Abstract
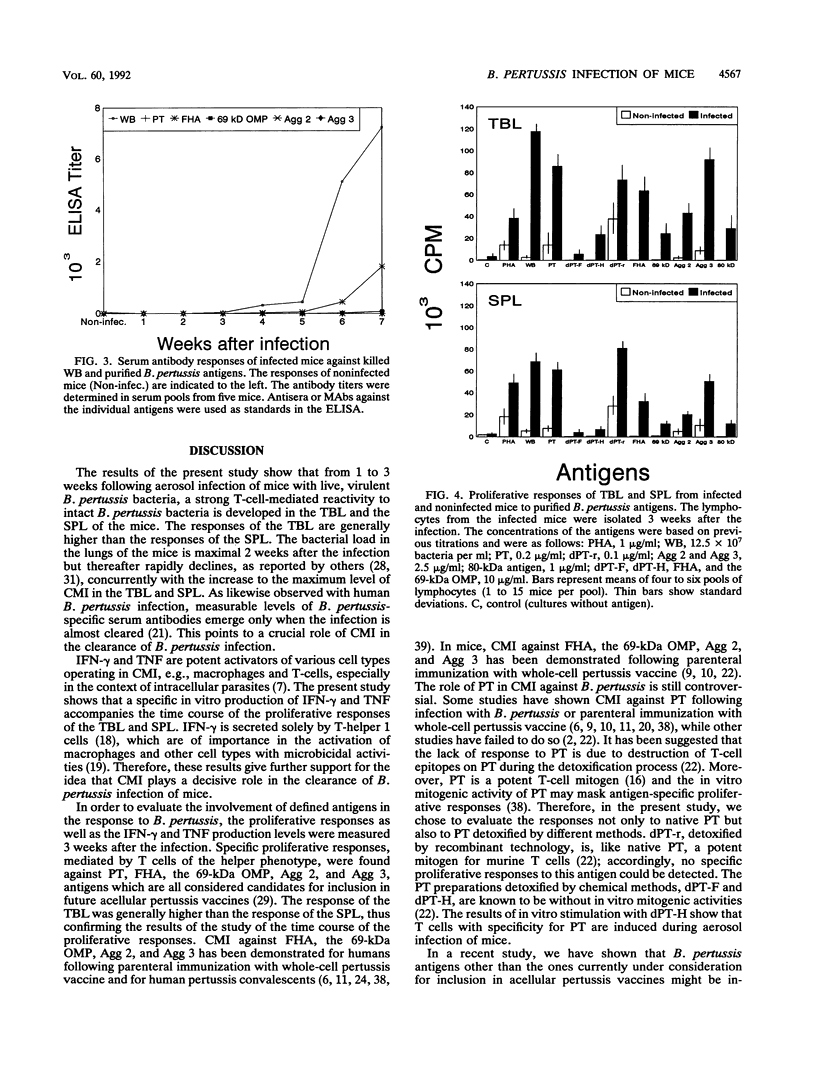
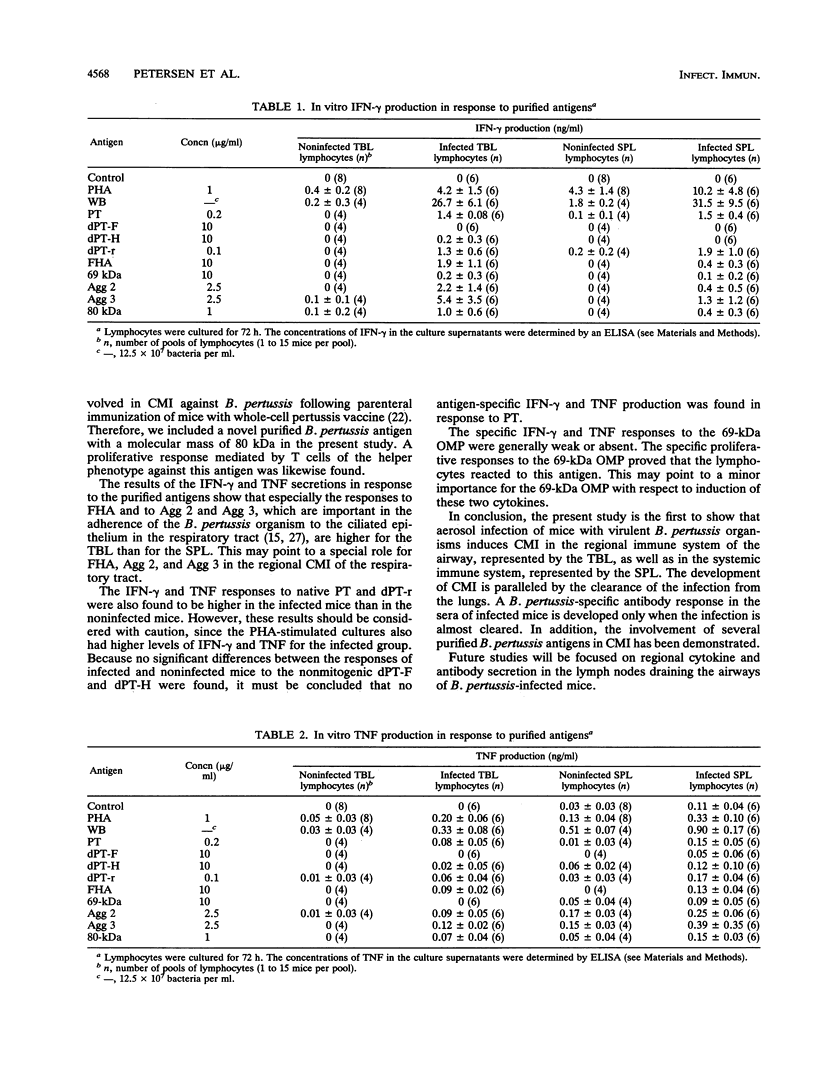
A group of mice was aerosol infected with live, virulent Bordetella pertussis bacteria. During a period of 7 weeks following the infection, with intervals of 1 week, lymphocytes were isolated from the tracheobroncheal lymph nodes (TBL) and the spleens (SPL) of the infected mice. The in vitro proliferative responses as well as the gamma interferon and tumor necrosis factor production levels of the isolated lymphocytes in response to stimulation with whole killed B. pertussis bacteria were measured as parameters for cell-mediated immunity (CMI). The course of the infection was monitored by counting of CFU in the lungs of the mice. Moreover, antibody responses in serum against a range of B. pertussis antigens were assessed. The results showed that a vigorous proliferative response of the TBL and SPL to stimulation with whole killed B. pertussis bacteria was induced by the infection. The proliferative response of the TBL was significantly higher than the response of the SPL. The proliferative responses were maximal 3 to 4 weeks after the infection and were paralleled by in vitro gamma interferon and tumor necrosis factor production upon specific stimulation. The development of the CMI was observed simultaneously with the clearance of the infection from the lungs. Antibody responses became measurable in the sera only after the infection was cleared. A specific CMI against pertussis toxin, the filamentous hemagglutinin, the 69-kDa outer membrane protein, and the agglutinogens 2 and 3, antigens which are under consideration for inclusion in future acellular pertussis vaccines, was successfully demonstrated in mice 3 weeks after the infection.
Full text
PDF

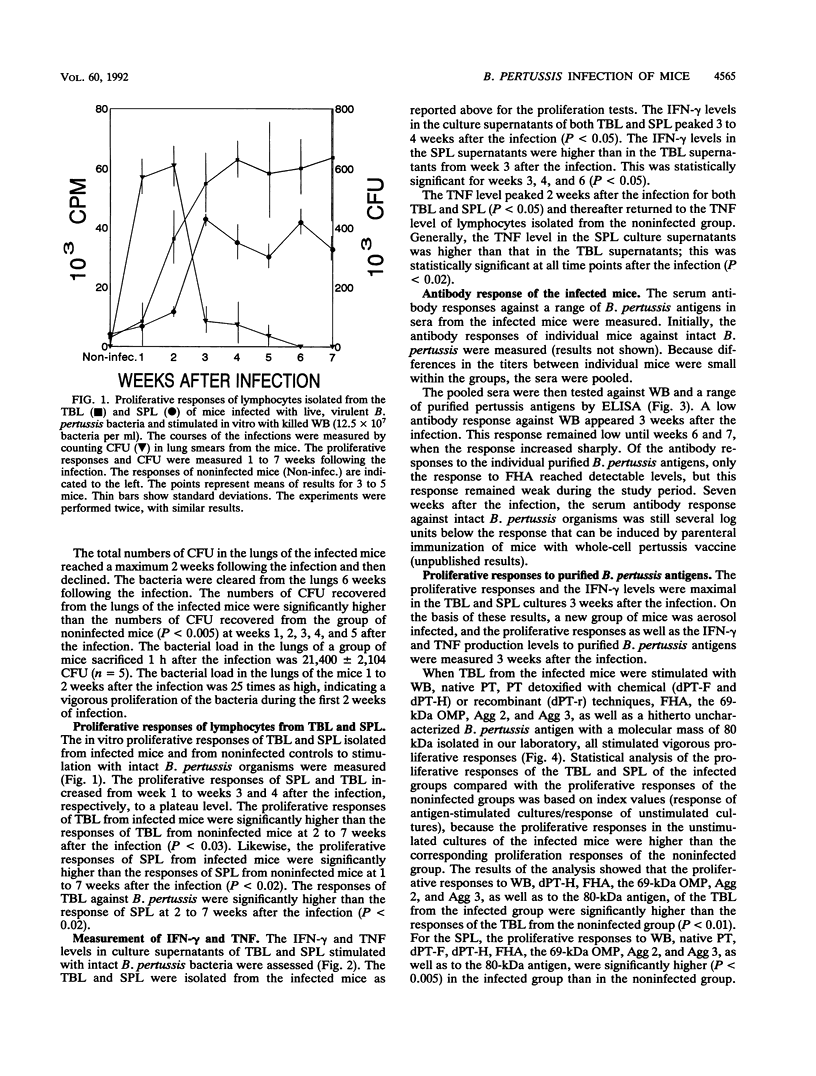

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Capiau C., Petre J., Van Damme J., Puype M., Vandekerckhove J. Protein-chemical analysis of pertussis toxin reveals homology between the subunits S2 and S3, between S1 and the A chains of enterotoxins of Vibrio cholerae and Escherichia coli and identifies S2 as the haptoglobin-binding subunit. FEBS Lett. 1986 Aug 18;204(2):336–340. doi: 10.1016/0014-5793(86)80839-0. [DOI] [PubMed] [Google Scholar]
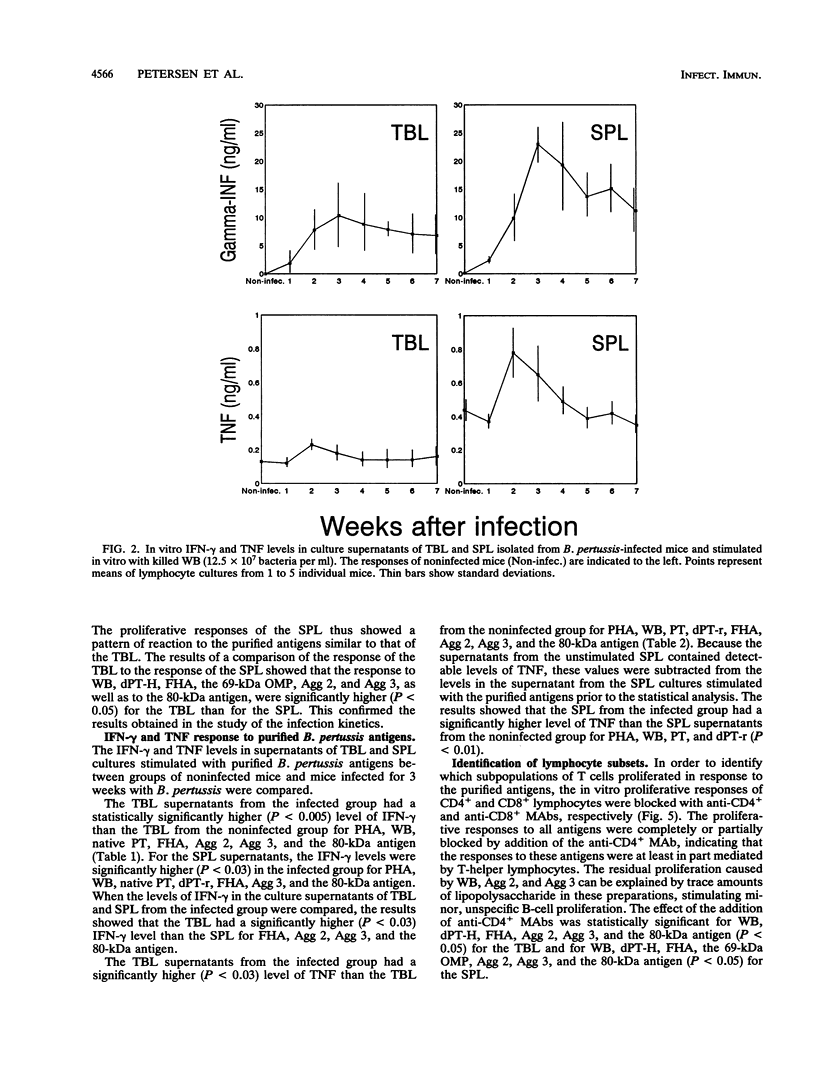
- De Magistris M. T., Romano M., Nuti S., Rappuoli R., Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988 Oct 1;168(4):1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza I., Männel D., Ruppel A., Falk W., Krammer P. H. Interferon gamma and lymphotoxin or tumor necrosis factor act synergistically to induce macrophage killing of tumor cells and schistosomula of Schistosoma mansoni. J Exp Med. 1987 Aug 1;166(2):589–594. doi: 10.1084/jem.166.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish F., Cowell J. L., Manclark C. R. Proliferative response of immune mouse T-lymphocytes to the lymphocytosis-promoting factor of Bordetella pertussis. Infect Immun. 1984 Apr;44(1):1–6. doi: 10.1128/iai.44.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing A. J., Bird C. R., Redhead K., Thomas M. Human cellular immune responses to Bordetella pertussis infection. FEMS Microbiol Immunol. 1989 Mar;1(4):205–211. doi: 10.1111/j.1574-6968.1989.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Bird C., Wadha M., Redhead K. The primary and secondary cellular immune responses to whole cell Bordetella pertussis vaccine and its components. Clin Exp Immunol. 1987 May;68(2):275–281. [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Harmsen A. G., Muggenburg B. A., Snipes M. B., Bice D. E. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985 Dec 13;230(4731):1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- Hasløv K., Møller S., Bentzon M. W. Effect of 2-mercaptoethanol and mycostatin on guinea pig lymphocyte transformation: interpretation of results depends on the method of calculation. J Immunol Methods. 1983 Jun 24;61(1):55–65. doi: 10.1016/0022-1759(83)90008-x. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A. S., Morse S. I. The in vitro effects of Bordetella pertussis lymphocytosis-promoting factor on murine lymphocytes: II. Nature of the responding cells. J Exp Med. 1977 Jan 1;145(1):163–174. doi: 10.1084/jem.145.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Roberts A. L., Finn T. M., Knapp S., Mekalanos J. J. A new assay for invasion of HeLa 229 cells by Bordetella pertussis: effects of inhibitors, phenotypic modulation, and genetic alterations. Infect Immun. 1990 Aug;58(8):2516–2522. doi: 10.1128/iai.58.8.2516-2522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Gamma interferon, cytokine-induced macrophage activation, and antimicrobial host defense. In vitro, in animal models, and in humans. Diagn Microbiol Infect Dis. 1990 Sep-Oct;13(5):411–421. doi: 10.1016/0732-8893(90)90012-k. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Judd A. K., Ko C., Lim M., Fernandez R., Schoolnik G. K., Steinman L. MHC-restricted recognition of immunogenic T cell epitopes of pertussis toxin reveals determinants in man distinct from the ADP-ribosylase active site. J Exp Med. 1988 Nov 1;168(5):1855–1864. doi: 10.1084/jem.168.5.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. C. Pertussis. Medicine (Baltimore) 1975 Nov;54(6):427–469. doi: 10.1097/00005792-197511000-00001. [DOI] [PubMed] [Google Scholar]
- Petersen J. W., Ibsen P. H., Bentzon M. W., Capiau C., Heron I. The cell mediated and humoral immune response to vaccination with acellular and whole cell pertussis vaccine in adult humans. FEMS Microbiol Immunol. 1991 Oct;3(5):279–287. doi: 10.1111/j.1574-6968.1991.tb04224.x. [DOI] [PubMed] [Google Scholar]
- Pizza M., Covacci A., Bartoloni A., Perugini M., Nencioni L., De Magistris M. T., Villa L., Nucci D., Manetti R., Bugnoli M. Mutants of pertussis toxin suitable for vaccine development. Science. 1989 Oct 27;246(4929):497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- Redhead K. Serum antibody responses to the outer membrane proteins of Bordetella pertussis. Infect Immun. 1984 Jun;44(3):724–729. doi: 10.1128/iai.44.3.724-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Tite J. P., Fairweather N. F., Dougan G., Charles I. G. Recombinant P.69/pertactin: immunogenicity and protection of mice against Bordetella pertussis infection. Vaccine. 1992;10(1):43–48. doi: 10.1016/0264-410x(92)90418-j. [DOI] [PubMed] [Google Scholar]
- Robinson A., Irons L. I., Ashworth L. A. Pertussis vaccine: present status and future prospects. Vaccine. 1985 Mar;3(1):11–22. doi: 10.1016/0264-410x(85)90004-0. [DOI] [PubMed] [Google Scholar]
- Sato H., Ito A., Chiba J., Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984 Nov;46(2):422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Role of antibody to leukocytosis-promoting factor hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect Immun. 1981 Mar;31(3):1223–1231. doi: 10.1128/iai.31.3.1223-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Cabellos C., Burroughs M., Prasad S., Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991 May 1;173(5):1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekura R. D., Zhang Y. L., Roberson R., Acton B., Trollfors B., Tolson N., Shiloach J., Bryla D., Muir-Nash J., Koeller D. Clinical, metabolic, and antibody responses of adult volunteers to an investigational vaccine composed of pertussis toxin inactivated by hydrogen peroxide. J Pediatr. 1988 Nov;113(5):806–813. doi: 10.1016/s0022-3476(88)80005-2. [DOI] [PubMed] [Google Scholar]
- Shahin R. D., Brennan M. J., Li Z. M., Meade B. D., Manclark C. R. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med. 1990 Jan 1;171(1):63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. G., Ashworth L. A., Miller E., Lambert H. P. Serum IgG, IgA, and IgM responses to pertussis toxin, filamentous hemagglutinin, and agglutinogens 2 and 3 after infection with Bordetella pertussis and immunization with whole-cell pertussis vaccine. J Infect Dis. 1989 Nov;160(5):838–845. doi: 10.1093/infdis/160.5.838. [DOI] [PubMed] [Google Scholar]
- Thomas M. G., Redhead K., Lambert H. P. Human serum antibody responses to Bordetella pertussis infection and pertussis vaccination. J Infect Dis. 1989 Feb;159(2):211–218. doi: 10.1093/infdis/159.2.211. [DOI] [PubMed] [Google Scholar]
- Tomoda T., Ogura H., Kurashige T. Immune responses to Bordetella pertussis infection and vaccination. J Infect Dis. 1991 Mar;163(3):559–563. doi: 10.1093/infdis/163.3.559. [DOI] [PubMed] [Google Scholar]
- Westphal Petersen J., Hasløv K., Lundberg L., Weis Bentzon M., Gehring T. Reactivity of lymphoid cells isolated from the tracheobroncheal lymph nodes of two guinea-pig strains with high or low susceptibility to respiratory anaphylaxis. Int Arch Allergy Appl Immunol. 1990;93(1):59–65. doi: 10.1159/000235280. [DOI] [PubMed] [Google Scholar]
- Wiertz E. J., Loggen H. G., Walvoort H. C., Kreeftenberg J. G. In vitro induction of antigen specific antibody synthesis and proliferation of T lymphocytes with acellular pertussis vaccines, pertussis toxin and filamentous haemagglutinin in humans. J Biol Stand. 1989 Apr;17(2):181–190. doi: 10.1016/0092-1157(89)90008-5. [DOI] [PubMed] [Google Scholar]
- Wiertz E. J., Walvoort H. C., Van Loveren H., Van Straaten-Van De Kappelle I., Van Der Gun J. W., Kreeftenberg J. G. Acellular and whole cell pertussis vaccines protect against the lethal effects of intracerebral challenge by two different T-cell dependent humoral routes. Biologicals. 1990 Jul;18(3):173–180. doi: 10.1016/1045-1056(90)90004-j. [DOI] [PubMed] [Google Scholar]


