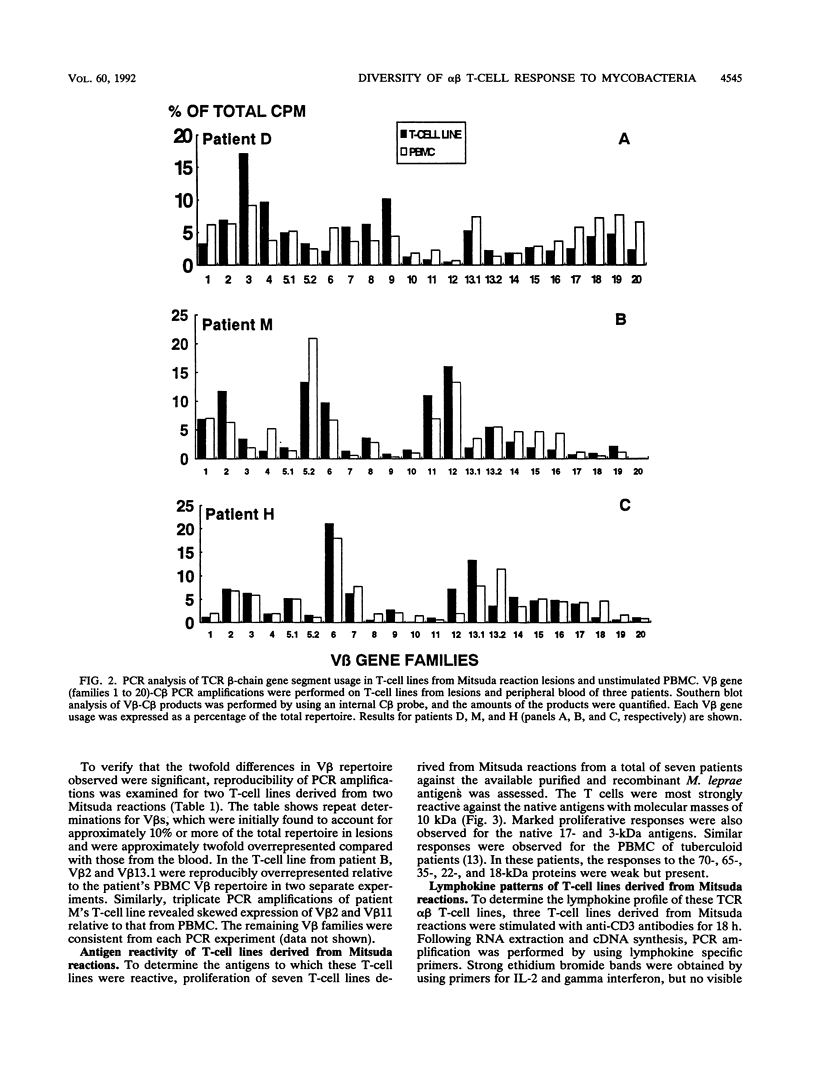
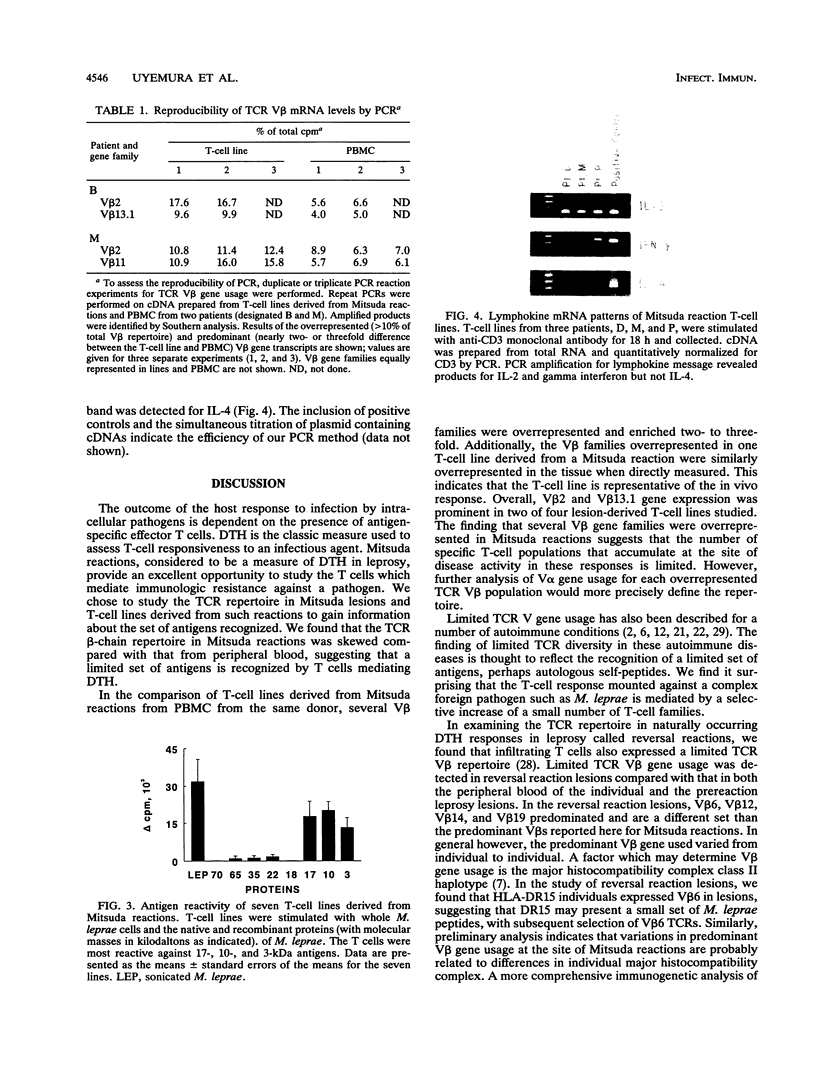
Delayed-type hypersensitivity (DTH) is the standard measure of T-cell responsiveness to infectious organisms. For leprosy, the Mitsuda reaction, a local immune response to cutaneous challenge with Mycobacterium leprae, is considered to represent a measure of DTH against the pathogen. We analyzed the diversity of the T-cell receptor beta-chain repertoire in Mitsuda reactions to determine the breadth of the mycobacterial antigens involved. The polymerase chain reaction was used to compare V beta usage in the Mitsuda reaction T-cell lines established and unstimulated peripheral blood. These molecular analyses revealed a skewed T-cell receptor V beta gene usage in the Mitsuda reaction and in T-cell lines from lesions. To examine the reactivity of T cells from these lesions, T-cell lines were tested against the available native and recombinant antigens of M. leprae. T-cell lines derived from Mitsuda reactions responded more strongly to the 10-kDa M. leprae antigen, a homolog of GroES in Escherichia coli, than to other M. leprae proteins. T-cell lines were also shown to proliferate strongly in response to the 17- and 3-kDa proteins. The pattern of the lymphokine mRNA of these cells was reminiscent of the pattern of murine TH1 cells, positive for interleukin-2 and gamma interferon and weakly positive for interleukin-4. These data indicate that a limited array of T cells, perhaps recognizing stress proteins, secrete a type 1 lymphokine profile in the DTH response to mycobacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. F., Grisso C. L., Abrams J. S., Band H., Rea T. H., Modlin R. L. Gamma delta T lymphocytes in human tuberculosis. J Infect Dis. 1992 Mar;165(3):506–512. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Liblau R. S., Cohen L., Lehmann D., Tournier-Lasserve E., Rosenzweig A., Zhang J. W., Raus J. C., Bach M. A. Restricted T-cell receptor V beta gene usage by myelin basic protein-specific T-cell clones in multiple sclerosis: predominant genes vary in individuals. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2466–2470. doi: 10.1073/pnas.88.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper C. L., Mueller C., Sinchaisri T. A., Pirmez C., Chan J., Kaplan G., Young S. M., Weissman I. L., Bloom B. R., Rea T. H. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989 May 1;169(5):1565–1581. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Concepcion E. S., Graves P., Cohen L., Ben-Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 1991 Jul 25;325(4):238–244. doi: 10.1056/NEJM199107253250404. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- DerSimonian H., Band H., Brenner M. B. Increased frequency of T cell receptor V alpha 12.1 expression on CD8+ T cells: evidence that V alpha participates in shaping the peripheral T cell repertoire. J Exp Med. 1991 Sep 1;174(3):639–648. doi: 10.1084/jem.174.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan E., Modlin R. L., Rea T. H. An in situ immunohistological study of Mitsuda reactions. Int J Lepr Other Mycobact Dis. 1985 Sep;53(3):404–409. [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Rivoire B., Mehra V., Bloom B. R., Brennan P. J. The major native proteins of the leprosy bacillus. J Biol Chem. 1990 Aug 25;265(24):14065–14068. [PubMed] [Google Scholar]
- Kotzin B. L., Karuturi S., Chou Y. K., Lafferty J., Forrester J. M., Better M., Nedwin G. E., Offner H., Vandenbark A. A. Preferential T-cell receptor beta-chain variable gene use in myelin basic protein-reactive T-cell clones from patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9161–9165. doi: 10.1073/pnas.88.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Bajardi A. C., Grisso C. L., Sieling P. A., Alland D., Convit J., Fan X. D., Hunter S. W., Brennan P. J. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J Exp Med. 1992 Jan 1;175(1):275–284. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Bloom B. R., Torigian V. K., Mandich D., Reichel M., Young S. M., Salgame P., Convit J., Hunter S. W., McNeil M. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J Immunol. 1989 Apr 15;142(8):2873–2878. [PubMed] [Google Scholar]
- Meyers W. M., Kvernes S., Binford C. H. Comparison of reactions to human and armadillo lepromins in leprosy. Int J Lepr Other Mycobact Dis. 1975 Jul-Sep;43(3):218–225. [PubMed] [Google Scholar]
- Modlin R. L., Kato H., Mehra V., Nelson E. E., Fan X. D., Rea T. H., Pattengale P. K., Bloom B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. 1986 Jul 31-Aug 6Nature. 322(6078):459–461. doi: 10.1038/322459a0. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Wong L., Fujimiya Y., Chang W. C., Horwitz D. A., Bloom B. R., Rea T. H., Pattengale P. K. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986 Nov 1;137(9):2831–2834. [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Ridley D. S. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51(5):451–465. [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Sumida T., Yonaha F., Maeda T., Tanabe E., Koike T., Tomioka H., Yoshida S. T cell receptor repertoire of infiltrating T cells in lips of Sjögren's syndrome patients. J Clin Invest. 1992 Feb;89(2):681–685. doi: 10.1172/JCI115635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
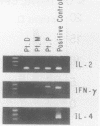
- Yamamura M., Wang X. H., Ohmen J. D., Uyemura K., Rea T. H., Bloom B. R., Modlin R. L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992 Aug 15;149(4):1470–1475. [PubMed] [Google Scholar]