Abstract
Patients with isolated ZIC4 antibodies usually have paraneoplastic cerebellar degeneration (PCD) and small cell lung cancer (SCLC) but the frequency is unknown. We analyzed the presence of ZIC1, ZIC2 and ZIC4 antibodies in 27 patients with PCD and SCLC negative for other onconeural antibodies. ZIC antibodies were detected in nitrocellulose filters with phage plaques. Four (15%) PCD sera recognized ZIC2. Three of these positive sera also reacted with ZIC1 and two with ZIC4. Our study suggests that 1) the incidence of isolated ZIC antibodies is low in PCD patients and SCLC and 2) ZIC antibodies are probably directed to epitopes shared by the three ZIC proteins.
Keywords: ZIC antibodies, Paraneoplastic cerebellar degeneration, Small cell lung cancer, Onconeural antibodies
1. Introduction
Around 50% of patients with paraneoplastic cerebellar degeneration (PCD) and small cell lung carcinoma (SCLC) do not harbor onconeural or voltage-gated calcium channel antibodies (Graus et al., 2002) and the diagnosis is based on the clinical picture and demonstration of the tumor (Graus et al., 2004). Immunity against ZIC (derived from zinc fingers of the cerebellum) proteins is common in patients with SCLC (Güre et al., 2000). The ZIC family includes five DNA-binding proteins that are structurally similar to each other (Grinberg and Millen, 2005). We previously found ZIC4 antibodies in 49 (29%) of 167 patients with paraneoplastic neurological syndromes and SCLC whereas the frequency of ZIC4 antibodies in patients with SCLC alone was significatively lower (16%) (Bataller et al., 2004). Most of the patients with ZIC4 antibodies also had Hu or CV2 antibodies and presented with different paraneoplastic neurological syndromes. In contrast, eight of the nine patients with isolated ZIC4 antibodies presented with PCD (Bataller et al., 2004). However, the frequency of antibodies against ZIC4 or other members of the ZIC family in PCD associated with SCLC is unknown. In the present study, we analyze the presence of antibodies against ZIC1, ZIC2 and ZIC4 in a series of PCD patients and SCLC to ascertain its value in the diagnosis of this disorder.
2. Materials and methods
2.1. Patients
We selected from our database, 27 patients with the final diagnosis of PCD with SCLC and no onconeural antibodies. The inclusion criteria were the presence of an isolated or clearly predominant cerebellar syndrome of unknown cause, the diagnosis of SCLC, and absence of onconeural antibodies. Serum and CSF, when available, were evaluated for the presence of onconeural antibodies (Hu, Yo, Ri, CV2, Tr, amphiphysin, Ma2, ANNA3, PCA2) by immunohistochemistry on frozen sections of rat cerebellum according to standardized techniques previously reported in detail (Saiz et al., 1997). Fourteen of them (52%) had voltage-gated calcium channel antibodies (VGCC) detected by radioimmunoassay (Graus et al., 2002).
2.2. Isolation of cDNA clones and detection of ZIC antibodies
A Uni-ZAP XR Library (Stratagene, La Jolla, CA) from human cerebellum was immunoscreened with a with a pool of five sera (each diluted 1:1000) of PCD patients as previously reported (Bataller et al., 2002, 2003). Four sera were negative by immunohistochemistry or immunoblot. The fifth serum was positive for ZIC4 antibodies from the previous study (Bataller et al., 2004). Phage ZIC positive clones were subcloned in pBluescript using the in vivo excision phage rescue protocol (Stratagene, La Jolla, CA) and they were full length sequenced.
The presence of ZIC antibodies in patient's sera was examined using nitrocellulose filters with mixed phage plaques (50% of plaques from positive clones and 50% from irrelevant clones). Filters were cut into four pieces, each one incubated with different patient's sera (dilution 1:1000) and developed by an avidin biotin immunoperoxidase technique as described (Bataller et al., 2003). Filters were scored by two investigators (LS and FG) blind to the clinical diagnosis.
3. Results
3.1. Analysis of the ZIC clones
We isolated by immunoscreening of a human cerebellar expression library 12 ZIC1 clones identical to NM_003412 (NCBI accession number), 11 ZIC2 clones (NM_007129), and four ZIC4 clones (NM_032153.3). The clones with the longest insert were selected to perform further studies. ZIC1 and ZIC2 clones included the complete predicted open reading whereas the analysis of the predicted translation of the ZIC4 gene revealed that the protein lacked the first 22 amino acids of the coding sequence but contained the zinc finger motifs.
3.2. Frequency of ZIC antibodies in PCD patients with SCLC
Filters with plaques containing ZIC2 protein reacted with the serum of 4 of 22 PCD patients (15%). Three of these positive sera also reacted with ZIC1 and two with ZIC4. The strongest immunoreactivity was always observed in plaques containing ZIC2 protein (Fig. 1). The serum with the weakest reaction with ZIC2 was the only one that was negative with ZIC1 and ZIC4 proteins. The clinical features of ZIC-positive patients were not different from the rest of the series. The presence of ZIC antibodies did not correlate with that of VGCC antibodies. ZIC antibodies were present in the serum of two patients without and two with VGCC antibodies. CSF of the ZIC-positive patients was not available for study. As expected from previous studies with other antibodies (Graus et al., 1997), the immunoblot assay to detect ZIC antibodies was more sensitive than the detection by immunohistochemistry and only two of the positive sera stained the granular cells of the cerebellum on rat sections (Bataller et al., 2002).
Fig. 1.
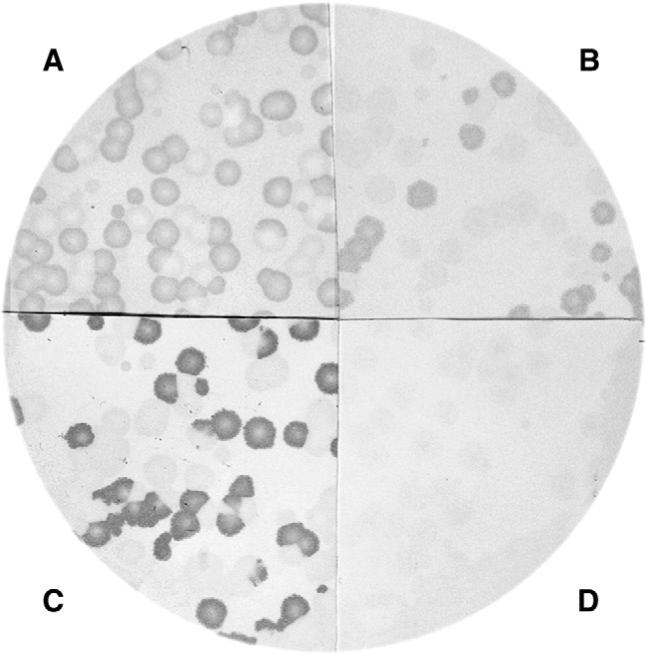
Detection of ZIC antibodies with phage plaques (see Materials and methods). The four quadrants contained A) ZIC4, B) ZIC1, C) ZIC2, and D) irrelevant phages that were immunoreacted with a positive serum that recognized the three ZIC proteins. Note that immunoreactivity was much stronger with the ZIC2 phage.
4. Discussion
The Purkinje cell of the cerebellum is a frequent target of the immune response raised against the underlying cancer. The antigen identified usually depends on the tumor type and the great majority of PCD patients associated with a particular tumor develop a predictable immune response (for example, anti-Yo antibodies in PCD and gynecological cancer) (Shams'ili et al., 2003). However, this is not the case when PCD associates with SCLC. In this clinical setting, up to 41% of PCD patients present anti-VGCC antibodies with or without associated Lambert–Eaton myasthenic syndrome, 23% anti-Hu antibodies (Graus et al., 2002), and a minority, other antibodies linked to SCLC such as anti-CV2, (also known as CRMP5) (Yu et al., 2001), anti-amphiphysin (Antoine et al., 1999), anti-PCA2 (Vernino and Lennon, 2000), and anti-ANNA3 (Chan et al., 2001).
In the present study, ZIC antibodies were present in 15% of PCD patients with SCLC and seronegative for known onconeural antibodies (Graus et al., 2004). We could not analyze the presence of intrathecal synthesis of ZIC antibodies in our seropositive patients, a data that would implicate the immune response against ZIC in the pathogenesis of PCD (Bataller et al., 2002). Therefore, we cannot exclude that the presence of ZIC antibodies in our patients was a simple indication that the underlying cancer was a SCLC. Despite this, detection of ZIC antibodies in a patient with symptoms of PCD should prompt a screening for SCLC.
The ZIC gene family includes five genes that highly conserved across evolution. They are involved in several development processes particularly the formation of the cerebellum (Aruga et al., 1994; Grinberg and Millen, 2005). All ZIC genes continue to be expressed in the granular cells of the adult cerebellum and heterozygous deletion of both ZIC1 and ZIC4 is implicated in the Dandy–Walker malformation a congenital disorder that shows hypoplasia of the cerebellar vermix (Merzdort, 2007). Consistent with this observation, homocygous deletion of ZIC1 in mice was found associated with cerebellar hypoplasia (Aruga et al., 1998). Taken together, these data emphasizes the important role of ZIC genes in cerebellar development.
ZIC genes encode proteins that contain five zinc-finger domains (Grinberg and Millen, 2005). Four domains are nearly identical in the five ZIC proteins whereas the first zinc-finger shows minor differences (Grinberg and Millen, 2005). In our previous work, we analyzed the presence of ZIC4 antibodies in a large series of patients with different paraneoplastic neurological syndromes (Bataller et al., 2004). The study did not clarify if the antibodies were specific to ZIC4 because reactivity against other ZIC family members was not done (Bataller et al., 2004). In our present screening, all ZIC1, ZIC2, and ZIC4 clones were recognized by the same serum. Sera that reacted more strongly with ZIC2 also recognized ZIC1 and ZIC4 suggesting that the immunoreactivity of these sera is directed primarily against the conserved zinc-finger domains of ZIC proteins. In line with this explanation, ZIC2 antibodies detected by serologic analysis of expression of cDNA libraries derived from SCLC lines, reacted with a ZIC2 clone that lacked the N-terminal 207 amino acids but contained the zinc-finger domain (Vural et al., 2005). This type of immune response has been observed with anti-Hu and other onconeural antibodies that recognize immunodominant epitopes present is specific domains shared by different members of the antigen family (Manley et al., 1995).
Which ZIC protein is preferentially expressed in SCLC is unclear. ZIC2 mRNA was detected in 80% of 11 SCLC cell lines (Güre et al., 2000). By contrast ZIC1 protein was not detected in six samples of lung cancer, including two with SCLC, that were immunoreacted with a ZIC1 monoclonal antibody (Yokota et al., 1996). These data suggest that the expression of ZIC2 in SCLC plays a critical role in triggering the anti-ZIC immune response in PCD patients. However, more studies on the expression of the different ZIC proteins in SCLC are needed to confirm this hypothesis.
To conclude, our data emphasize that the determination of ZIC antibodies identify an additional 15% of patients with PCD and SCLC that do not show onconeural antibodies. Future studies using new strategies to isolate novel antibodies not detected by conventional immunohistochemistry will be necessary to increase our knowledge on the immunomic repertoire of the PCD associated with SCLC and provide new tools to improve our diagnostic accuracy of this disorder.
Acknowledgements
Supported by grant PI030028 Fondo de Investigaciones Sanitarias, Madrid, Spain (FG), and by RO1CA107192 NCI/NIH (JD). The authors thank Eva Caballero for excellent technical assistance.
Footnotes
This study is dedicated to the memory of Professor John Newsom-Davis whose work inspired our interest in neuroimmunology.
References
- Antoine JC, Absi L, Honnorat J, Boulesteix J, de Brouker T, Vial C, Butler M, De Camilli P, Michel D. Antiamphiphysin antibodies are associated with various paraneoplastic syndromes and tumors. Arch. Neurol. 1999;56:172–177. doi: 10.1001/archneur.56.2.172. [DOI] [PubMed] [Google Scholar]
- Aruga J, Yokota N, Hashimoto M, Furuichi T, Fukuda M, Mikoshiba K. A novel zinc finger protein, ZIC, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J. Neurochem. 1994;63:1880–1890. doi: 10.1046/j.1471-4159.1994.63051880.x. [DOI] [PubMed] [Google Scholar]
- Aruga J, Minowa O, Yaginuma H, Kuno J, Nagai T, Noda T, Mikoshiba K. Mouse Zic1 is involved in cerebellar development. J. Neurosci. 1998;18:284–293. doi: 10.1523/JNEUROSCI.18-01-00284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller L, Wade DF, Fuller GN, Rosenfeld MR, Dalmau J. Cerebellar degeneration and autoimmunity to zinc-finger proteins of the cerebellum. Neurology. 2002;59:1985–1987. doi: 10.1212/01.wnl.0000038352.01415.ce. [DOI] [PubMed] [Google Scholar]
- Bataller L, Wade DF, Graus F, Rosenfeld MR, Dalmau J. The MAZ protein is an autoantigen of Hodgkin's disease and paraneoplastic cerebellar dysfunction. Ann. Neurol. 2003;53:123–127. doi: 10.1002/ana.10434. [DOI] [PubMed] [Google Scholar]
- Bataller L, Wade DF, Graus F, Stacey HD, Rosenfeld MR, Dalmau J. Antibodies to Zic4 in paraneoplastic neurologic disorders and small-cell lung cancer. Neurology. 2004;62:778–782. doi: 10.1212/01.wnl.0000113749.77217.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Vernino S, Lennon VA. ANNA-3 anti-neuronal nuclear antibody: marker of lung cancer-related autoimmunity. Ann. Neurol. 2001;50:301–311. doi: 10.1002/ana.1127. [DOI] [PubMed] [Google Scholar]
- Graus F, Dalmau J, Reñé R, Tora M, Malats N, Verschuuren JJ, Cardenal F, Viñolas N, Gracía del Muro J, Vadell C, Mason WP, Rosell R, Posner JB, Real FX. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J. Clin. Oncol. 1997;15:2866–2872. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- Graus F, Lang B, Pozo-Rosich P, Saiz A, Casamitjana R, Vincent A. P/Q type calcium channel antibodies in paraneoplastic in paraneoplastic cerebellar degeneration with lung cancer. Neurology. 2002;59:764–766. doi: 10.1212/wnl.59.5.764. [DOI] [PubMed] [Google Scholar]
- Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Sillevis Smitt P, Vedeler Ch., Verschuuren JJGM, Vincent A, Voltz R, for the paraneoplastic neurological syndrome Euronetwork Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg I, Millen KJ. The ZIC gene family in development and disease. Clin. Genet. 2005;67:290–296. doi: 10.1111/j.1399-0004.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- Güre AO, Stockert E, Scanlan MJ, Keresztes RS, Jäger D, Altorki NK, Old LJ, Chen Y. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4198–4203. doi: 10.1073/pnas.97.8.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Sillevis Smitt P, Dalmau J, Posner JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Neurology. 1995;38:102–110. doi: 10.1002/ana.410380117. [DOI] [PubMed] [Google Scholar]
- Merzdort C. Emerging roles of ZIC genes in early development. Dev. Dyn. 2007;236:922–940. doi: 10.1002/dvdy.21098. [DOI] [PubMed] [Google Scholar]
- Saiz A, Arpa J, Sagasta A, Casamitjana R, Zarranz JJ, Tolosa E, Graus F. Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late-onset insulin-dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology. 1997;49:1026–1030. doi: 10.1212/wnl.49.4.1026. [DOI] [PubMed] [Google Scholar]
- Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, van der Holt B, Vecht Ch., Sillevis Smitt P. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies:analysis of 50 patients. Brain. 2003;126:1409–1418. doi: 10.1093/brain/awg133. [DOI] [PubMed] [Google Scholar]
- Vernino S, Lennon VA. New Purkinje cell antibody (PCA-2): marker of lung cancer-related neurological autoimmunity. Ann. Neurol. 2000;47:297–305. [PubMed] [Google Scholar]
- Vural B, Chen L, Saip P, Chen Y, Ustuner Z, Gonen M, Simpson AJG, Old LJ, Ozbek U, Güre AO. Frequency of SOX group B (SOX 1, 2, 3) and ZIC2 antibodies in Turkish patients with small cell lung carcinoma and their correlation with clinical parameters. Cancer. 2005;103:2575–2583. doi: 10.1002/cncr.21088. [DOI] [PubMed] [Google Scholar]
- Yokota N, Aruga J, Takai S, Yamada K, Hamazaki M, Iwase T, Sugimura H, Mikoshiba K. Predominant expression of human Zic in cerebellar granule cell lineage and medulloblastoma. Cancer Res. 1996;56:377–383. [PubMed] [Google Scholar]
- Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann. Neurol. 2001;49:146–154. [PubMed] [Google Scholar]


