Abstract
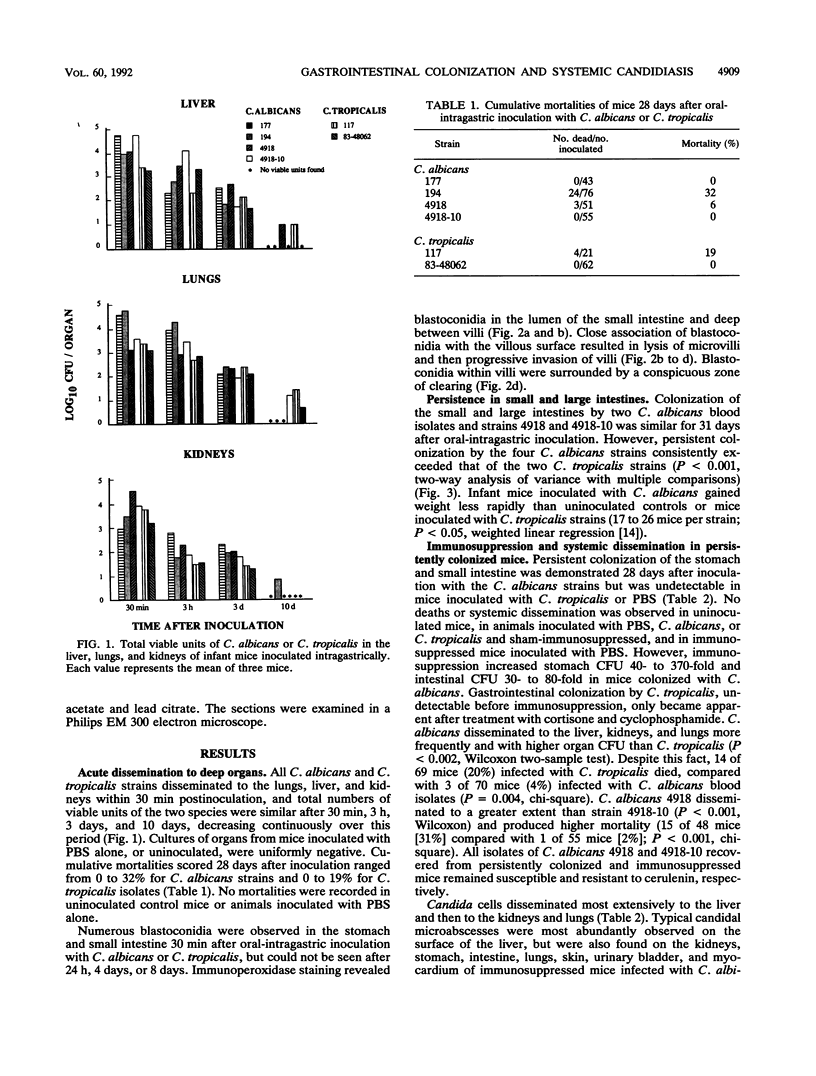
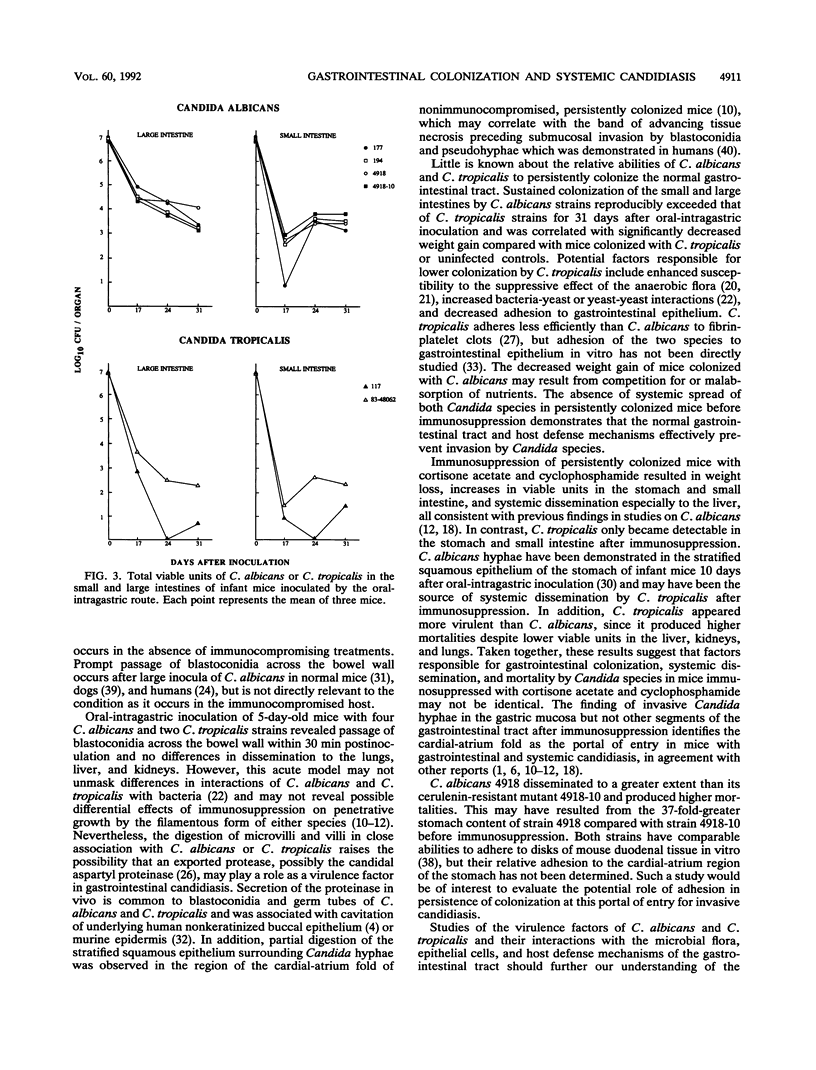
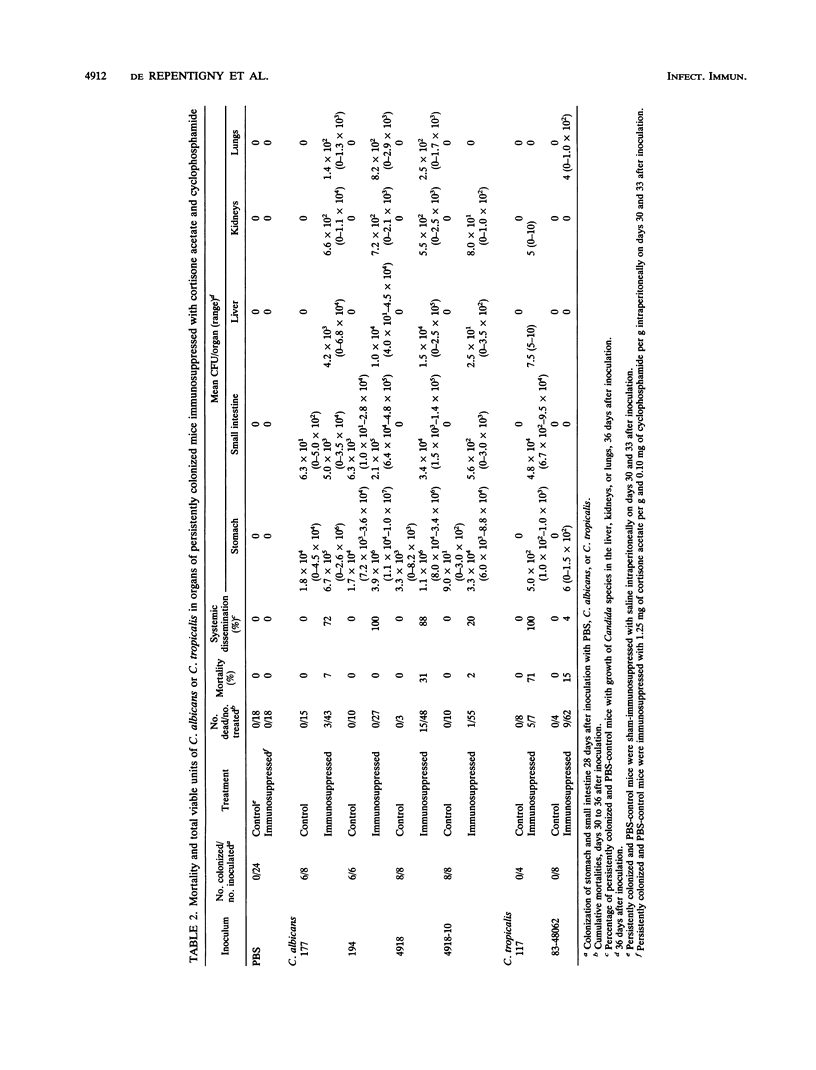
Gastrointestinal colonization and systemic dissemination by Candida albicans and Candida tropicalis were compared in intact and immunocompromised mice. Five-day-old CFW mice were inoculated by the oral-intragastric route with 1.0 x 10(7) CFU of two C. albicans and two C. tropicalis strains isolated from the blood of patients with acute leukemia and with C. albicans 4918 and its cerulenin-resistant mutant 4918-10. C. albicans and C. tropicalis spread to the lungs, liver, and kidneys within 30 min postinoculation, and organ CFU of the two species were comparable over the following 10 days. Close association of blastoconidia with the villous surface of the small intestine resulted in lysis of microvilli and then progressive invasion of villi. Blastoconidia within villi were surrounded by a conspicuous zone of clearing. Persistent colonization of the small and large intestines by C. albicans blood isolates and strains 4918 and 4918-10 was similar for 31 days after inoculation, but consistently exceeded that of C. tropicalis. In mice colonized with C. albicans, immunosuppression with cortisone acetate and cyclophosphamide on days 30 and 33 after inoculation increased stomach CFU 40- to 370-fold and intestinal CFU 30- to 80-fold. In contrast, persistent colonization by C. tropicalis was undetectable before immunosuppression and only became apparent after treatment. C. albicans disseminated more frequently and with higher organ CFU than C. tropicalis. Despite this fact, 20% of mice infected with C. tropicalis died, compared with 4% infected with C. albicans blood isolates. Indirect immunofluorescence revealed penetrative growth by Candida hyphae exclusively in the mucosa and submucosa of the stomach from immunosuppressed, persistently colonized mice. Taken together, the data indicate that C. tropicalis appears to be more virulent than C. albicans and that factors responsible for gastrointestinal colonization, systemic dissemination, and mortality in immunocompromised mice may not be identical.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balish E., Filutowicz H., Oberley T. D. Correlates of cell-mediated immunity in Candida albicans-colonized gnotobiotic mice. Infect Immun. 1990 Jan;58(1):107–113. doi: 10.1128/iai.58.1.107-113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Sbaraglia G., Perito S., Cassone A. A comparison of experimental pathogenicity of Candida species in cyclophosphamide-immunodepressed mice. Sabouraudia. 1984;22(5):409–418. doi: 10.1080/00362178485380661. [DOI] [PubMed] [Google Scholar]
- Bodey G. P. Candidiasis in cancer patients. Am J Med. 1984 Oct 30;77(4D):13–19. [PubMed] [Google Scholar]
- Borg M., Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988 Mar;56(3):626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R. A., Cihlar R. L., Lee D. D., Hoberg K., Scheld W. M. Yeast adhesion in the pathogenesis of endocarditis due to Candida albicans: studies with adherence-negative mutants. J Infect Dis. 1985 Oct;152(4):710–715. doi: 10.1093/infdis/152.4.710. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Acquired immunity to systemic candidiasis in immunodeficient mice. J Infect Dis. 1991 Nov;164(5):936–943. doi: 10.1093/infdis/164.5.936. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990 Apr;58(4):1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect Immun. 1991 Jul;59(7):2447–2455. doi: 10.1128/iai.59.7.2447-2455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Lynn K. T., Seshan K. R. An animal model for oropharyngeal, esophageal and gastric candidosis. Mycoses. 1990 Jan;33(1):7–19. doi: 10.1111/myc.1990.33.1.7. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Lynn K. T., Seshan K. R. Evaluation of a murine model of hepatic candidiasis. J Clin Microbiol. 1990 Aug;28(8):1828–1841. doi: 10.1128/jcm.28.8.1828-1841.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Lynn K. T., Seshan K. R., Pope L. M. Gastrointestinal and systemic candidosis in immunocompromised mice. J Med Vet Mycol. 1989;27(6):363–380. doi: 10.1080/02681218980000491. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Seshan K. R., Pope L. M., Yancey R. J. Morphological aspects of gastrointestinal tract invasion by Candida albicans in the infant mouse. J Med Vet Mycol. 1988 Jun;26(3):173–185. [PubMed] [Google Scholar]
- Ekenna O., Sherertz R. J. Factors affecting colonization and dissemination of Candida albicans from the gastrointestinal tract of mice. Infect Immun. 1987 Jul;55(7):1558–1563. doi: 10.1128/iai.55.7.1558-1563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. H., Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Persistence and spread of Candida albicans after intragastric inoculation of infant mice. Infect Immun. 1981 Feb;31(2):783–791. doi: 10.1128/iai.31.2.783-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R. A., Abruzzo G. K., Giltinan D. M. Candida tropicalis infection in normal, diabetic, and neutropenic mice. J Clin Microbiol. 1987 Aug;25(8):1416–1420. doi: 10.1128/jcm.25.8.1416-1420.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Herrera C. Effects of compromising agents on candidosis in mice with persistent infections initiated in infancy. Infect Immun. 1982 Jan;35(1):222–228. doi: 10.1128/iai.35.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J. Models for studying the role of fungal attachment in colonization and pathogenesis. Mycopathologia. 1990 Feb;109(2):123–137. doi: 10.1007/BF00436792. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A. Dissemination of yeasts after gastrointestinal inoculation in antibiotic-treated mice. Sabouraudia. 1983 Mar;21(1):27–33. doi: 10.1080/00362178385380051. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985 Sep;49(3):654–663. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A. Effect of various antibiotics on gastrointestinal colonization and dissemination by Candida albicans. Sabouraudia. 1985 Aug;23(4):265–273. doi: 10.1080/00362178585380391. [DOI] [PubMed] [Google Scholar]
- Kinsman O. S., Pitblado K. Candida albicans gastrointestinal colonization and invasion in the mouse: effect of antibacterial dosing, antifungal therapy and immunosuppression. Mycoses. 1989 Dec;32(12):664–674. doi: 10.1111/j.1439-0507.1989.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Krause W., Matheis H., Wulf K. Fungaemia and funguria after oral administration of Candida albicans. Lancet. 1969 Mar 22;1(7595):598–599. doi: 10.1016/s0140-6736(69)91534-7. [DOI] [PubMed] [Google Scholar]
- Lehrer N., Segal E., Cihlar R. L., Calderone R. A. Pathogenesis of vaginal candidiasis: studies with a mutant which has reduced ability to adhere in vitro. J Med Vet Mycol. 1986 Apr;24(2):127–131. doi: 10.1080/02681218680000191. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Pazin G. J., Allen C. M. Disseminated candidiasis. Changes in incidence, underlying diseases, and pathology. Am J Clin Pathol. 1977 Jul;68(1):29–38. doi: 10.1093/ajcp/68.1.29. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Joyce W. A., Greenfield R. A. Gastrointestinal candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1991 Jun;59(6):2116–2119. doi: 10.1128/iai.59.6.2116-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T. Comparative studies of gastrointestinal colonization and systemic spread by Candida albicans and nonlethal yeast in the infant mouse. Scan Electron Microsc. 1982;(Pt 4):1667–1676. [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect Immun. 1988 Aug;56(8):1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Samonis G., Anaissie E. J., Rosenbaum B., Bodey G. P. A model of sustained gastrointestinal colonization by Candida albicans in healthy adult mice. Infect Immun. 1990 Jun;58(6):1514–1517. doi: 10.1128/iai.58.6.1514-1517.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford G. R., Merz W. G., Wingard J. R., Charache P., Saral R. The value of fungal surveillance cultures as predictors of systemic fungal infections. J Infect Dis. 1980 Oct;142(4):503–509. doi: 10.1093/infdis/142.4.503. [DOI] [PubMed] [Google Scholar]
- Sandovsky-Losica H., Segal E. Interaction of Candida albicans with murine gastrointestinal mucosa from methotrexate and 5-fluorouracil treated animals: in vitro adhesion and prevention. J Med Vet Mycol. 1990;28(4):279–287. [PubMed] [Google Scholar]
- Sandovsky-Losica H., Segal E. Interaction of Candida albicans with murine gastrointestinal mucosa: effect of irradiation on adherence in vitro. J Med Vet Mycol. 1989;27(6):345–352. [PubMed] [Google Scholar]
- Segal E., Savage D. C. Adhesion of Candida albicans to mouse intestinal mucosa in vitro: development of the assay and test of inhibitors. J Med Vet Mycol. 1986 Dec;24(6):477–479. [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D., Currie C. A., Geheber C. E., Cuzzell J. Z. Candida sepsis: pathogenesis and principles of treatments. Ann Surg. 1974 May;179(5):697–711. doi: 10.1097/00000658-197405000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. J., Merz W. G. Pathologic features in the human alimentary tract associated with invasiveness of Candida tropicalis. Am J Clin Pathol. 1986 Apr;85(4):498–502. doi: 10.1093/ajcp/85.4.498. [DOI] [PubMed] [Google Scholar]
- Wingard J. R., Dick J. D., Merz W. G., Sandford G. R., Saral R., Burns W. H. Differences in virulence of clinical isolates of Candida tropicalis and Candida albicans in mice. Infect Immun. 1982 Aug;37(2):833–836. doi: 10.1128/iai.37.2.833-836.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard J. R., Dick J. D., Merz W. G., Sandford G. R., Saral R., Burns W. H. Pathogenicity of Candida tropicalis and Candida albicans after gastrointestinal inoculation in mice. Infect Immun. 1980 Aug;29(2):808–813. doi: 10.1128/iai.29.2.808-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard J. R., Merz W. G., Saral R. Candida tropicalis: a major pathogen in immunocompromised patients. Ann Intern Med. 1979 Oct;91(4):539–543. doi: 10.7326/0003-4819-91-4-539. [DOI] [PubMed] [Google Scholar]



