Abstract
Reversine (RV) is the synthetic purine identified from a protein kinase-based screen of purine mimetics and it has been shown to induce muscle myoblast differentiation into progenitor cells that can be further converted into other cell lineages. Since protein kinases play a pivotal role in cell cycle control, we hypothesize that RV might affect the proliferation of cancer cells. Herein we report that RV inhibited growth of cultured human tumor cells, respectively, PC-3, HeLa, CWR22Rv1, and DU-145 cells, and induced accumulation of polyploidal cells with ≥4N DNA content. However, RV was without effect on growth of normal prostate epithelial cells. RV-treated PC-3 cells showed enlarged nuclei and an estimated 100-fold increase in cell size. Moreover, PC-3 cells treated with RV for 2-4 days were accompanied by a marked increase in the expression of p21WAF1, a modest elevation in the levels of cyclin D3 and CDK6 and concomitantly, also a substantial reduction in cyclin B and CDK1. These results suggest that RV may induce polyploidy and increase in cell size by up-regulating p21WAF1 and cyclin D3/CDK6, while simultaneously suppressing the expression of cyclin B and CDK1.
Keywords: reversine, cell cycle control, polyploidy, human cancer cells
Introduction
Reversine [2-(4-morpholinoanilino)-6-cyclohexylaminopurine, herein referred to as RV, Fig. 1A] is a novel synthetic purine recently discovered at Scripps Research Institute (1). RV was identified from a murine cell-based screen of chemically synthesized heterocyclic purines aimed at revealing information on the molecular pathways in cell specialization. These studies resulted in the proposal that RV may have regeneration-inductive potential (1). Since identification of RV utilized assays targeting the modulation of protein kinases as the functional read-out (1), and because kinases play a pivotal role in cell cycle control, it is possible that RV might affect the proliferation of cancer cells. We tested this hypothesis by adding RV to cultured human tumor cells. We found that sub-μM RV inhibited growth of PC-3, HeLa, CWR22Rv1, and DU-145 cells with accompanying accumulation of polyploidal cells showing ≥4N DNA content, but had no effect on normal prostate epithelial cells. Exposure of PC-3 cells to RV for 2-6 days resulted in nuclei enlargement and an estimated 100-fold increase in cell size. Measurement of cell cycle regulatory cyclins, cyclin-dependent protein kinases and their inhibitors in treated PC-3 cells showed that RV significantly increased the expression of p21WAF1 and also modestly elevated the levels of cyclin D3 and CDK6. In contrast, RV-treated PC-3 cells showed lowered temporal expression of cyclin B and CDK1, with little to no commensurate change in levels of G1/S and S phase regulatory protein molecules. Collectively, these results suggest that RV may act preferentially on malignant cells, via mechanisms encompassing the induction of polyploidy, expansion of cell size, and repeated rounds of S and Gap phases. The observed cellular effects of RV may in part attribute to its ability to up-regulate p21WAF1 and cyclin D3/CDK6 and concomitantly, to suppress cyclin B and CDK1.
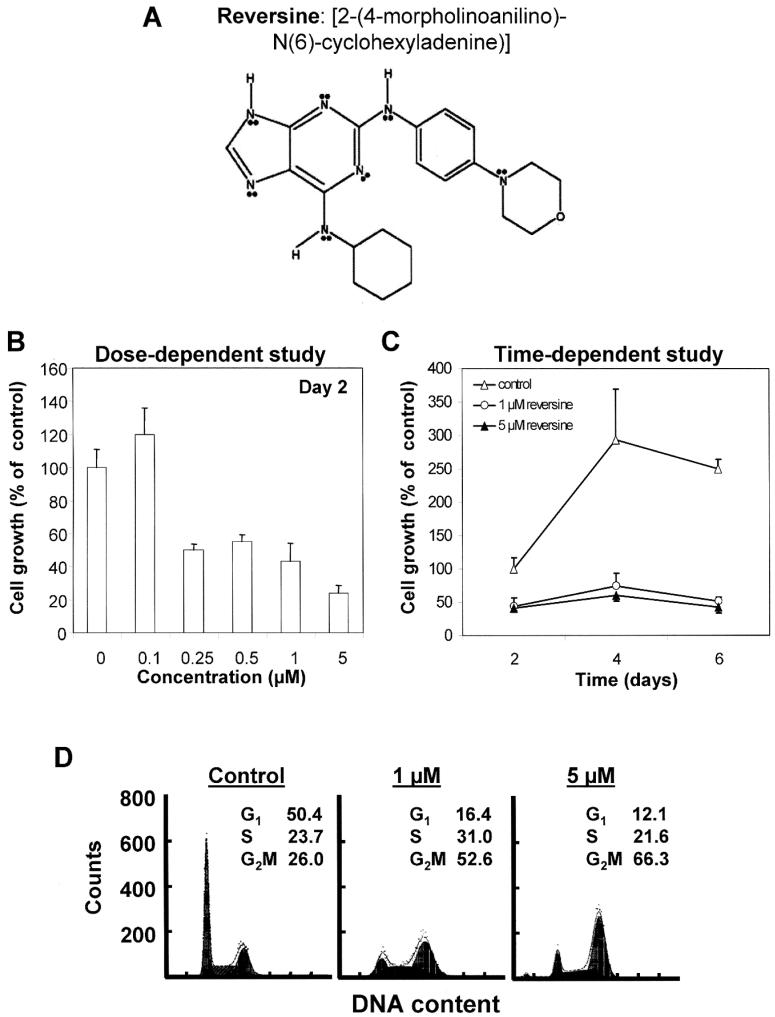
Figure 1.
Structure of RV and its effects on proliferation and cell cycle changes (B-F) in PC-3 cells. (A) Structure of RV. (B) Dose-dependent effects (2-day treatment) of RV on PC-3 cell growth (mean of 2-3 experiments). (C) Time-dependent effects of RV on PC-3 cell proliferation (mean of 2-3 experiments). (D) Effects of RV on cell cycle phase distribution. (E) Flow analysis showing formation of polyploidal cells (with ≥4N DNA) upon treatment by RV. (F) Quantitative determination of hyperploid cells with >4N DNA in RV-treated cells.
Materials and methods
RV was purchased from Calbiochem (St. Louis, MO) and was prepared as a 1 mM stock in dimethyl sulfoxide and kept at -20°C. PC-3, DU-145, HeLa and CWR22Rv1 cells were from American Type Culture Collection (ATCC, Rockville, MD). Cryopreserved and cultured prostate epithelial cells were obtained from Cambrex.
Cell cultures
Cultured human tumor cells were maintained as described previously (2,3). Normal human prostate epithelial cells were cultured in prostate epithelial cell basal media supplemented with Clonetics PrEGM™ bullet kit, as recommended by the manufacturer.
Cell proliferation
Cultured cells were incubated with increasing doses of RV and harvested at various times post-treatment. Measurement of cell proliferation and viability was as detailed in (4) while effects of RV on PC-3 cell clonogenicity was determined as described previously (5).
Flow cytometry
Cell cycle analyses were performed in various cancer cell lines, treated with 0 and ≤5 μM RV for 2, 4, and 6 days. Control and treated cells were analyzed by flow cytometry, using the procedure and method described previously (5). To determine the full range of changes in DNA content distribution, including hyperdiploid cells, the fluorescent intensity of cells from the RV-treated cultures was additionally measured at lower photomultiplier sensitivity (voltage). Since diploid G2/M and tetraploid G1 cells cannot be identified separately based on differences in DNA content alone, polyploidy was determined by gating cells with DNA content above that of G2/M (4N) cells as revealed on DNA content frequency histograms (6).
Analysis of nuclei and cell size changes in RV-treated PC-3 cells
Cells were cultured in slide chambers and fixed with 3.7% formaldehyde in PBS. Fixed cells were permeabilized with 0.1% Triton X-100, stained with 4′,6-diamidino-2-phenylindole (DAPI, 100 ng/ml) in PBS, and analyzed by fluorescent microscopy. To quantify cell size changes, images of 0, 0.5, 1.0, and 5.0 μM RV treated cells were captured by differential interference contrast (DIC) microscopy on days 2, 4, and 6 post-treatment. The observed RV-elicited cell size changes were presented as aggregate mean area calculated from the traced outline of 25-40 randomly selected cells. The significance in cell size change was determined by analysis of variance (ANOVA) and Tukey-Kramer multiple-comparison tests.
Preparation of whole cell extracts and Western blot analysis
Whole cell lysates were prepared by freeze/thaw cycles, as previously reported (5,7). Separation of proteins and immunoblot analysis using antibodies against cyclin D1 and D3, cyclin E, cyclin A, cyclin B1, CDKs 2/4/6, CDK1, and p21WAF1, and with signal for actin as control for protein loading, were as previously described (5,7). Results are presented as densitometrically quantified, β-actin-adjusted specific immunoreactive signals and are representative of two to three independent experiments.
Results
RV suppressed proliferation of tumor cells but had no effect on growth of normal prostate epithelial cells
We determined the effects of RV on growth of human tumor cells. Increasing doses of RV were added to asynchronously growing cells for 2 days and proliferation was measured using the trypan blue exclusion assay. Typical results observed are illustrated using PC-3 cells (Fig. 1B-E). RV significantly suppressed PC-3 cell proliferation in a dose-dependent manner, with 0.25 μM being sufficient to elicit a 50% decrease (Fig. 1B). Results of time course studies using 1 and 5 μM RV are shown in Fig. 1C. Untreated PC-3 cells proliferated rapidly, with growth peaking on day 4. The growth suppressive effects of either dose of RV were clearly evident on day 2 and persisted throughout the duration of the six-day experiment. To obtain information on the mechanisms for the observed anti-proliferative effects of RV, we analyzed cell cycle phase distribution changes by flow cytometry. Fig. 1D and E show flow cytograms for the distribution of DNA content in control and 1 and 5 μM RV-treated cells. At first glance, the results suggested that RV induced cell accumulation in G2/M, with 1 and 5 μM RV apparently eliciting a ≥2-fold expansion in the percentage of cells in the G2/M phase (Fig. 1D). Since diploid G2/M and tetraploid G1 cells cannot be identified separately based on differences in DNA content alone, the fluorescent intensity of cells from the RV-treated cultures was measured at lower photomultiplier sensitivity (voltage) in order to demonstrate the full range of changes in DNA content distribution, including hyperdiploid cells. Notably, this refined flow cytometric approach enabled ascertainment of polyploidy by gating cells with DNA content above that of G2/M (4N) cells as revealed on DNA content frequency histograms (6). Fig. 1E and F show a progressive, time- and dose-dependent increase in ≥4N DNA cycling cells exposed to RV, as compared to 2N/4N DNA content distribution in untreated cells. These analyses revealed that in cultures treated with 1 μM RV, presence of cells with ≥4N DNA content was evident by day 2, and became increasingly more pronounced on days 4 and 6. With 5 μM RV treatment, ≥4N DNA cells already reached a maximum on day 2 and noticeably declined after day 4 (Fig. 1F). These results suggest that RV induces polyploidy in treated cells.
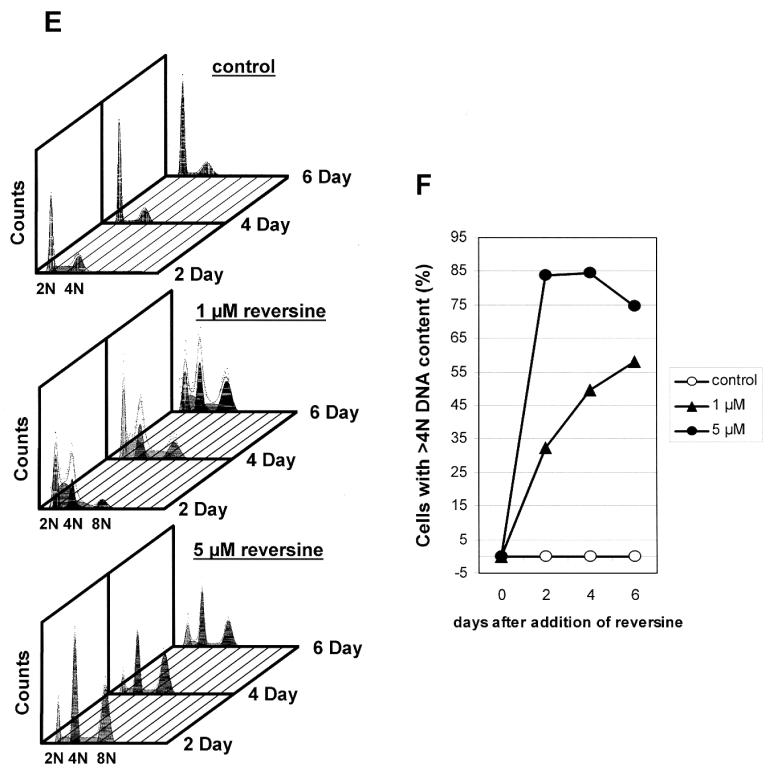
The ability of RV to affect cell growth and induce polyploidy was also tested in other malignant cell types using the trypan blue exclusion assay and by flow cytometry. Significant suppression of cell proliferation (data not shown) together with an expansion in proportion of ≥4N DNA content, polyploidal cells as demonstrated by flow cytometric analysis were also observed in HeLa, CWR22Rv1 cells, and DU-145 cells after a 2-day treatment with 1 and 5 μM RV (Fig. 2A-C).
Figure 2.
Induction of polyploidy in 2 day RV-treated HeLa (A), CWR22Rv1 (B), DU-145 (C) and normal prostate epithelial PrEc (D) cells. Flow cytometry was used to demonstrate the presence of polyploidal cells (≥4N DNA). N.D., not determined.
Since RV resulted in potent inhibition of proliferation and cell cycle control in various human malignant cells, it was of interest to determine whether it also affected growth of non-malignant cells. Accordingly, prostate epithelial cells obtained from commercial sources were incubated with media supplemented with 1 or 5 μM RV and effects on cell cycle distribution were monitored by flow cytometry. Fig. 2D showed that at these concentrations, RV had negligible effect on their cellular DNA content profile.
Induction of polyploidy and cell enlargement in PC-3 cells by RV
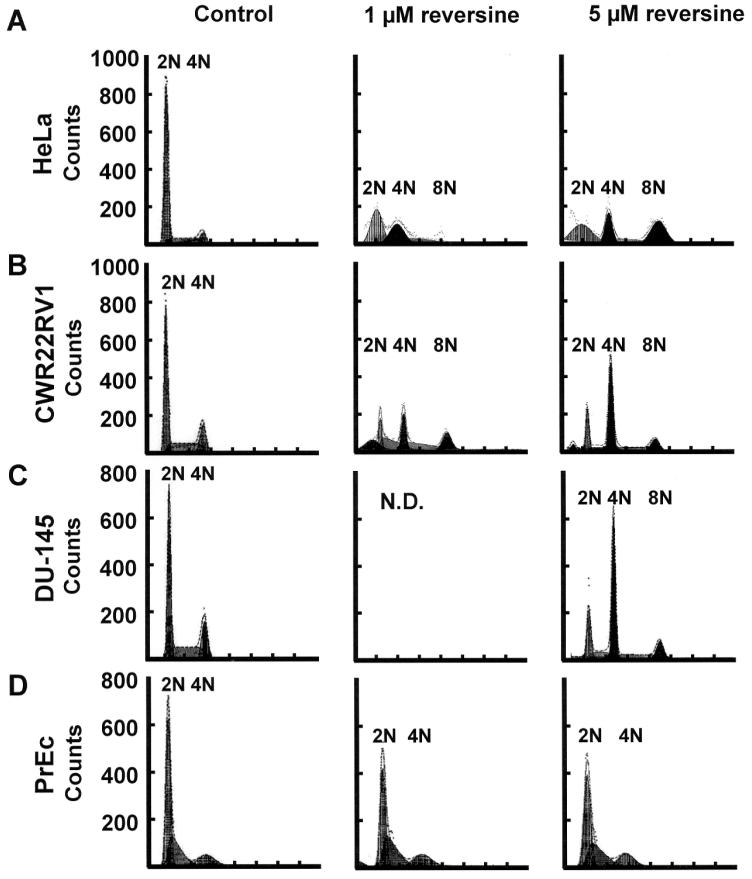
To confirm that RV induces polyploidy, control and RV-treated PC-3 cells were stained with DAPI, visualized, and analyzed by microscopy. Multi-lobed shaped, larger nuclei were found on day 4, 1 μM RV-treated cells (Fig. 3A, right panel), compared to the control cells (left panel). Cells treated with 5 μM RV showed stronger DAPI-stained nuclei with multi-lobed shapes (not shown). These results suggest that RV induced the formation of polyploidal cells. Polyploidy was evident at 8 h of exposure to 5 μM RV (not shown) and increased over time, clearly becoming dominant (≥65%) by day 4.
Figure 3.
Induction of polyploidy in RV-treated PC-3 cells. (A) Analysis of day 4, 1 μM RV (top right panel)-treated cells compared to the control cells (top left panel). Cells were stained with DAPI to reveal nuclei and with anti-actin to identify the entire outline of cells. Actin staining showed enlarged RV-treated cells (middle right panel), compared to control cells (middle left panel). Merge images (bottom panel) localize the nuclei and the cytoplasm. (B) Morphological [as assayed by differential interference contrast (DIC) microscopy] analysis illustrating cell size changes in PC-3 cells induced by increasing concentrations of RV at 2, 4, and 6 days of treatment. (C) Quantitation of cell size changes in PC-3 cells induced by increasing concentrations of RV at 2, 4, and 6 days of treatment.
Quantitative morphological and quantitative cell size changes were also observed in RV-treated cells, as analyzed by DIC microscopy (Fig. 3B and C). The gross enlargement of RV-treated cells was most vividly illustrated by a dose- and time-dependent increase in measured area, confirming the appearance of ‘giant’ cells that peaked on days 6 and 4 for 1 and 5 μM RV-treated cells, respectively. Notably, the proportion of ‘giant’ cells treated with either dose of RV was reduced on day 6, compared to day 2- and 4-treated cells, suggesting that cell viability may be compromised by prolonged exposure to RV (Fig. 3B). To accurately determine overall cell size changes, multiple DIC image sets of control and RV-treated cells were taken on days 2, 4, and 6, and a mean area was calculated as described in Materials and methods. Statistical analysis showed that on day 4, the mean areas of 1 and 5 μM RV-treated cells were significantly increased compared to the control. For day 6 samples, all doses of RV-treated cells showed a significant increase in cell size. The results of cell size measurements in two separate experiments are shown in Fig. 3C.
Expression of regulatory proteins involved in G1, G1/S and S phase transition in RV-treated PC-3 cells
Treatment of PC-3 cells by RV results in enlarged cell size, aberrant cell cycling, and polyploidization. These observations raise the possibility that RV exerts a pronounced modulatory effect on the expression of cell cycle phase regulatory proteins. This hypothesis was tested by quantifying temporal changes in the expression of several cyclins, cyclin-dependent protein kinases and their inhibitors in control and RV-treated PC-3 cells. Robust expression of cyclin D3 occurred on day 2, followed by a precipitous decline on day 4 (Fig. 4A and B) and day 6 (data not shown). Treatment by RV did not affect cyclin D3 level on day 2 but attenuated the subsequent time-dependent decline in cyclin D3. A similar result was also observed in CDK6. However, RV treatment had only minimal effect on the temporal expression of cyclins D1, E and CDK2 and 4, as well as on the level of phosphorylated and total Rb (data not shown). Conceivably, the elevation of cyclin D3 and CDK6 on day 4 could enable the RV-treated cells to traverse the G1 checkpoint for engagement in a new round of DNA synthesis.
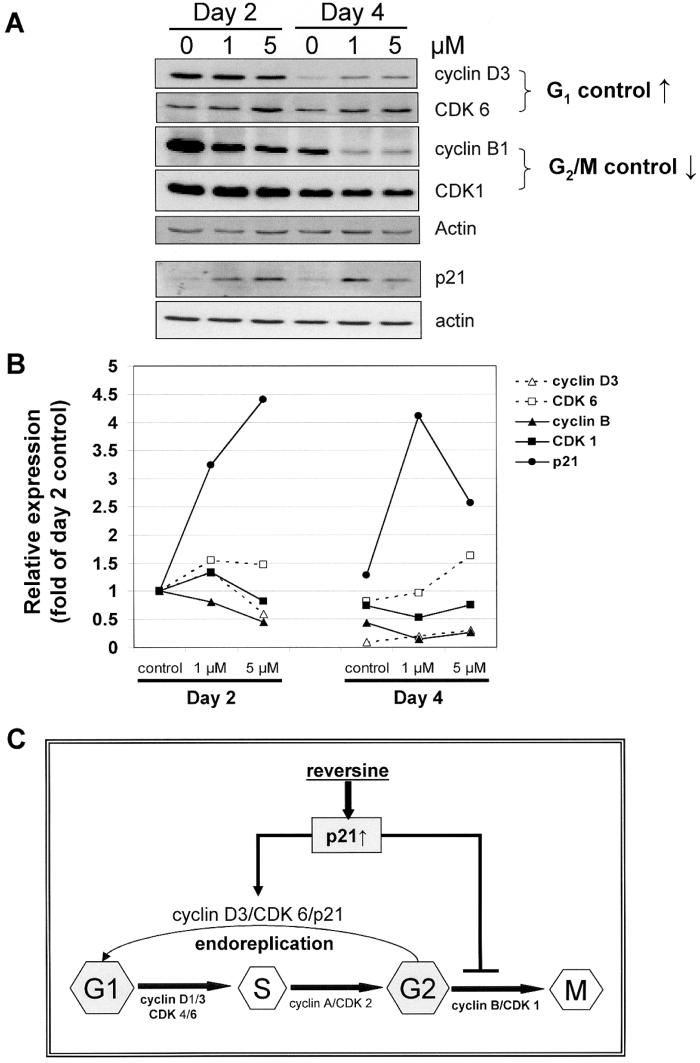
Figure 4.
Changes in cell cycle regulatory proteins involved in G1, G1/S to S, and S to G2/M transition in control and 2- and 4-day RV-treated PC-3 cells. (A) Expression of cyclins D3, CDK 6, cyclin B1, CDK1, p21 and actin was determined by immunoblot analysis as detailed in Materials and methods, and then quantitatively determined, with actin expression used to adjust for equal protein loading for each of the proteins analyzed. Results shown are representative of two independent experiments. (B) Quantification of temporal changes in cyclin D3, CDK 6, cyclin B1, CDK1, and p21 in control and RV-treated PC-3 cells, using procedures identical to those described in (A). (C) A model for induction of polyploidy by RV. RV is proposed to increase degradation of CDK1/cyclin B1, in coordination with up-regulation of p21WAF1, which promote the exit from faulty mitosis followed by entry of cells into the G1 phase and re-commitment to additional rounds of DNA replication without going through cytokinesis. These changes have the net effect of increasing the propensity of RV-treated cells for mitotic slippage and induction of polyploidy.
We next analyzed the expression of the cyclin B1, which, functioning in complex with CDK1, serves as the switch for entry and transition through mitosis, also known as the M phase. Copious expression of cyclin B1 was found in day 2 followed by a time-dependent decline on day 4 in control PC-3 cells, whereas cyclin B1 level was dose-dependently suppressed by 1 and 5 μM RV on day 2 and more substantially reduced on day 4 (Fig. 4A and B) and day 6 (data not shown).
We also measured changes in total CDK1, a key sensor for the G2/M phase transition. In control PC-3 cells, CDK1 was maximally expressed on day 2, after which its level vividly declined on days 4 and 6. Treatment by 1 and 5 μM RV slightly increased the expression of CDK1 on day 2, and also accelerated the time-dependent decrease in CDK1 (Fig. 4A and B).
Changes in p21WAF1 were next investigated. As a member of the Cip/Kip family (8), p21WAF1 has been reported to control DNA endoreduplication and the expression of proteins critical for mitosis (9-11). RV treatment resulted in copious, dose-dependent induction of p21WAF1 expression; 1 and 5 μM RV elicited a 3- and 4.5-fold increase in p21WAF1 levels on day 2 (Fig. 4A and B). Long-term exposure to a high dose of RV significantly reduced p21WAF1 levels. Other CDK inhibitors, e.g., p27 and p16 were not affected by RV (data not shown). The increased expression of p21WAF1 together with the modest elevation in the levels of cyclin D3 and CDK6, and reduced amount of cyclin B1 and CDK1, supports the interpretation that RV-treated cells may progress from S into G2 for entry into new rounds of cell cycle without completion of mitosis.
Discussion
RV was identified by Schultz and coworkers in 2004 as a substituted purine that, in murine C2C12 cells, induced their transformation into progenitors that were further directed to undergo osteogenesis or adipogenesis, depending on conditions of the culture media (1). Similar results were reported by Venerando and coworkers who additionally showed that RV converted primary murine and human dermal fibroblasts into myogenic-competent cells, both in vitro and in vivo (12). However, Jacobson and coworkers failed to show the dedifferentiation properties of RV using the same culture system (13).
Although the actual mechanisms for the dedifferentiation effects of RV have not been totally elucidated, possible involvement of protein kinases and phosphorylation events was proposed since the identity of RV was based on assays using modulation of protein kinases as the functional readout. Protein kinases play a pivotal role in cell cycle control, suggesting the possibility that RV might affect the proliferation of cancer cells. Therefore, we tested the growth modulatory effects of RV in a variety of human malignant cells. Using asynchronous PC-3 and other cancer cell cultures, we showed that a 2 day exposure to 0.25-0.5 μM RV (Fig. 1B) inhibited cell proliferation and induced polyploidal cell formation, characterized by a progressive enlargement of cell size and nuclei, the prevalence of cells with ≥4N DNA, and uncompleted mitosis. Similar results were also observed in HeLa, CWR22Rv1 and DU-145 malignant cells (Figs. 1-3). Additionally, RV-treated PC-3 cells showed significantly increased expression of p21WAF1, with accompanying, temporal decreases of CDK1 as well as cyclin B1 (Fig. 4).
Formation of giant polyploidy cells has been most frequently shown in tumors with mutated, inactivated, or totally absent p53, particularly upon exposure to ionizing radiation (6,14), and various antitumor agents including taxol, vincristine and nocodazole (15,16). Such giant cells can be formed by cell fusion, endoreplication (also known as endomitosis), and by abortive cell cycling (17,18). In normal cells, polyploidy may function as an adaptative response to metabolic stress and genotoxic damage. Because of their intrinsic genetic instability, polyploidal cells may also promote aneuploidy, a status that increases the risk for cancer (19). Notably, polyploidal tumor cells also show increased sensitivity to chemotherapeutic agents or γ-irradiation by facilitating delayed apoptotic or mitotic cell death (6,20).
The induction of polyploidy in p53-null PC-3 cells in response to RV may occur by an endoreplication mechanism, i.e., uncoupling DNA replication from cell division as a result of mitotic slippage and/or deficiency in the completion of mitosis. We propose that RV acts by a mechanism involving the accelerated degradation of the complex CDK1/cyclin B1, coordinated with the up-regulation of p21WAF1, which promote the exit from faulty mitosis followed by entry of cells into the G1 phase and re-commitment to DNA replication without cytokinesis. These changes have the net effect of increasing the propensity for mitotic slippage and induction of polyploidy (Fig. 4C). In the case of PC-3 cells, considered as a model of hormone refractory prostate cancer (HRPC), targeting the G2/M phase of the cell cycle by RV may be particularly significant since the G1/S checkpoint is largely defective in HRPC cells, thus requiring that the G2/M checkpoint take on greater importance for monitoring accuracy and timeliness of DNA replication and repair in order to minimize passing DNA damages to the daughter cells.
On first glance, the proposed mechanism appears to contradict the most extensively studied function of p21WAF1 as an inhibitor of key cell cycle regulatory cyclin-dependent kinases, which in principle, should serve to impose restrictions on DNA endoreplication. We suggest that the observed p21WAF1 increase is sufficiently robust to overcome the threshold level required to block S-phase entry and subsequent DNA endoreplication, as well as for the proposed roles of p21WAF1 in retarding cell growth, inducing abnormal mitosis and endoreduplication (9,11), and inhibiting a set of genes involved in mitosis, DNA replication, segregation, and repair (21).
Induction of p21WAF1 occurs both by p53-dependent and p53-independent mechanisms. By and large, the timely expression of p21WAF1 is controlled by the availability of the tumor suppressor, p53 (22); however, ample evidence has been obtained showing that p21WAF1 can also be induced in p53-null cells, via a transcription factor SP1-mediated mechanism (23). Because of the p53 null status of PC-3 cells, it is likely that induction of p21WAF1 in response to RV, involves a p53-independent mechanism.
The mechanisms by which RV up-regulates p21WAF1 remain undetermined. Since RV was identified using a kinase-directed scaffold screen (1), one might surmise that RV may affect the state of phosphorylation and/or dephosphorylation of kinases and phosphatases including ones with an integral role in the expression of p21WAF1. In this context, it is notable that the transcription of p21WAF1 has been shown to coordinate with a number of kinase-mediated signaling pathways. For instance, c-Jun, a co-activator of SP1 in the control of p21WAF1 transcription (24), as well as SP1 is known to be regulated by kinases and phosphatases (25,26). Studies are currently underway to investigate these possibilities in the up-regulation of p21WAF1 by RV.
Acknowledgements
Wilson D. Lee provided assistance in the analysis of DIC captured images. Dr Zhirong Wang determined some of the changes in cell cycle regulatory proteins. Jan Kunicki contributed in the cell cycle analysis.
References
- 1.Chen S, Zhang Q, Wu X, Schultz PG, Ding S. Dedifferentiation of lineage-committed cells by a small molecule. J Am Chem Soc. 2004;126:410–411. doi: 10.1021/ja037390k. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh TC, Wu JM. Effects of the retinoid 4-HPR on human prostate growth, induction of apoptosis and modulation of androgen receptor (AR) and prostate specific antigen (PSA) expression. Prostate. 1997;33:97–104. doi: 10.1002/(sici)1097-0045(19971001)33:2<97::aid-pros3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh TC, Lu X, Guo J, Xiong W, Kunicki J, Darzynkiewicz Z, Wu JM. Effects of herbal preparation Equiguard on hormone-responsive and hormone-refractory prostate carcinoma cells: Mechanistic studies. Int J Oncol. 2002;20:681–689. [PubMed] [Google Scholar]
- 5.Hsieh TC, Wang Z, Hamby CV, Wu JM. Inhibition of melanoma cell proliferation by resveratrol is correlated with upregulation of quinone reductase 2 and p53. Biochem Biophys Res Commun. 2005;334:223–230. doi: 10.1016/j.bbrc.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 6.Kim JY, Kim CH, Stratford IJ, Patterson AV, Hendry JH. The bioreductive agent RH1 and gamma-irradiation both cause G2/M cell cycle phase arrest and polyploidy in a p53-mutated human breast cancer cell line. Int J Radiat Oncol Biol Phys. 2004;58:376–385. doi: 10.1016/j.ijrobp.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 7.Di Pietrantonio AM, Hsieh TC, Olson SC, Wu JM. Regulation of G1/S transition and induction of apoptosis in HL-60 leukemia cells by fenretinide (4HPR) Int J Cancer. 1998;78:53–61. doi: 10.1002/(sici)1097-0215(19980925)78:1<53::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Nurse PM. Nobel Lecture. Cyclin dependent kinases and cell cycle control. Biosci Rep. 2002;22:487–499. doi: 10.1023/a:1022017701871. [DOI] [PubMed] [Google Scholar]
- 9.Bates S, Ryan KM, Phillips AC, Vousden KH. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–1703. doi: 10.1038/sj.onc.1202104. [DOI] [PubMed] [Google Scholar]
- 10.Niculescu AB, III, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang BD, Broude EV, Fang J, Kalinichenko TV, Abdryashitov R, Poole JC, Roninson IB. p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene. 2000;19:2165–2170. doi: 10.1038/sj.onc.1203573. [DOI] [PubMed] [Google Scholar]
- 12.Anastasia L, Sampaolesi M, Papini N, Oleari D, Lamorte G, Tringali C, Monti E, Galli D, Tettamanti G, Cossu G, Venerando B. Reversine-treated fibroblasts acquire myogenic competence in vitro and in regenerating skeletal muscle. Cell Death Differ. doi: 10.1038/sj.cdd.4401958. In press. [DOI] [PubMed] [Google Scholar]
- 13.Perreira M, Jiang JK, Klutz AM, Gao ZG, Shainberg A, Lu C, Thomas CJ, Jacobson KA. ‘Reversine’ and its 2-substituted adenine derivatives as potent and selective A3 adenosine receptor antagonists. J Med Chem. 2005;48:4910–4918. doi: 10.1021/jm050221l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ianzini F, Cherubini R, Mackey MA. Mitotic catastrophe induced by exposure of V79 Chinese hamster cells to low-energy protons. Int J Radiat Biol. 1999;75:717–723. doi: 10.1080/095530099140050. [DOI] [PubMed] [Google Scholar]
- 15.Hong FD, Chen J, Donovan S, Schneider N, Nisen PD. Taxol, vincristine or nocodazole induces lethality in G1-checkpoint-defective human astrocytoma U373MG cells by triggering hyperploid progression. Carcinogenesis. 1999;20:1161–1168. doi: 10.1093/carcin/20.7.1161. [DOI] [PubMed] [Google Scholar]
- 16.Michalakis J, Georgatos SD, Romanos J, Koutala H, Georgoulias V, Tsiftsis D, Theodoropoulos PA. Micromolar taxol, with or without hyperthermia, induces mitotic catastrophe and cell necrosis in HeLa cells. Cancer Chemother Pharmacol. 2005;56:615–622. doi: 10.1007/s00280-005-1002-7. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(Suppl 2):1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- 18.Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 19.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 20.Lanzi C, Cassinelli G, Cuccuru G, Supino R, Zuco V, Ferlini C, Scambia G, Zunino F. Cell cycle checkpoint efficiency and cellular response to paclitaxel in prostate cancer cells. Prostate. 2001;48:254–264. doi: 10.1002/pros.1105. [DOI] [PubMed] [Google Scholar]
- 21.Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcino-genesis, senescence, and age-related diseases. Proc Natl Acad Sci USA. 2000;97:4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, Fujita T, Ohtani-Fujita N, Matsukawa Y, Tokino T, Yamagishi H, Oka T, Nomura H, Sakai T. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J Biol Chem. 1997;272:22199–22206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- 23.Sowa Y, Orita T, Minamikawa S, Nakano K, Mizuno T, Nomura H, Sakai T. Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem Biophys Res Commun. 1997;241:142–150. doi: 10.1006/bbrc.1997.7786. [DOI] [PubMed] [Google Scholar]
- 24.Kardassis D, Papakosta P, Pardali K, Moustakas A. c-Jun transactivates the promoter of the human p21(WAF1/Cip1) gene by acting as a superactivator of the ubiquitous transcription factor Sp1. J Biol Chem. 1999;274:29572–29581. doi: 10.1074/jbc.274.41.29572. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- 26.Inostroza J, Saenz L, Calaf G, Cabello G, Parra E. Role of the phosphatase PP4 in the activation of JNK-1 in prostate carcinoma cell lines PC-3 and LNCaP resulting in increased AP-1 and EGR-1 activity. Biol Res. 2005;38:163–178. doi: 10.4067/s0716-97602005000200006. [DOI] [PubMed] [Google Scholar]