Abstract
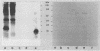
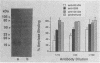
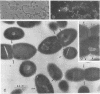
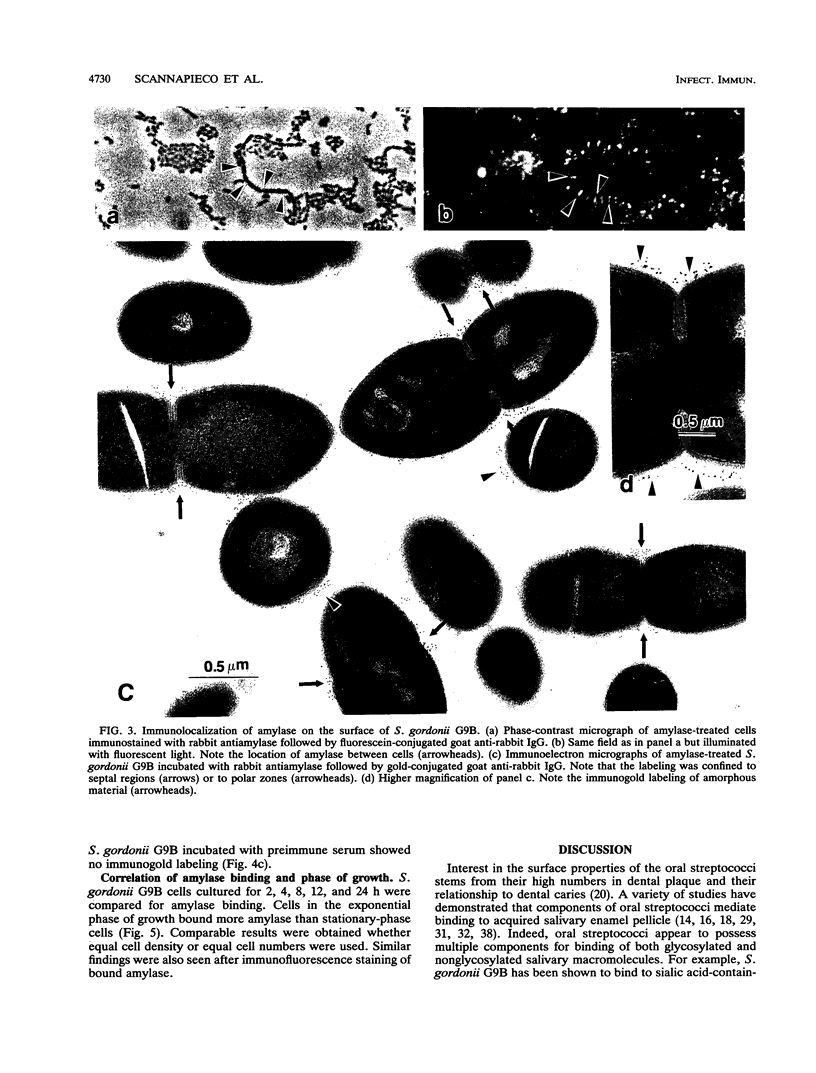
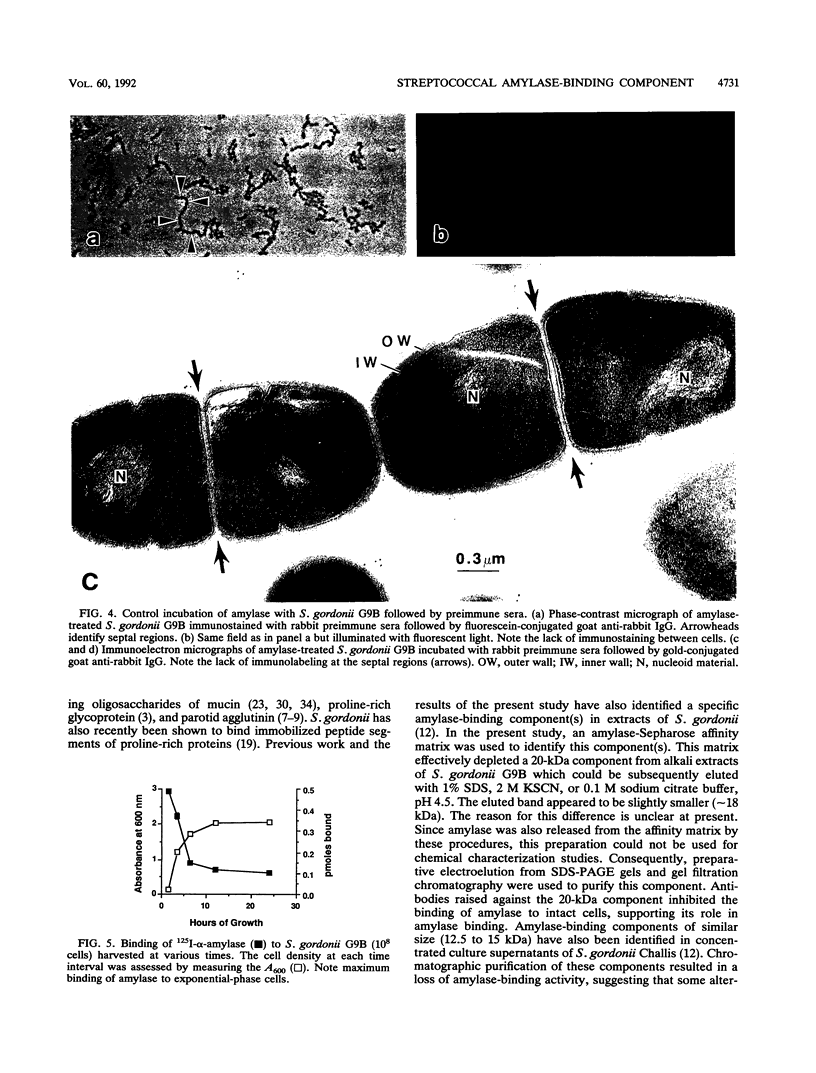
The goal of the present study was to begin characterizing the amylase-binding component(s) on the surface of Streptococcus gordonii G9B. Alkali extracts but not phenol-water extracts of this bacterium inhibited 125I-amylase binding to S. gordonii G9B. To identify the bacterial components involved in amylase binding, the alkali extract was subjected to affinity chromatography on amylase-Sepharose. Immunoblotting with a rabbit antiserum against S. gordonii G9B revealed that a 20-kDa streptococcal component was eluted from the amylase-Sepharose with 1% sodium dodecyl sulfate (SDS), 2 M KSCN, or 0.1 M sodium citrate buffer, pH 4.5. Subsequently, the 20-kDa component was prepared from alkali extracts by electroelution from preparative SDS electrophoresis or by gel filtration chromatography. This component was trypsin sensitive, and an antibody raised against it inhibited the binding of 125I-amylase to S. gordonii G9B. Indirect immunofluorescence microscopy and immunogold electron microscopy demonstrated that both bound amylase and the 20-kDa component were localized to the cell division septum on dividing cells or to polar zones on single cells. In addition, exponentially growing bacteria bound more 125I-amylase than stationary-phase cells did. Collectively, these results suggest that a 20-kDa amylase-binding component is present on the surface of the nascent streptococcal cell wall.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre A., Levine M. J., Cohen R. E., Tabak L. A. Immunochemical quantitation of alpha-amylase and secretory IgA in parotid saliva from people of various ages. Arch Oral Biol. 1987;32(4):297–301. doi: 10.1016/0003-9969(87)90024-0. [DOI] [PubMed] [Google Scholar]
- Al-Hashimi I., Levine M. J. Characterization of in vivo salivary-derived enamel pellicle. Arch Oral Biol. 1989;34(4):289–295. doi: 10.1016/0003-9969(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Bergey E. J., Levine M. J., Reddy M. S., Bradway S. D., Al-Hashimi I. Use of the photoaffinity cross-linking agent N-hydroxysuccinimidyl-4-azidosalicylic acid to characterize salivary-glycoprotein-bacterial interactions. Biochem J. 1986 Feb 15;234(1):43–48. doi: 10.1042/bj2340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D., Skude G. Relation of amylase to starch and Lycasin metabolism in human dental plaque in vitro. Scand J Dent Res. 1978 Jul;86(4):248–258. doi: 10.1111/j.1600-0722.1978.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Zooglea-forming streptococci, resembling Streptococcus sanguis, isolated from dental plaque in man. Odontol Revy. 1965;16(4):348–358. [PubMed] [Google Scholar]
- Demuth D. R., Berthold P., Leboy P. S., Golub E. E., Davis C. A., Malamud D. Saliva-mediated aggregation of Enterococcus faecalis transformed with a Streptococcus sanguis gene encoding the SSP-5 surface antigen. Infect Immun. 1989 May;57(5):1470–1475. doi: 10.1128/iai.57.5.1470-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Davis C. A., Corner A. M., Lamont R. J., Leboy P. S., Malamud D. Cloning and expression of a Streptococcus sanguis surface antigen that interacts with a human salivary agglutinin. Infect Immun. 1988 Sep;56(9):2484–2490. doi: 10.1128/iai.56.9.2484-2490.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- DiPaola C., Herrera M. S., Mandel I. D. Immunochemical study of host proteins in human supragingival compared with denture plaque. Arch Oral Biol. 1984;29(2):161–163. doi: 10.1016/0003-9969(84)90122-5. [DOI] [PubMed] [Google Scholar]
- Douglas C. W. Characterization of the alpha-amylase receptor of Streptococcus gordonii NCTC 7868. J Dent Res. 1990 Nov;69(11):1746–1752. doi: 10.1177/00220345900690110701. [DOI] [PubMed] [Google Scholar]
- Douglas C. W., Pease A. A., Whiley R. A. Amylase-binding as a discriminator among oral streptococci. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):193–197. doi: 10.1016/0378-1097(90)90281-t. [DOI] [PubMed] [Google Scholar]
- Douglas C. W. The binding of human salivary alpha-amylase by oral strains of streptococcal bacteria. Arch Oral Biol. 1983;28(7):567–573. doi: 10.1016/0003-9969(83)90003-1. [DOI] [PubMed] [Google Scholar]
- Fenno J. C., LeBlanc D. J., Fives-Taylor P. Nucleotide sequence analysis of a type 1 fimbrial gene of Streptococcus sanguis FW213. Infect Immun. 1989 Nov;57(11):3527–3533. doi: 10.1128/iai.57.11.3527-3533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fives-Taylor P. M., Thompson D. W. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect Immun. 1985 Mar;47(3):752–759. doi: 10.1128/iai.47.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. V., Pedrazzoli V., Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991 Jun;6(3):129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Ganeshkumar N., Song M., McBride B. C. Cloning of a Streptococcus sanguis adhesin which mediates binding to saliva-coated hydroxyapatite. Infect Immun. 1988 May;56(5):1150–1157. doi: 10.1128/iai.56.5.1150-1157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Schlesinger D. H. Delineation of a segment of adsorbed salivary acidic proline-rich proteins which promotes adhesion of Streptococcus gordonii to apatitic surfaces. Infect Immun. 1991 Sep;59(9):2948–2954. doi: 10.1128/iai.59.9.2948-2954.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Handley P. S., Carter P. L., Wyatt J. E., Hesketh L. M. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sanguis strains may be related to their ability to coaggregate with other oral genera. Infect Immun. 1985 Jan;47(1):217–227. doi: 10.1128/iai.47.1.217-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P., Coykendall A., Beighton D., Hardie J. M., Whiley R. A. Streptococcus crista sp. nov., a viridans streptococcus with tufted fibrils, isolated from the human oral cavity and throat. Int J Syst Bacteriol. 1991 Oct;41(4):543–547. doi: 10.1099/00207713-41-4-543. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Haines M., Whalen M., Glaser D., Bylund J. Relationship between changes in buoyant density and formation of new sites of cell wall growth in cultures of streptococci (Enterococcus hirae ATCC 9790) undergoing a nutritional shift-up. J Bacteriol. 1990 Aug;172(8):4415–4419. doi: 10.1128/jb.172.8.4415-4419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Nyvad B. Ability to bind salivary alpha-amylase discriminates certain viridans group streptococcal species. J Clin Microbiol. 1990 Nov;28(11):2576–2577. doi: 10.1128/jcm.28.11.2576-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamont R. J., Rosan B., Murphy G. M., Baker C. T. Streptococcus sanguis surface antigens and their interactions with saliva. Infect Immun. 1988 Jan;56(1):64–70. doi: 10.1128/iai.56.1.64-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. J., Ganeshkumar N., McBride B. C. Cell surface components of Streptococcus sanguis: relationship to aggregation, adherence, and hydrophobicity. J Bacteriol. 1985 Oct;164(1):255–262. doi: 10.1128/jb.164.1.255-262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. J., Ganeshkumar N., Song M., McBride B. C. Identification and preliminary characterization of a Streptococcus sanguis fibrillar glycoprotein. J Bacteriol. 1987 Jan;169(1):164–171. doi: 10.1128/jb.169.1.164-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Reynolds H. S., Genco R. J. Characterization of tufted streptococci isolated from the "corn cob" configuration of human dental plaque. Infect Immun. 1980 Jan;27(1):235–245. doi: 10.1128/iai.27.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Reddy M. S., Tabak L. A., Bergey E. J. Preparation of a sialic acid-binding protein from Streptococcus mitis KS32AR. Infect Immun. 1986 Aug;53(2):359–365. doi: 10.1128/iai.53.2.359-365.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987 Oct;95(5):369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W. The acquired pellicle: immunofluorescent demonstration of specific proteins. J Oral Pathol. 1973;2(1):68–76. doi: 10.1111/j.1600-0714.1973.tb01675.x. [DOI] [PubMed] [Google Scholar]
- Rosan B., Baker C. T., Nelson G. M., Berman R., Lamont R. J., Demuth D. R. Cloning and expression of an adhesin antigen of Streptococcus sanguis G9B in Escherichia coli. J Gen Microbiol. 1989 Mar;135(3):531–538. doi: 10.1099/00221287-135-3-531. [DOI] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco F. A., Bergey E. J., Reddy M. S., Levine M. J. Characterization of salivary alpha-amylase binding to Streptococcus sanguis. Infect Immun. 1989 Sep;57(9):2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco F. A., Bhandary K., Ramasubbu N., Levine M. J. Structural relationship between the enzymatic and streptococcal binding sites of human salivary alpha-amylase. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1109–1115. doi: 10.1016/s0006-291x(05)80900-3. [DOI] [PubMed] [Google Scholar]
- Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977 May;85(4):247–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Bergey E. J. Isolation of heart- and kidney-binding protein from group A streptococci. Infect Immun. 1982 Jan;35(1):335–342. doi: 10.1128/iai.35.1.335-342.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. C., Scannapieco F. A., Levine M. J. Use of a replica-plate assay for the rapid assessment of salivary protein-bacteria interactions. Oral Microbiol Immunol. 1992 Feb;7(1):53–56. doi: 10.1111/j.1399-302x.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]