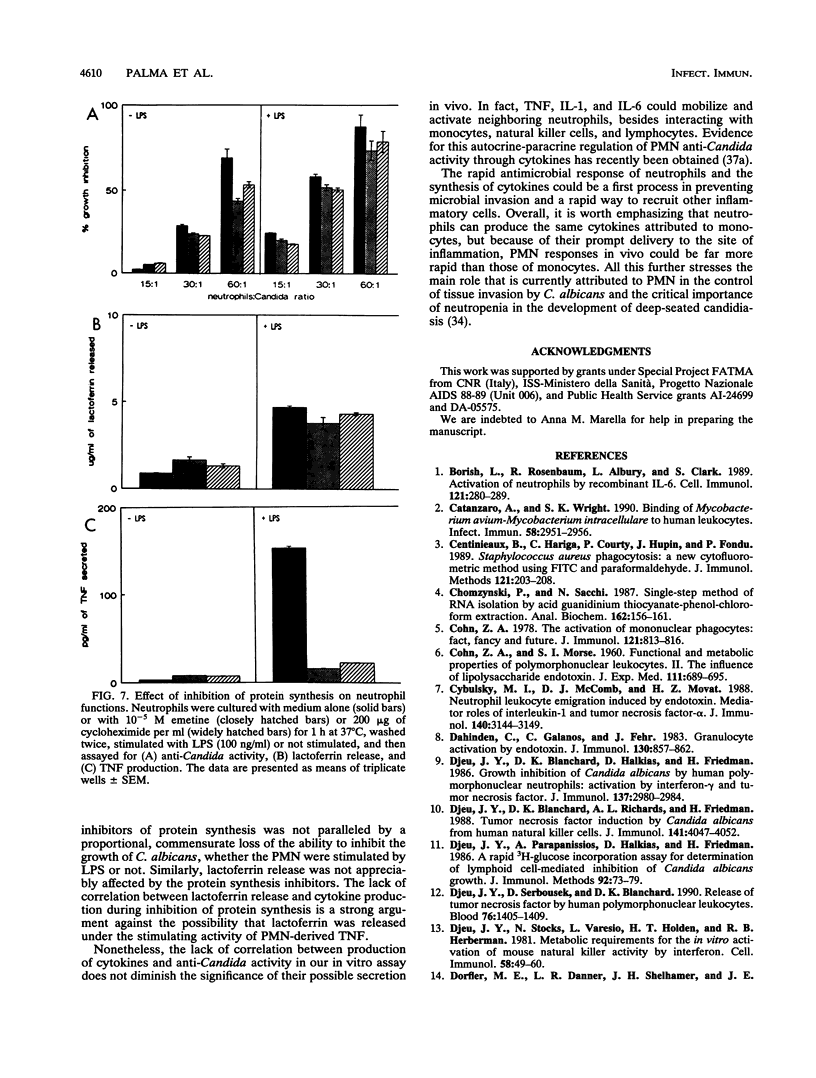
Abstract
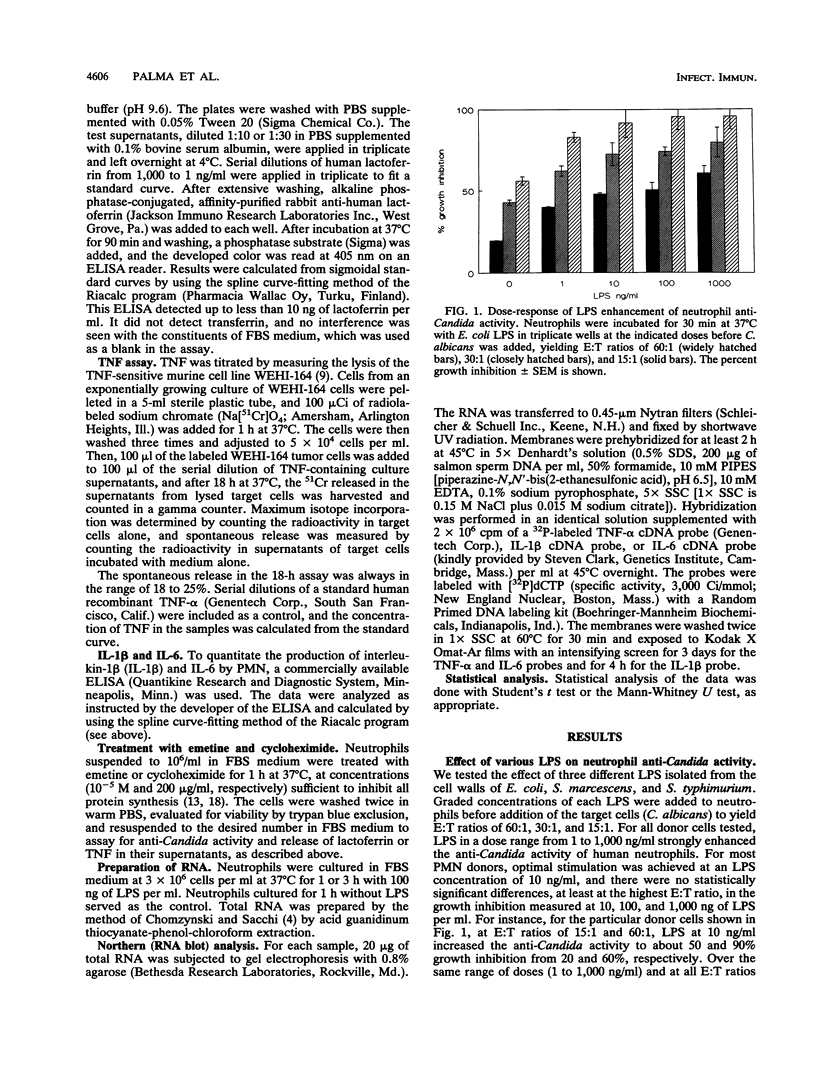
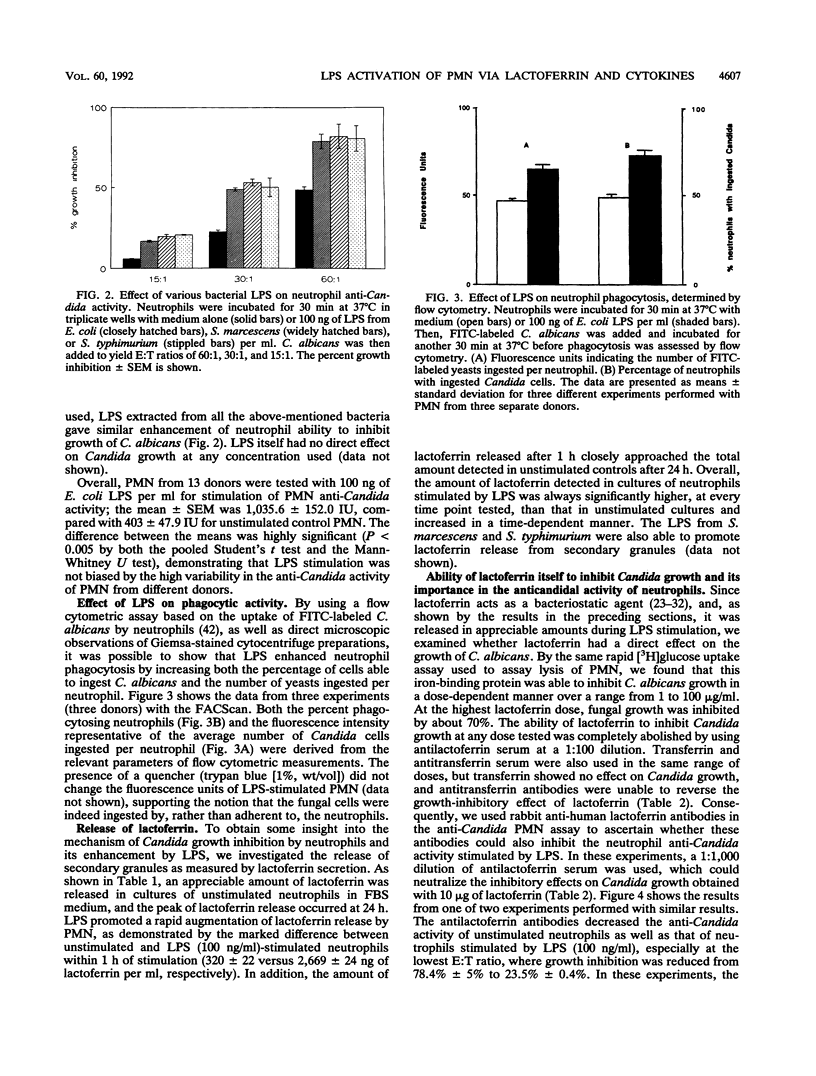
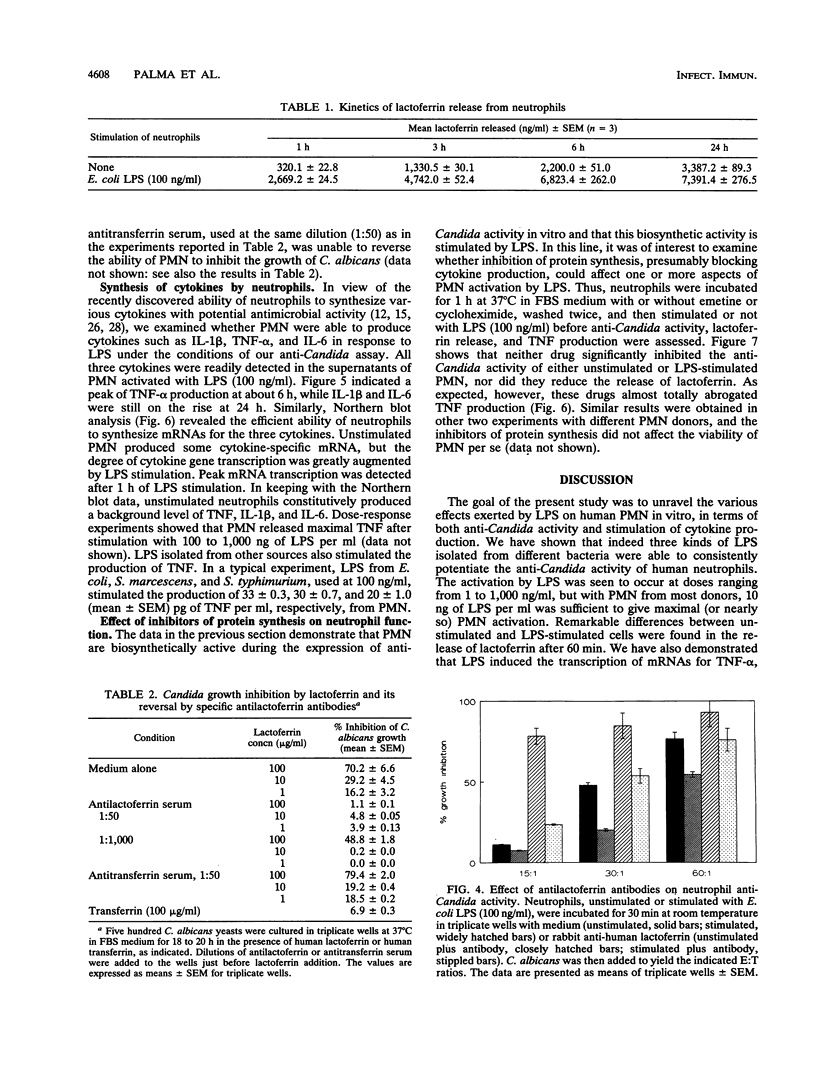
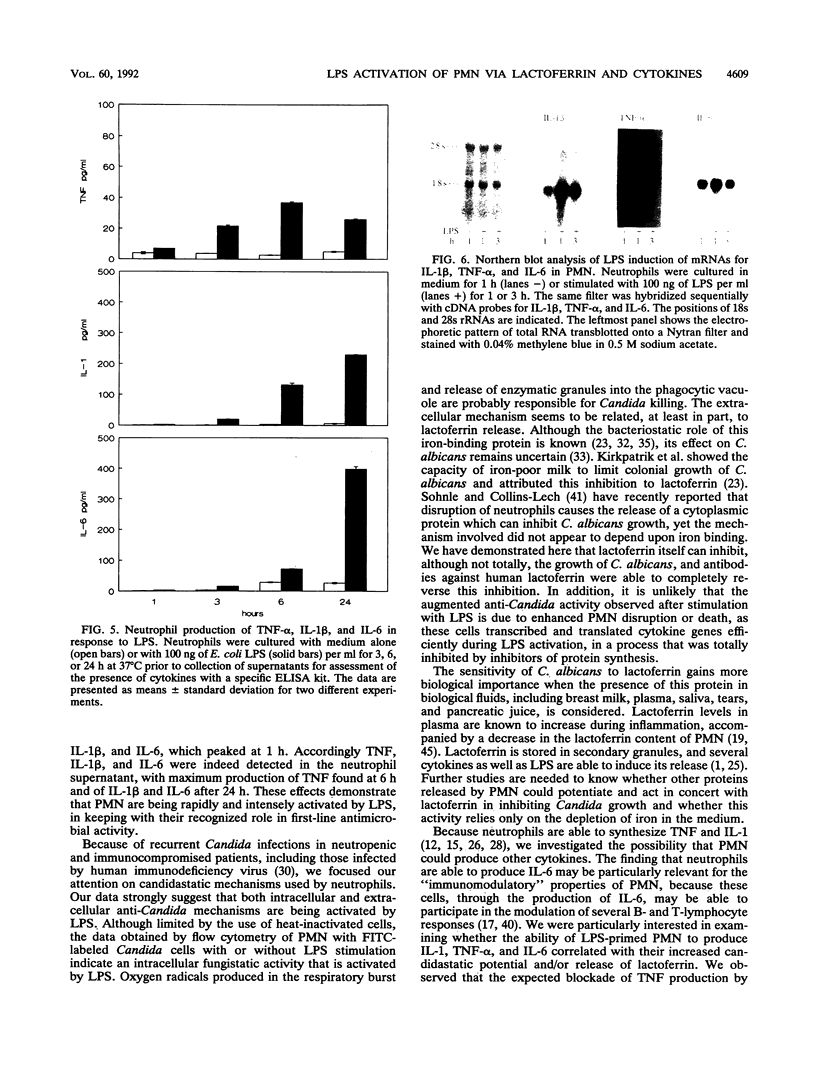
Lipopolysaccharides (LPSs) from Escherichia coli, Serratia marcescens, and Salmonella typhimurium, at doses from 1 to 100 ng/ml, strongly enhanced growth inhibition of Candida albicans by human polymorphonuclear leukocytes (PMN) in vitro. Flow cytometry analysis demonstrated that LPS markedly augmented phagocytosis of Candida cells by increasing the number of yeasts ingested per neutrophil as well as the number of neutrophils capable of ingesting fungal cells. LPS activation caused augmented release of lactoferrin, an iron-binding protein which itself could inhibit the growth of C. albicans in vitro. Antibodies against lactoferrin effectively and specifically reduced the anti-C. albicans activity of both LPS-stimulated and unstimulated PMN. Northern (RNA blot) analysis showed enhanced production of mRNAs for interleukin-1 beta, tumor necrosis factor alpha, and interleukin-6 and in neutrophils within 1 h of stimulation with LPS. The cytokines were also detected in the supernatant of the activated PMN, and their synthesis was prevented by pretreatment of LPS-stimulated PMN with protein synthesis inhibitors, such as emetine and cycloheximide. These inhibitors, however, did not block either lactoferrin release or the anti-Candida activity of LPS-stimulated PMN. These results demonstrate the ability of various bacterial LPSs to augment neutrophil function against C. albicans and suggest that the release of a candidastatic, iron-binding protein, lactoferrin, may contribute to the antifungal effect of PMN. Moreover, the ability to produce cytokines upon stimulation by ubiquitous microbial products such as the endotoxins points to an extraphagocytic, immunomodulatory role of PMN during infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borish L., Rosenbaum R., Albury L., Clark S. Activation of neutrophils by recombinant interleukin 6. Cell Immunol. 1989 Jul;121(2):280–289. doi: 10.1016/0008-8749(89)90026-9. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. II. The influence of a lipopolysaccharide endotoxin. J Exp Med. 1960 May 1;111:689–704. doi: 10.1084/jem.111.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantinieaux B., Hariga C., Courtoy P., Hupin J., Fondu P. Staphylococcus aureus phagocytosis. A new cytofluorometric method using FITC and paraformaldehyde. J Immunol Methods. 1989 Jul 26;121(2):203–208. doi: 10.1016/0022-1759(89)90161-0. [DOI] [PubMed] [Google Scholar]
- Catanzaro A., Wright S. D. Binding of Mycobacterium avium-Mycobacterium intracellulare to human leukocytes. Infect Immun. 1990 Sep;58(9):2951–2956. doi: 10.1128/iai.58.9.2951-2956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Cybulsky M. I., McComb D. J., Movat H. Z. Neutrophil leukocyte emigration induced by endotoxin. Mediator roles of interleukin 1 and tumor necrosis factor alpha 1. J Immunol. 1988 May 1;140(9):3144–3149. [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Djeu J. Y., Parapanissios A., Halkias D., Friedman H. A rapid [3H]glucose incorporation assay for determination of lymphoid cell-mediated inhibition of Candida albicans growth. J Immunol Methods. 1986 Aug 21;92(1):73–77. doi: 10.1016/0022-1759(86)90505-3. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Serbousek D., Blanchard D. K. Release of tumor necrosis factor by human polymorphonuclear leukocytes. Blood. 1990 Oct 1;76(7):1405–1409. [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Varesio L., Holden H. T., Herberman R. B. Metabolic requirements for the in vitro augmentation of mouse natural killer activity by interferon. Cell Immunol. 1981 Feb;58(1):49–60. doi: 10.1016/0008-8749(81)90148-9. [DOI] [PubMed] [Google Scholar]
- Doerfler M. E., Danner R. L., Shelhamer J. H., Parrillo J. E. Bacterial lipopolysaccharides prime human neutrophils for enhanced production of leukotriene B4. J Clin Invest. 1989 Mar;83(3):970–977. doi: 10.1172/JCI113983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubravec D. B., Spriggs D. R., Mannick J. A., Rodrick M. L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6758–6761. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Nandoskar M., Walz A., Goh D. H., Kowanko I. C. Effects of tumour necrosis factor alpha and interleukin-1 alpha and beta on human neutrophil migration, respiratory burst and degranulation. Int Arch Allergy Appl Immunol. 1988;86(1):82–91. doi: 10.1159/000234610. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N. E., Karle H., Andersen V., Malmquist J., Hoff G. E. Neutrophilic granulocytes in acute bacterial infection. Sequential studies on lysozyme, myeloperoxidase and lactoferrin. Clin Exp Immunol. 1976 Dec;26(3):463–468. [PMC free article] [PubMed] [Google Scholar]
- Hart D. H. Polymorphonuclear leukocyte elastase activity is increased by bacterial lipopolysaccharide: a response inhibited by glucocorticoids. Blood. 1984 Feb;63(2):421–426. [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van der Tol M. E., Thyssen R. M., van Asbeck B. S., Verhoef J. Escherichia coli lipopolysaccharides diminish and enhance cell function of human polymorphonuclear leukocytes. Infect Immun. 1983 Jul;41(1):294–301. doi: 10.1128/iai.41.1.294-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Green I., Rich R. R., Schade A. L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J Infect Dis. 1971 Dec;124(6):539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Koivuranta-Vaara P., Banda D., Goldstein I. M. Bacterial-lipopolysaccharide-induced release of lactoferrin from human polymorphonuclear leukocytes: role of monocyte-derived tumor necrosis factor alpha. Infect Immun. 1987 Dec;55(12):2956–2961. doi: 10.1128/iai.55.12.2956-2961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann A., Riedel D., Oster W., Meuer S. C., Blohm D., Mertelsmann R. H., Herrmann F. Granulocyte/macrophage colony-stimulating factor induces interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1988 Feb 1;140(3):837–839. [PubMed] [Google Scholar]
- Marucha P. T., Zeff R. A., Kreutzer D. L. Cytokine regulation of IL-1 beta gene expression in the human polymorphonuclear leukocyte. J Immunol. 1990 Nov 1;145(9):2932–2937. [PubMed] [Google Scholar]
- Michalek S. M., Moore R. N., McGhee J. R., Rosenstreich D. L., Mergenhagen S. E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980 Jan;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Mildvan D., Mathur U., Enlow R. W., Romain P. L., Winchester R. J., Colp C., Singman H., Adelsberg B. R., Spigland I. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):700–704. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Kume S. Potentiation of neutrophil function by recombinant DNA-produced interleukin 1a. J Leukoc Biol. 1987 Dec;42(6):621–627. doi: 10.1002/jlb.42.6.621. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Niwa Y., Kume S. Effect of recombinant DNA-produced tumor necrosis factor on various parameters of neutrophil function. Inflammation. 1988 Aug;12(4):297–309. doi: 10.1007/BF00915767. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Schade U., Jensen M., Wollenweber H. W., Lüderitz O., Greisman S. G. Bacterial endotoxins: chemical structure, biological activity and role in septicaemia. Scand J Infect Dis Suppl. 1982;31:8–21. [PubMed] [Google Scholar]
- Sagar A. D., Sehgal P. B., Slate D. L., Ruddle F. H. Multiple human beta interferon genes. J Exp Med. 1982 Sep 1;156(3):744–755. doi: 10.1084/jem.156.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohnle P. G., Collins-Lech C. Comparison of candidacidal and candidastatic activities of human neutrophils. Infect Immun. 1990 Aug;58(8):2696–2698. doi: 10.1128/iai.58.8.2696-2698.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. C., Lehnert B. E., Steinkamp J. A. In vitro and in vivo measurement of phagocytosis by flow cytometry. Methods Enzymol. 1986;132:183–192. doi: 10.1016/s0076-6879(86)32006-8. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Worthen G. S., Seccombe J. F., Clay K. L., Guthrie L. A., Johnston R. B., Jr The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988 May 15;140(10):3553–3559. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]



