Abstract
Isoprene is the most abundant volatile compound emitted by vegetation. It influences air chemistry and is part of plant defense against abiotic stresses. However, whether isoprene influences biotic interactions between plants and other organisms has not been investigated to date. Here we show a new effect of isoprene, namely its influence on interactions between plants and insects. Herbivory induces the release of plant volatiles that attract the herbivore's enemies, such as parasitic wasps, as a kind of bodyguard. We used transgenic isoprene-emitting Arabidopsis plants in behavioral, chemical, and electrophysiological studies to investigate the effects of isoprene on ecological interactions in 2 tritrophic systems. We demonstrate that isoprene is perceived by the chemoreceptors of the parasitic wasp Diadegma semiclausum and interferes with the attraction of this parasitic wasp to volatiles from herbivore-infested plants. We verified this repellent effect on D. semiclausum female wasps by adding external isoprene to the volatile blend of wild-type plants. In contrast, the antennae of the parasitic wasp Cotesia rubecula do not perceive isoprene and the behavior of this wasp was not altered by isoprene emission. In addition, the performance of the 2 examined lepidopteran herbivores (Pieris rapae and Plutella xylostella) was not affected by isoprene emission. Therefore, attraction of parasitic wasps to host-infested herbaceous plants in the neighborhood of high isoprene emitters, such as poplar or willow, may be hampered by the isoprene emission that repels plant bodyguards.
Keywords: Arabidopsis, isoprene emission, plant-insect interactions, tritrophic interactions, parasitoid
Plants interact with their abiotic and biotic environments through volatile organic compounds, of which terpenes [isoprene (C5), mono- (C10), sesqui- (C15), and homoterpenes (C11, C16)] form the most prominent group (1, 2). Global emission of the highly reactive hemiterpene isoprene is estimated to be 440–660 Tg carbon per year (3), which amounts to 44% of the overall nonmethane volatiles released from ecosystems. Some plants, mainly tree species (1), may even emit up to 15% of photosynthetically fixed carbon back to the atmosphere as isoprene (4). The compound may help the photosynthetic apparatus to recover from brief, high-temperature episodes (5). This effect was recently demonstrated with transgenic isoprene nonemitting poplar leaves in which gene expression of isoprene synthase (PcISPS) was knocked down (6). Isoprene is thought to act physically by stabilizing the thylakoid membranes at high temperatures (5) or by quenching reactive oxygen species, such as ozone, which can lead to membrane damage (7).
Isoprene emission was first observed 51 years ago (8) and has been widely studied by atmospheric chemists and plant physiologists (5, 9). To our knowledge, a potential effect of isoprene on interactions between the emitting plants and other biota remains unexplored to date. Attack by insect herbivores results in the biosynthesis of a plant-and-herbivore-specific blend of volatiles that mediates plant defense by repelling herbivores and/or attracting carnivorous arthropods, such as predators and parasitic wasps (10, 11). The attraction of carnivores provides plants with a top-down control of herbivore populations, which was first observed for Lima bean plants that recruited predatory mites in response to spider-mite infestation (12). It has later been shown to be a more general phenomenon (13), also observed for isoprene-emitting tree species, such as poplar (14, 15). Especially higher isoprenoids, like mono- and sesquiterpenes, are shown to play important roles in attracting bodyguards to herbivore-infested plants. They are derived from the 2 isoprenoid pathways localized in the cytosol and chloroplasts of plant cells. Isoprene (C5) as well as monoterpenes [C10, e.g., (E)-β-ocimene] originate from the chloroplastidic methylerythritol-phosphate (MEP) pathway, whereas sesquiterpenes (C15; e.g., α-farnesene) are of cytosolic origin (16). The homoterpene TMTT [C16; (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetradiene] is thought to have a cytosolic origin (17), but Mumm et al. (18) showed that its synthesis depends on substrate supply from the MEP pathway. Thus, monoterpenes and, to some extent, TMTT may compete with isoprene for substrate supply. However, although many roles in plant–insect interactions are known for higher isoprenoids (10–15, 19–21), the role of isoprene in the recruitment of carnivorous arthropods to herbivore-induced plant volatiles remains unknown.
Arabidopsis thaliana, the model plant of molecular biology, has proven to be a valuable tool to investigate the effect of volatiles on plant-insect interactions (20, 22). Just like many other plant species, it responds to herbivory with the release of volatiles that attract carnivorous enemies of the herbivores (23). The rate of terpene emission from Arabidopsis is comparatively low relative to insect-pollinated species (24). However, when induced by herbivory, the volatiles emitted from the leaves are abundant enough to be recorded by GC-MS and to attract carnivorous enemies of the herbivores (23). The low constitutive emission of volatiles from its leaf rosettes (23, 24) makes Arabidopsis an interesting tool for studying the ecological effects of specific compounds by transforming them with genes that code for terpene synthases (20, 25, 26).
Arabidopsis does not naturally emit isoprene. Here, we exploit a transgenic isoprene-emitting Arabidopsis line (27) to study the function of isoprene in plant–insect interactions. We investigated the effects of the inserted Populus× canescens isoprene synthase gene (PcISPS), under the constitutive control of the 35S promoter, on Arabidopsis–insect interactions in 2 well-studied tritrophic systems. We analyzed the behavior of the small cabbage white butterfly Pieris rapae L. (Lepidoptera, Pieridae) and its specialist parasitic wasp Cotesia rubecula Marshall (Hymenoptera, Braconidae), which is attracted by the volatile blend of P. rapae-infested Arabidopsis plants (23). As a second model, we studied the behavior of Diadegma semiclausum Hellén (Hymenoptera, Ichneumonidae), a specialist parasitic wasp of the diamondback moth Plutella xylostella L. (Lepidoptera, Plutellidae) [supporting information (SI) Fig. S1]. Diamondback larvae are not commonly observed to feed on Arabidopsis, but the plant was recently shown to be a suitable host for this herbivore (28). Both herbivore species are specialists on plants in the Brassicaceae family, and the parasitoids C. rubecula and D. semiclausum are specialist parasitoids of P. rapae and Pl. xylostella, respectively.
Results
Choice of Parasitic Wasps in Y-Tube Olfactometer.
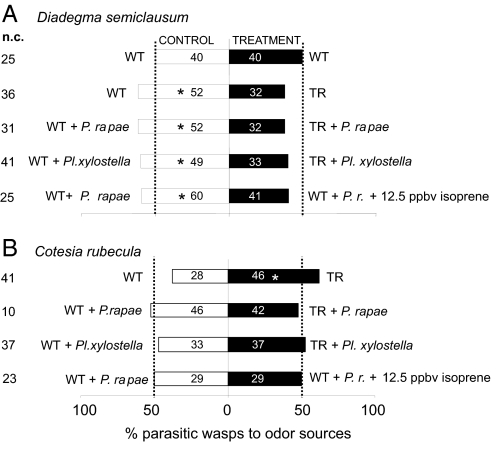
In a Y-tube olfactometer choice assay, female D. semiclausum wasps preferred the volatiles from wild-type (WT) Arabidopsis plants to the volatiles from isoprene-emitting transgenic ones when both were uninfested (binomial test, z = −2.07, P = 0.019, n = 80) as well as when both were infested by either P. rapae (binomial test, z = −2.07, P = 0.019, n = 84) or Pl. xylostella caterpillars (binomial test, z = −1.66, P = 0.485, n = 82) (Fig. 1A).
Fig. 1.
Behavioral responses of parasitoids to isoprene-emitting plants. Response of naïve D. semiclausum females (A) and naïve C. rubecula females (B) to volatiles released by A. thaliana in a Y-tube olfactometer. Bars represent the overall percentages of wasps choosing either of the odor sources; numbers in bars are the total numbers of wasps choosing that odor source. Choices between odor sources were analyzed with a binomial test. *, P < 0.05; n.c., no choice.
Conversely, the endoparasitic wasp C. rubecula was little affected by the presence of isoprene. C. rubecula, in contrast to D. semiclausum, preferred uninfested isoprene-emitting transgenic plants to uninfested WT plants (binomial test, z = −1.98, P = 0.023, n = 74). However, when both plant types were infested by either of the herbivores, C. rubecula showed no preference for isoprene-emitting plants versus WT plants anymore (Fig. 1B).
We further examined the effects of isoprene on the behavior of the parasitic wasps by adding 12.5 ppbv isoprene (from an isoprene standard with 10 ppmv isoprene in N2) into the odor flow downstream from uninfested WT Arabidopsis rosettes compared with similar control plants without isoprene in the cuvette air. This independent external control gave similar results as obtained with isoprene-emitting transgenic plants: D. semiclausum wasps preferred WT plants without isoprene over WT plants whose odor blend was supplemented with isoprene (binomial test, z = −1.79, P = 0.036, n = 101) (Fig. 1A), whereas C. rubecula showed no preference between the 2 odor sources (Fig. 1B).
Volatile Blend from Isoprene-Emitting Transgenic Arabidopsis Plants.
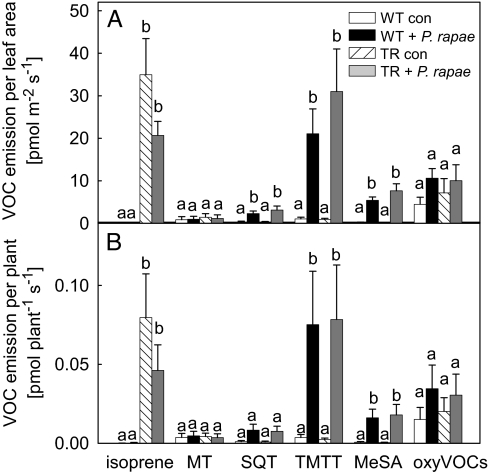
The analyses of the volatiles emitted from transgenic and WT Arabidopsis plants showed that isoprene is the predominant volatile compound (78% of overall emission) (for compounds list see Table S1) emitted from the uninfested transgenic plants (Fig. 2, see also gas chromatography-mass spectrometry profiles of emitted volatiles in Fig. S2B) with an emission rate of ≈35 ± 8.5 pmol m−2 leaf area s−1 (Fig. 2A) equivalent to 0.08 ± 0.028 pmol plant−1 s−1 (Fig. 2B). As expected, uninfested and caterpillar-infested Arabidopsis WT plants emitted no isoprene. Moreover, isoprene emission from transgenic plants infested by P. rapae caterpillars was still 27% of the sum of all of the other compounds (Fig. 2 and Fig. S2D). Our data further show that P. rapae herbivory significantly (Mann–Whitney U test, n = 4, P < 0.05) (see Table S2 for details) induced the release of 2 other terpenoids, the sesquiterpene α-farnesene and the homoterpene TMTT, as well as methyl salicylate (23). The constitutive, high-emission rate of isoprene in transgenic plants did not result in a significantly reduced constitutive or induced emission of other terpenoid compounds.
Fig. 2.
Headspace analysis of WT and transgenic Arabidopsis rosettes with and without herbivory. Volatile emission rates based on leaf area basis (A) and per plant (B). White, uninfested WT plant; black, P. rapae-infested WT plant; hatched, uninfested transgenic plant; gray, P. rapae infested transgenic plant. MT, monoterpenes (α-pinene, camphene, β-myrcene, limonene, linalool); SQT, sesquiterpene (α-farnesene); oxyVOCs, other oxygenated volatile organic compounds (10 compounds). Four independent experiments per treatment and line were analyzed, and means ± SE are given. Significant differences (P < 0.05, Mann–Whitney U test) between treatments are indicated by different letters per compound class. (For GC-MS profiles and compound list see Fig. S2 and Table S1).
Electrophysiological Recognition of Isoprene by D. semiclausum and C. rubecula Antennae.
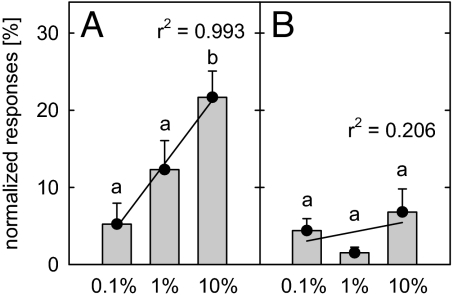
It was further ascertained that isoprene is physiologically active and is detected by the insect antennae. In D. semiclausum antennae, isoprene evoked significant electroantennographic (EAG) responses relative to responses attained with (1%) (Z)-3-hexen-1-yl acetate, a green leaf volatile that is well recognized by the antennae of numerous insect species (29). The antennae of D. semiclausum females responded to isoprene in a dose-dependent manner (Fig. 3A). Higher concentrations of isoprene (10%) evoked a significantly higher response in the insect antennae than the lower isoprene concentrations (Paired t test; P < 0.05) (see for details Table S2). In contrast, the antennae of C. rubecula females showed no response to the different isoprene concentrations applied (Fig. 3B). In addition, when the responses of C. rubecula to isoprene (in hexadecane) were compared with responses to pure hexadecane [both compounds relative to (Z)-3-hexen-1-yl acetate], no significant differences were found (paired samples t test, P > 0.05).
Fig. 3.
EAG response of antennae of D. semiclausum (A) and C. rubecula (B) females to isoprene diluted in hexadecane. Response values are normalized relative to the response value of 1% (Z)-3-hexen-1-yl acetate. The mean (± SE) response of 6 (D. semiclausum) and 8 (C. rubecula) different antennae is given. The responses were analyzed with paired samples t test (different letters above bars indicate significant differences at P < 0.05, for details see Table S3) and with linear regression analysis (x-axis common log).
The Herbivore Performance on Isoprene-Emitting Arabidopsis.
The performance of Pl. xylostella and P. rapae was not affected by transgenic isoprene-emitting Arabidopsis plants compared with WT plants. After 5 days (Pl. xylostella) or 1 week (P. rapae) of feeding on either WT or transgenic Arabidopsis plants, the larvae of both species had gained equal weights on the 2 plant types (Table 1).
Table 1.
P. rapae and Pl. xylostella behavior on WT or transgenic (TR) isoprene-emitting Arabidopsis plants
| Line | Development |
Cafeteria |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight gain, mg | n | t test | Choice, % |
n | Binomial test* | ||||
| 1st | 0.5 h | 1 h | 2 h | ||||||
| P. rapae | |||||||||
| WT | 46.9 ± 1.72 | 29 | t = −0.741 | 54 | 52 | 53 | 53 | 100 | z = −0.7 |
| TR | 49.8 ± 3.43 | 33 | P = 0.462 | 46 | 48 | 47 | 47 | 100 | P = 0.242 |
| Pl. xylostella | |||||||||
| WT | 6.6 ± 0.26 | 22 | t = −0.919 | 46 | 42 | 40 | 43 | 100 | z = −1.9 |
| TR | 7.0 ± 0.33 | 23 | P = 0.364 | 54 | 58 | 60 | 57 | 100 | P = 0.028 |
Mean ± SE is shown for larval weight gain and percentage for the cafeteria choice-experiment. In the cafeteria experiment, the 1st choices as well as choices after 0.5 h, 1 h, and 2 h after the start of the experiment are shown. The number of independent replications is indicated by n.
*The results from binomial tests are shown for the time point, in which highest difference in choices between WT and TR were found. For the other time points P > 0.05.
When caterpillars of Pl. xylostella and P. rapae were given a free choice in a cafeteria test setup (30) to feed either on WT or on transgenic Arabidopsis leaves, they did not prefer either plant type in the beginning (first choice), 0.5 h, or 2 h after the beginning of the experiment (Table 1). Nevertheless, Pl. xylostella preferred to feed on transgenic plants at 1 time point: 1 h after the beginning of the experiment (P < 0.05, Table 1).
In addition, ovipositing P. rapae and Pl. xylostella females did not discriminate between a WT and an isoprene-emitting transgenic plant when given a choice. On average, female P. rapae butterflies laid 22.7 ± 1.8 eggs on WT and 22.9 ± 1.6 eggs on transgenic plants, respectively (paired samples t test, t = 0.194, P = 0.847, n = 81, mean ± SE). Pl. xylostella females laid on average 13.6 ± 1.3 eggs on WT and 11.0 ± 1.4 eggs on transgenic plants, respectively (paired samples t test, t = 1.29, P = 0.205, n = 35, mean ± SE).
Discussion
In the present study, we demonstrate that isoprene is perceived by the antennae of D. semiclausum and that it interferes with the attraction of this parasitic wasp to volatiles emitted by herbivore-infested Arabidopsis plants. This observation adds a new ecophysiological component to the previously proposed biological roles of isoprene (5, 6). The parasitic wasp being repelled by isoprene is remarkable, because many higher terpenes, such as monoterpenes, homoterpenes, and sesquiterpenes, are observed to function as attractants to carnivorous enemies of herbivores (18, 20, 26). In contrast to D. semiclausum, the antennae of Cotesia rubecula did not show a similar dose-dependent response to isoprene, and we also did not observe a behavioral discrimination between WT and isoprene-emitting plants. Although both parasitic wasp species parasitize herbivores that are specialist feeders on brassicaceous plant species, isoprene apparently has different effects on D. semiclausum and C. rubecula. Indeed, it appears that the 2 wasp species use different plant volatiles during host location: Cotesia spp. is particularly attracted to green leaf volatiles (GLV: C6 aldehydes, alcohols, and esters derived from unsaturated fatty acids) and glucosinolate breakdown products (31–33), whereas for D. semiclausum isoprenoids apart from isoprene also seem to be important for host finding (R.M. and M.D., unpublished work).
We hypothesize that this different recognition of volatile isoprenoids for the orientation of D. semiclausum and C. rubecula might be causally linked to the isoprene insensitivity of C. rubecula antennae and the absence of behavioral changes of this wasp species in the presence of isoprene.
It is important to keep in mind that alteration of metabolic fluxes in transgenic isoprene-emitting Arabidopsis might have pleiotropic effects, as the isoprene synthase may serve as a sink of substrates that the plant synthesized for other use. Such an alteration could have resulted in, for example, lower monoterpene emission from transgenic plants, thus changing the attractiveness of the plant to bodyguards. Indeed, DMADP levels in Arabidopsis leaves are 5–10 times lower (27) compared with the levels in isoprene emitters like poplar (6), and isoprene emission in the transgenic Arabidopsis plants seems to be substrate-limited because isoprene emission from transgenic Arabidopsis could be enhanced by 1-deoxy-D-xylulose feeding (27). However, our data show that isoprene is the most prominent compound emitted by our uninfested transgenic Arabidopsis plants. Furthermore, the emission rates of chloroplast-derived terpenes did not differ between WT and isoprene-emitting Arabidopsis, indicating that the biosynthesis of these feeding-induced volatiles was not impaired by isoprene formation. The results show that the repellence of D. semiclausum by isoprene-emitting transgenic plants was caused by isoprene perceived by the antennae of this parasitic wasp. As the electrophysiological response of live insects can be significantly higher than the recorded EAG response of a head preparation (34), it is likely that under natural conditions the response to isoprene is even more sensitive than measured in our experiments. Together, these results suggest that the indirect defense of plants that do not emit isoprene themselves can be compromised when they are in an isoprene-rich environment, for example, in mixed forests or nearby field edges.
Our results urge for future studies to investigate whether the perception of isoprene by carnivorous insects is a more common phenomenon and what the real roles are for the plant itself. Is isoprene interfering with the attraction of parasitic wasps, as shown here for D. semiclausum females, or may isoprene simply be a “non-host-related volatile” (NHV) for the wasps because it is naturally not emitted by the food plants of its hosts? NHVs have been shown to modulate host location of phytophagous insects in mixed conifer and deciduous forests (35). Odors collected from nonhost plants (e.g., birch and oak) contained mono- and sesquiterpenes and GLV, several of which elicited EAG responses and reduced the attraction of bark beetles to a pheromone in the field (36). Specialist parasitoids like D. semiclausum may benefit from an innate avoidance response to an NHV because the ability to discriminate between patches with predominantly non-host-related plants and patches containing plants with suitable hosts is essential for the initial host-finding stages of a parasitoid (37). Thus, isoprene may have an antiattractive effect similar to these volatile compounds from broad-leaved angiosperm trees, which can repel various conifer bark beetle species (35).
This possibility should be investigated by addressing the role of isoprene in the foraging behavior of carnivorous arthropods that search for herbivorous victims on naturally isoprene-emitting plants, such as poplar or oak. Independent of the underlying mechanism, however, it is important to note that isoprene can give an ecological advantage to herbivores feeding on herbaceous plants in an isoprene-containing environment, as parasitic wasps searching for hosts may be hampered whereas herbivore feeding seems not to be disturbed.
The research on tritrophic interactions so far has mainly focused on isolated systems without including the effects of background volatiles (but see 21, 38, 39). Isoprene emission, also in transgenic isoprene-emitting Arabidopsis, strongly increases with temperature, reaching maximal rates around 40 °C. Under these conditions, isoprene-emission rates of Arabidopsis leaves raise up to 3 nmol m−2 s−1 (27). Contrasting with these drastic temperature conditions, Arabidopsis plants in the present study were cultivated at room temperature (24–25 °C) with consequently much lower isoprene emission rates. Even if isoprene emission from Arabidopsis is low (20–30 pmol m−2 s−1) compared with that of poplar leaves at similar temperature (5–10 nmol m−2 s−1) (40) it does clearly affect the orientation behavior of D. semiclausum wasps.
Isoprene emission by poplar leaves can result in concentrations up to 100 ppbv close to the isoprene-emitting leaves (6). Depending on turbulence and wind speed, plant volatiles become rapidly diluted within and above the canopy (41). However, atmospheric isoprene concentrations up to 12 ppbv are possible in mixed forest canopies with a high proportion of isoprene emitters (41). At more heterogeneous sites, isoprene concentrations/fluxes vary with daytime, but also change with wind speed and direction (42). Moreover, photooxidation of isoprene and other reactive mono- and sesquiterpenes in nitric-oxides-enriched suburban atmospheres (41) makes the distribution and concentration of isoprene even more variable. In addition to transgenic isoprene-emitting plants, we used external isoprene in a concentration of 12.5 ppbv to prove that such a concentration of isoprene in the surrounding environment interferes with the parasitic wasp's orientation to Arabidopsis plants. Moreover, similar experiments with volatiles of Brassica oleracea, a natural host plant of D. semiclausum's hosts, supplemented with isoprene gave comparable results (data not shown).
Future studies should address the functions of isoprene in plant-herbivore and tritrophic interactions with a natural isoprene emitter, for example, by using isoprene emission knock-down lines of gray poplar (6). Poplars are attacked by a large variety of insects and mites (15, 43–45), for example, the cottonwood leaf beetle (Chrysomela scripta F.) (46), the forest tent caterpillar (Malacosoma disstria Hübner) (15), or the gypsy moth (Lymantria dispar L.) (43). In poplar plantations, damage by insect defoliators is responsible for enormous economic losses (43, 47). Thus, there is an urgent need to elucidate the role of isoprene in tritrophic interactions of a real isoprene emitter. Indeed, plant–herbivore–parasitic wasp interactions depend on so-far overlooked environmental aspects, such as those shown here for isoprene. Moreover, given that isoprene emission is positively correlated with temperature, climate change may aggravate the interference with the attraction of bodyguards by plants.
Materials and Methods
Plant Treatments and Growth Condition.
Arabidopsis thaliana (Col-0) and transgenic plants in the Col-0 background constitutively expressing PcISPS derived from gray poplar (27) were investigated. Experiments were carried out with 6- to 10-week-old Arabidopsis rosettes grown at 21 ± 1 °C, 55 ± 5% relative humidity (RH), L8:D16, and a photosynthetic photon flux density (PPFD) of 95 ± 15 μmol photons m−2 s−1. Plants were infested by placing 20 first instar larvae (either P. rapae or Pl. xylostella) over several leaves of each plant for 24 h.
Insects.
P. rapae and Pl. xylostella were reared on B. oleracea var. gemmifera cultivar Cyrus in climate rooms (21 ± 1 °C, RH 60 ± 10%, L16:D8) (18). The parasitic wasp C. rubecula was reared on P. rapae larvae feeding on B. oleracea in a greenhouse (24 ± 4 °C, RH 60 ± 20%, L16:D8) (23). D. semiclausum (Fig. S1) was reared on Pl. xylostella larvae feeding on B. oleracea. For bioassays, either C. rubecula or D. semiclausum adults were each transferred to a separate cage in which they were provided with honey and water. Female wasps had no oviposition experience and are, therefore, referred to as naïve wasps.
Olfactometer Bioassays.
The behavioral response of female parasitic wasps to plant volatiles was investigated in a Y-tube olfactometer (48) under constant conditions (22 ± 2 °C, 60 ± 5 μmol photons m−2 s−1). One parasitoid at a time was introduced to the olfactometer by using a glass vial, and its behavior was observed for a maximum of 10 min. To correct for unforeseen asymmetry in the set-up, the position of the odor sources was switched after 5 tested parasitoids. Wasps not making a choice within this period were discarded from the statistical analysis. For isoprene fumigation, isoprene [10 ppmv in N2 (Air Liquide)] was added (5 ml min−1) to the outlet air (4 l min−1) of 1 side arm of the Y-tube olfactometer ≈5 cm downstream of the plant odor source, resulting in a final concentration of 12.5 ppbv isoprene.
Headspace Collection and Analysis.
For dynamic headspace collection, 4 independent experiments were performed. In each experiment, 4 plants of each line and treatment were placed in 2.5-L glass jars 24 h before sampling. Inlet air was filtered by passing through tubes filled with 200 mg of Tenax TA (Grace-Alltech). The system was purged for 1 h with filtered air before trapping volatiles onto the adsorbents. Air was sucked out of the jar with 100 ml min−1 by passing first through a tube filled with 200 mg of Tenax TA and subsequently through a tube containing 200 mg of Carbopack X (Grace-Alltech). Headspace volatiles from different treatments were collected for a period of 5 h between 11:00 AM and 4:00 PM. Fresh weights of all rosettes were determined immediately after the experiments. For calculating emission rates according to international standards (SI system), the fresh weight of Arabidopsis rosettes was converted to leaf area by using the correlation shown in Fig. S3.
Chemical Analysis of Headspace Volatiles.
Headspace samples were analyzed with a Thermo TraceGC Ultra connected to a Thermo TraceDSQ quadrupole mass spectrometer (Thermo Fisher Scientific). Before thermodesorption, traps were flushed with helium at 50 ml min−1 for 20 min. After flushing, Tenax traps were desorbed at 250 °C (Model Ultra; Markes) for 5 min with a helium flow at 30 ml min−1. Carbopack X traps were desorbed at 320 °C for 7 min with a helium flow of 30 ml min−1. Volatiles were focused on a sorbent trap (Unity; Markes) at 0 °C (Tenax) or 30 °C (Carbopack X). For injection into the analytical column (RTX-5ms, 30 m × 0.25 mm ID, 1.0-μm film thickness; Restek), the cold trap was rapidly heated to 250 °C with a split flow of 5 ml min−1. The temperature program started at 40 °C (Tenax) or 32 °C (Carbopack X) (4-min hold) and rose with a rate of 10 °C min−1 to 280 °C (2-min hold). The column effluent was ionized by electron impact ionization at 70 eV. Mass scanning was done from 25 to 300 m/z with a scan rate of 3.8 scans s−1. Compounds were identified by comparing the mass spectra with those of authentic standards or with NIST 05 and Wiley library spectra. Linear retention indices were calculated for each compound according to van den Dool and Kratz (49). Calibration (isoprene, α-pinene, limonene, methyl salicylate, β-caryophyllene) was performed according to Schuh et al. (50).
Herbivore Choice and Performance Experiments.
The experiments were carried out under controlled conditions (21 ± 1 °C, 55 ± 5% RH, L16:D8 photoperiod with 80–110 μmol photons m−2 s−1 PPFD). Arabidopsis plants were individually placed in Magenta GA-7 vessels (Sigma–Aldrich) with an insect-proof mesh lid. The weight of first instar larvae of P. rapae (weight of the larvae at the start of the experiment: on WT plants = 0.48 ± 0.15 mg; on transgenic plants = 0.45 ± 0.18 mg) and Pl. xylostella (weight of the larvae at the start of the experiment: WT = 0.26 ± 0.10 mg; transgenic = 0.26 ± 0.13 mg) was measured, and caterpillars were then individually transferred either onto a WT or a transgenic plant. Larvae were allowed to feed for 4–5 days (Pl. xylostella) or 7 days (P. rapae) and were weighed again.
In a cafeteria experiment (30), 100 first instar caterpillars of P. rapae and Pl. xylostella were given a free choice to feed on transgenic or on WT Arabidopsis leaves. The petiole of each leaf was placed in a 0.5-ml vial filled with tap water. Two transgenic and two WT leaves were placed on moisturized filter paper in a Petri dish (90-mm diameter) ≈2 cm away from each other in a rectangular distribution. An individual caterpillar was then placed in the middle. The feeding choice of 20 caterpillars was investigated simultaneously, and the experiment was replicated on 5 different days.
In 2-choice experiments, female P. rapae butterflies were given the opportunity to lay eggs on either transgenic or WT Arabidopsis plants. Forty-eight hours before the experiment, freshly emerged male and female P. rapae-butterflies were given the possibility to mate for 24 h in a cage, after which a single untreated Brussels sprouts leaf was placed into the cage for 6 h as an oviposition substrate to reduce the egg load of the female butterflies. One male and one female were then transferred into individual experimental cages (67 × 50 × 75 cm), 16 ± 2 h before starting the experiment. One WT and one transgenic plant were placed in the cage, ≈15 cm from each other. The number of eggs deposited during 4 h (10:00 AM–2:00 PM) on either of the offered lines was counted. The oviposition behavior of 10 to 12 butterflies was investigated simultaneously. The experiment was replicated 8 times on different days with new plants and new butterflies. Oviposition choice experiments with Pl. xylostella were carried out in plastic cylinders (height: 21 cm, inner diameter: 13.5 cm) with an insect-proof mesh lid under controlled conditions (22 ± 1 °C, 60 ± 5% RH, L16:D8 photoperiod with 80–110 μmol photons m−2 s−1 PPFD). One Pl. xylostella male and one female (3–4 days old) were transferred into the experimental cylinders 16 ± 2 h before starting the experiment. One WT and one transgenic plant were placed in the cylinder. The number of eggs deposited during 24 h (starting at 11:00 AM) on either of the offered lines was counted. The oviposition behavior of 17 to 18 moths was investigated simultaneously. The experiment was replicated 2 times on different days with new plants and new moths.
EAG.
EAG recordings were made as described in Smid et al. (29). The response of individual D. semiclausum and C. rubecula females to 0.1%, 1%, or 10% (v:v) isoprene in hexadecane (99% purity, Sigma–Aldrich) was recorded. Ten microliters of each dilution was applied on a strip of filter paper, which was inserted into a Pasteur pipette. Stimulus puffs (0.5 sec, 100 ml min−1) were injected into a continuous air stream of humidified, charcoal-filtered air of 500 ml min−1 running over the antennal preparation. (Z)-3-hexen-1-yl acetate (≥98% purity, Sigma–Aldrich; 1% solution in hexadecane) was used as a standard odor for normalization. The standard odor was applied in the beginning and at the end of one series that involved the 3 different isoprene concentrations in ascending order and the control stimulations. Control stimulations were performed with 10 μL of hexadecane, which was applied 3 times within a series. The average of the control stimulations was subtracted from each EAG-response value. The obtained response values were converted to a percentage by using the mean of the 2 responses to the standard odor.
Statistical Analysis.
Binomial tests were performed to analyze the Y-tube olfactometer and cafeteria experiments. Wasps that did not make a choice were excluded from the analysis. Paired samples t tests were applied to analyze the EAG data. In the oviposition data, the egg numbers on each treatment per individual were considered as paired samples and therefore analyzed with a paired samples t test. An independent samples t test was applied to analyze the gained weight of the larvae in larval performance experiments.
Supplementary Material
Acknowledgments.
We thank Hans Smid for help with EAG experiments; Jürgen Wildt for advice and support with GC-MS calibration; Wim van Ieperen for providing some technical equipment; Iris Kappers for advice and support with the headspace collection; Sandrine Louis, Hans Peter Schmid, and 2 anonymous reviewers for constructive comments that were valuable in improving a previous version of the manuscript; and Leo Koopman, Frans van Aggelen, and André Gidding for culturing the insects. This work was supported by the European Commission in the frame of the Marie-Curie Research Training Network Ecological and Physiological Functions of Biogenic Isoprenoids and Their Impact on the Environment (to M.L. and R.M.), an exchange grant from the European Science Foundation within the scientific program Volatile Organic Compounds in the Biosphere-Atmosphere System (to M.L.), and Netherlands Organization for Scientific Research NWO/ALW VICI Grant 865.03.002 (to R.M. and M.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17211.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804488105/DCSupplemental.
References
- 1.Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J Atmos Chem. 1999;33:23–88. [Google Scholar]
- 2.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 3.Guenther A, et al. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature) Atmos Chem Phys. 2006;6:3181–3210. [Google Scholar]
- 4.Sharkey TD, Singsaas EL, Vanderveer PJ, Geron C. Field measurements of isoprene emission from trees in response to temperature and light. Tree Physiol. 1996;16:649–654. doi: 10.1093/treephys/16.7.649. [DOI] [PubMed] [Google Scholar]
- 5.Sharkey TD, Singsaas EL. Why plants emit isoprene. Nature. 1995;374:769. [Google Scholar]
- 6.Behnke K, et al. Transgenic, non-isoprene emitting poplars don't like it hot. Plant J. 2007;51:485–499. doi: 10.1111/j.1365-313X.2007.03157.x. [DOI] [PubMed] [Google Scholar]
- 7.Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]
- 8.Sanadze JA. Emission of organic matters by leaves of Robinia pseudoacacia L. Soobshch Akad Nauk Gruz SSR. 1957;19:83. [Google Scholar]
- 9.Claeys M, et al. Formation of secondary organic aerosols through photooxidation of isoprene. Science. 2004;303:1173–1176. doi: 10.1126/science.1092805. [DOI] [PubMed] [Google Scholar]
- 10.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;30:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 11.De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 12.Dicke M, Sabelis MW. How plants obtain predatory mites as bodyguards. Neth J Zool. 1988;38:148–165. [Google Scholar]
- 13.Degenhardt J, Gershenzon J, Baldwin IT, Kessler A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol. 2003;14:169–176. doi: 10.1016/s0958-1669(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 14.Havill NP, Raffa KF. Compound effects of induced plant responses on insect herbivores and parasitoids: implications for tritrophic interactions. Ecol Entomol. 2000;25:171–179. [Google Scholar]
- 15.Arimura G, Huber DPW, Bohlmann J. Forest tent caterpillars (Malacosoma disstria) induce systemic and diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1. Plant J. 2004;37:603–616. doi: 10.1111/j.1365-313x.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- 16.Aharoni A, Jongsma MA, Bouwmeester HJ. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plants Sci. 2005;10:594–602. doi: 10.1016/j.tplants.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Herde M, et al. Identification and regulation of TPS04/GES, an Arabidopsis geranyllinalool synthase catalyzing the first step in the formation of the insect-induced volatile C16-homoterpene TMTT. Plant Cell. 2008;20:1152–1168. doi: 10.1105/tpc.106.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mumm R, Posthumus MA, Dicke M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008;31:575–585. doi: 10.1111/j.1365-3040.2008.01783.x. [DOI] [PubMed] [Google Scholar]
- 19.Dicke M, et al. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions. Involvement of host plant in its production. J Chem Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- 20.Kappers IF, et al. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science. 2005;309:2070–2072. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- 21.Mumm R, Hilker M. The significance of background odor for an egg parasitoid to detect plants with host eggs. Chem Senses. 2005;30:337–343. doi: 10.1093/chemse/bji028. [DOI] [PubMed] [Google Scholar]
- 22.Van Poecke RMP, Dicke M. Indirect defence of plants against herbivores: Using Arabidopsis thaliana as a model plant. Plant Biol. 2004;6:387–401. doi: 10.1055/s-2004-820887. [DOI] [PubMed] [Google Scholar]
- 23.Van Poecke RMP, Posthumus MA, Dicke M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: Chemical, behavioral, and gene-expression analysis. J Chem Ecol. 2001;27:1911–1928. doi: 10.1023/a:1012213116515. [DOI] [PubMed] [Google Scholar]
- 24.Chen F, et al. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell. 2003;15:481–494. doi: 10.1105/tpc.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aharoni A, et al. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell. 2003;15:2866–2884. doi: 10.1105/tpc.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnee C, et al. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA. 2006;24:1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loivamäki M, et al. Arabidopsis, a model to study biological functions of isoprene emission? Plant Physiol. 2007;144:1–13. doi: 10.1104/pp.107.098509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker JE, Poppy GM, Payne CC. Suitability of Arabidopsis thaliana as a model for host plant-Plutella xylostella-Cotesia plutellae interactions. Entomol Exp Appl. 2007;122:17–26. [Google Scholar]
- 29.Smid HM, van Loon JJA, Posthumus MA, Vet LEM. GC-EAG-analysis of volatiles from Brussels sprouts plants damaged by two species of Pieris caterpillars: Olfactory receptive range of a specialist and generalist parasitoid wasp species. Chemoecology. 2002;12:169–176. [Google Scholar]
- 30.Jermy T, Hanson FE, Dethier VG. Induction of specific food preference in lepidopterous larvae. Entomol Exp Appl. 1968;11:211–230. [Google Scholar]
- 31.Reddy GVP, Holopainen JK, Guerrero A. Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J Chem Ecol. 2002;28:131–143. doi: 10.1023/a:1013519003944. [DOI] [PubMed] [Google Scholar]
- 32.Shiojiri K, et al. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc Natl Acad Sci USA. 2006a;103:16672–16676. doi: 10.1073/pnas.0607780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiojiri K, et al. Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J Chem Ecol. 2006b;32:969–979. doi: 10.1007/s10886-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 34.Malo EA, Renou M, Guerrero A. Analytical studies of Spodoptera littoralis sex pheromone components by electroantennography and coupled gas chromatography-electroantennographic detection. Talanta. 2000;52:525–532. doi: 10.1016/s0039-9140(00)00401-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang QH, Schlyter F. Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric Forest Entomol. 2004;6:1–20. [Google Scholar]
- 36.Byers JA, Zhang QH, Birgersson G. Avoidance of nonhost plants by bark beetle, Pityogenes bidentatus, in a forest of odors. Naturwissenschaften. 2004;91:215–219. doi: 10.1007/s00114-004-0520-1. [DOI] [PubMed] [Google Scholar]
- 37.Vet LEM, Dicke M. Ecology of infochemicals use by natural enemies in a tritrophic context. Annu Rev Entomol. 1992;37:141–172. [Google Scholar]
- 38.Schröder R, Hilker M. The relevance of background odor in resource location by insects: A behavioral approach. BioScience. 2008;58:308–316. [Google Scholar]
- 39.Gols R, et al. Reduced foraging efficiency of a parasitoid under habitat complexity: Implication for population stability and species coexistence. J Anim Ecol. 2005;74:1059–1068. [Google Scholar]
- 40.Magel E, et al. Determination of the role of products of photosynthesis in substrate supply of isoprenoid biosynthesis in poplar leaves. Atmos Environ. 2006;40:S138–S151. [Google Scholar]
- 41.Fuentes JD, et al. Biogenic hydrocarbon chemistry within and above a mixed deciduous forest. J Atmos Chem. 2007;56:165–185. [Google Scholar]
- 42.Kaharabata SK, Schuepp PH, Fuentes JD. Source footprint considerations in the determination of volatile organic compound fluxes from forest canopies. J Appl Meteorol. 1999;38:878–884. [Google Scholar]
- 43.Russell CA, Kosala KA, Paul EA, Robertson GP. Nitrogen cycling in poplar stands defoliated by insects. Biogeochem. 2004;68:365–381. [Google Scholar]
- 44.Mattson WJ, Hart EA, Volney WJA. Insect pests of Populus: Coping with the inevitable. In: Dickmann DJ, Isebrands JG, Eckenwalder JE, Richardson J, editors. Poplar Culture in North America. Ottawa, Canada: NRC Research Press; 2001. pp. 219–248. [Google Scholar]
- 45.Philippe RN, Bohlmann J. Poplar defense against insect herbivores. Can J Bot. 2008;85:1111–1126. [Google Scholar]
- 46.Reichenbacker RR, Schultz RC, Hart ER. Artificial defoliation effect on Populus growth, biomass production, and total nonstructural carbohydrate concentration. Environ Entomol. 1996;25:632–642. [Google Scholar]
- 47.Coyle DR, Nebeker TE, Hart ER, Mattson WJ. Biology and management of insect pests in North American intensively managed hardwood forest systems. Annu Rev Entomol. 2005;50:1–29. doi: 10.1146/annurev.ento.50.071803.130431. [DOI] [PubMed] [Google Scholar]
- 48.Takabayashi J, Dicke M. Response of predatory mites with different rearing histories to volatiles of un-infested plants. Entomol Exp Appl. 1992;64:187–193. [Google Scholar]
- 49.van den Dool J, Kratz PD. A generalization of the retention index system including linear programmed gas-liquid partition chromatography. J Chromatogr. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 50.Schuh G, et al. Emissions of volatile organic compounds from sunflower and beech: Dependence on temperature and light intensity. J Atmos Chem. 1997;27:291–318. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.