Abstract
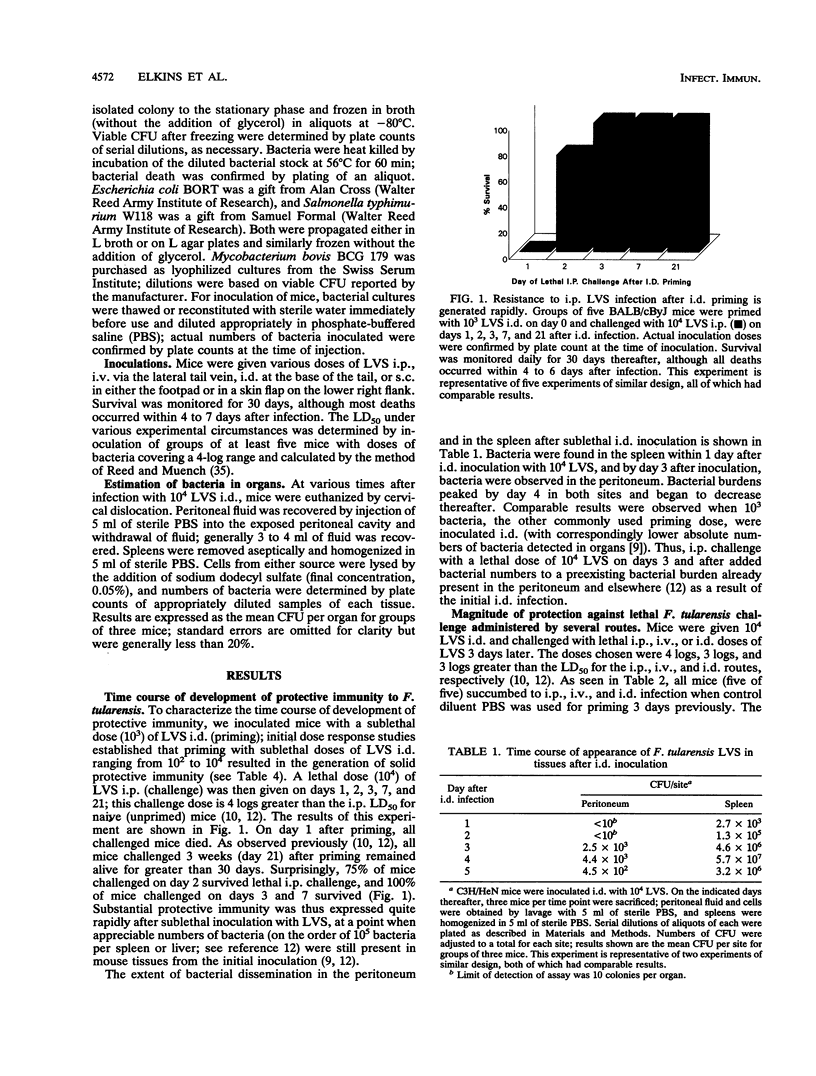
Mice inoculated either subcutaneously (s.c.) or intradermally (i.d.) with a sublethal dose of Francisella tularensis LVS are immune to a lethal intraperitoneal (i.p.) or intravenous (i.v.) challenge of LVS. Here, we show that this immunity developed quite rapidly: mice given a sublethal dose of live LVS s.c. or i.d. (but not i.v.) withstood lethal i.p., i.v., or i.d. challenge as early as 2 days after the initial inoculation, despite the presence of bacterial burdens already in tissues. The magnitude of this early protection was quite impressive. The i.p. 50% lethal dose (LD50) in naive C3H/HeN mice was only 2 bacteria, while the i.p. LD50 in mice given 10(4) LVS i.d. 3 days previously was 3 x 10(6) bacteria. Similarly, the i.v. LD50 in C3H/HeN mice shifted from 3 x 10(2) in naive mice to 5 x 10(6) in primed mice within 3 days after i.d. LVS infection. Comparable changes in the i.p. and i.v. LD50 were observed in C57BL/6J mice. This rapid generation of protective immunity was specific for LVS, in that mice given a sublethal i.d. inoculation of LVS did not survive a lethal challenge with either Salmonella typhimurium W118 or Escherichia coli O118 BORT at any time, nor could mice given sublethal doses of S. typhimurium, E. coli, or Mycobacterium bovis BCG survive lethal doses of LVS. Although an increase in the mean time to death from S. typhimurium infection was noted when mice were given a sublethal i.d. dose of LVS 4 to 14 days earlier, no overall increase in protection or change in the S. typhimurium LD50 was observed. Thus, sublethal infection with LVS at skin sites induced rapid and specific protective immunity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansel J., Perry P., Brown J., Damm D., Phan T., Hart C., Luger T., Hefeneider S. Cytokine modulation of keratinocyte cytokines. J Invest Dermatol. 1990 Jun;94(6 Suppl):101S–107S. doi: 10.1111/1523-1747.ep12876053. [DOI] [PubMed] [Google Scholar]
- Baker C. N., Hollis D. G., Thornsberry C. Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J Clin Microbiol. 1985 Aug;22(2):212–215. doi: 10.1128/jcm.22.2.212-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Burke D. S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977 Jan;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Kelly N., Bernton E., Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989 Jun 1;169(6):2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Flesch I., Kaufmann S. H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988 Jan;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Follows G. A., Munk M. E., Gatrill A. J., Conradt P., Kaufmann S. H. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in gamma/delta T-cell cultures after activation with bacteria. Infect Immun. 1992 Mar;60(3):1229–1231. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Slayter M. V., Ziemba R., Meltzer M. S., Nacy C. A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991 Sep;59(9):2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Peñarrubia P., Koster F. T., Kelley R. O., McDowell T. D., Bankhurst A. D. Antibacterial activity of human natural killer cells. J Exp Med. 1989 Jan 1;169(1):99–113. doi: 10.1084/jem.169.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. P., Harshan K. V., Born W. K., Orme I. M. Kinetics of accumulation of gamma delta receptor-bearing T lymphocytes in mice infected with live mycobacteria. Infect Immun. 1991 Nov;59(11):4263–4265. doi: 10.1128/iai.59.11.4263-4265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E., Mastroeni P., Arena A., Uddin J., Joysey H. S. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology. 1990 Jun;70(2):247–250. [PMC free article] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985 Mar;47(3):605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990 Jun;94(6 Suppl):146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- Kuramoto E., Toizumi S., Shimada S., Tokunaga T. In situ infiltration of natural killer-like cells induced by intradermal injection of the nucleic acid fraction from BCG. Microbiol Immunol. 1989;33(11):929–940. doi: 10.1111/j.1348-0421.1989.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Leiby D. A., Fortier A. H., Crawford R. M., Schreiber R. D., Nacy C. A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992 Jan;60(1):84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann R. A. Roles of interferon and cellular adhesion molecules in bacterial activation of human natural killer cells. Infect Immun. 1989 Jun;57(6):1702–1706. doi: 10.1128/iai.57.6.1702-1706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. E., Ziegler H. K. Role of bacterial hemolysin production in induction of macrophage Ia expression during infection with Listeria monocytogenes. J Immunol. 1991 Oct 1;147(7):2324–2332. [PubMed] [Google Scholar]
- Muotiala A., Mäkelä P. H. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb Pathog. 1990 Feb;8(2):135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- Nakano Y., Onozuka K., Terada Y., Shinomiya H., Nakano M. Protective effect of recombinant tumor necrosis factor-alpha in murine salmonellosis. J Immunol. 1990 Mar 1;144(5):1935–1941. [PubMed] [Google Scholar]
- Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992 Feb;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J Immunol. 1990 Aug 15;145(4):1265–1269. [PubMed] [Google Scholar]
- Nelson B. J., Ralph P., Green S. J., Nacy C. A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991 Mar 15;146(6):1849–1857. [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L. Genetic control of the susceptibility of C3HeB/FeJ mice to Salmonella typhimurium is regulated by a locus distinct from known salmonella response genes. J Immunol. 1983 Dec;131(6):2613–2615. [PubMed] [Google Scholar]
- Orme I. M., Miller E. S., Roberts A. D., Furney S. K., Griffin J. P., Dobos K. M., Chi D., Rivoire B., Brennan P. J. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992 Jan 1;148(1):189–196. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: induction of tumoricidal macrophages by supernatants of PPD-stimulated Bacillus Calmette-Guérin-immune spleen cell cultures. J Immunol. 1977 Sep;119(3):889–896. [PubMed] [Google Scholar]
- Schafer R., Eisenstein T. K. Natural killer cells mediate protection induced by a Salmonella aroA mutant. Infect Immun. 1992 Mar;60(3):791–797. doi: 10.1128/iai.60.3.791-797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein J. W. Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol. 1983 Jun;80 (Suppl):12s–16s. doi: 10.1111/1523-1747.ep12536743. [DOI] [PubMed] [Google Scholar]
- TIGERTT W. D. Soviet viable Pasteurella tularensis vaccines. A review of selected articles. Bacteriol Rev. 1962 Sep;26:354–373. doi: 10.1128/br.26.3.354-373.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigelaar R. E., Lewis J. M., Bergstresser P. R. TCR gamma/delta+ dendritic epidermal T cells as constituents of skin-associated lymphoid tissue. J Invest Dermatol. 1990 Jun;94(6 Suppl):58S–63S. doi: 10.1111/1523-1747.ep12875138. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Dougan G., Chatfield S. N. The involvement of tumor necrosis factor in immunity to Salmonella infection. J Immunol. 1991 Nov 1;147(9):3161–3164. [PubMed] [Google Scholar]
- Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989 May-Jun;11(3):440–451. [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. Cell-mediated immune response to Salmonella typhimurium infection in mice: development of nonspecific bactericidal activity against Listeria monocytogenes. Infect Immun. 1976 Apr;13(4):1069–1073. doi: 10.1128/iai.13.4.1069-1073.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]


