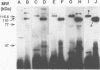
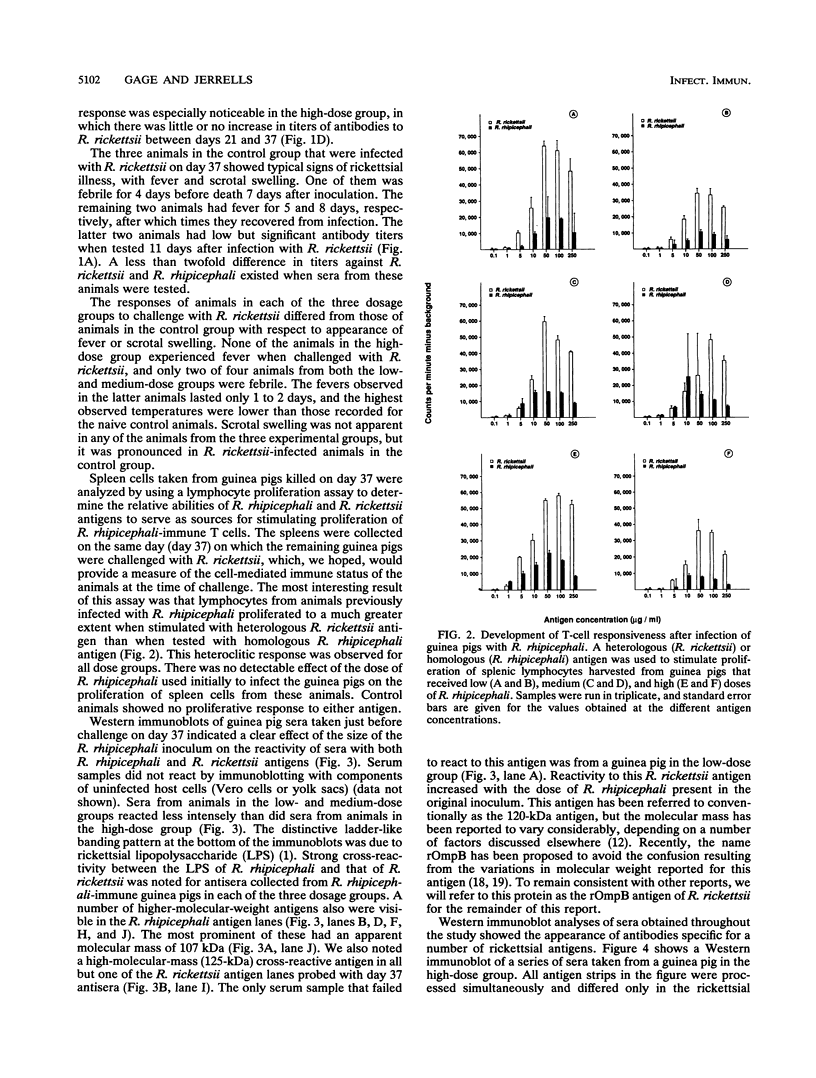
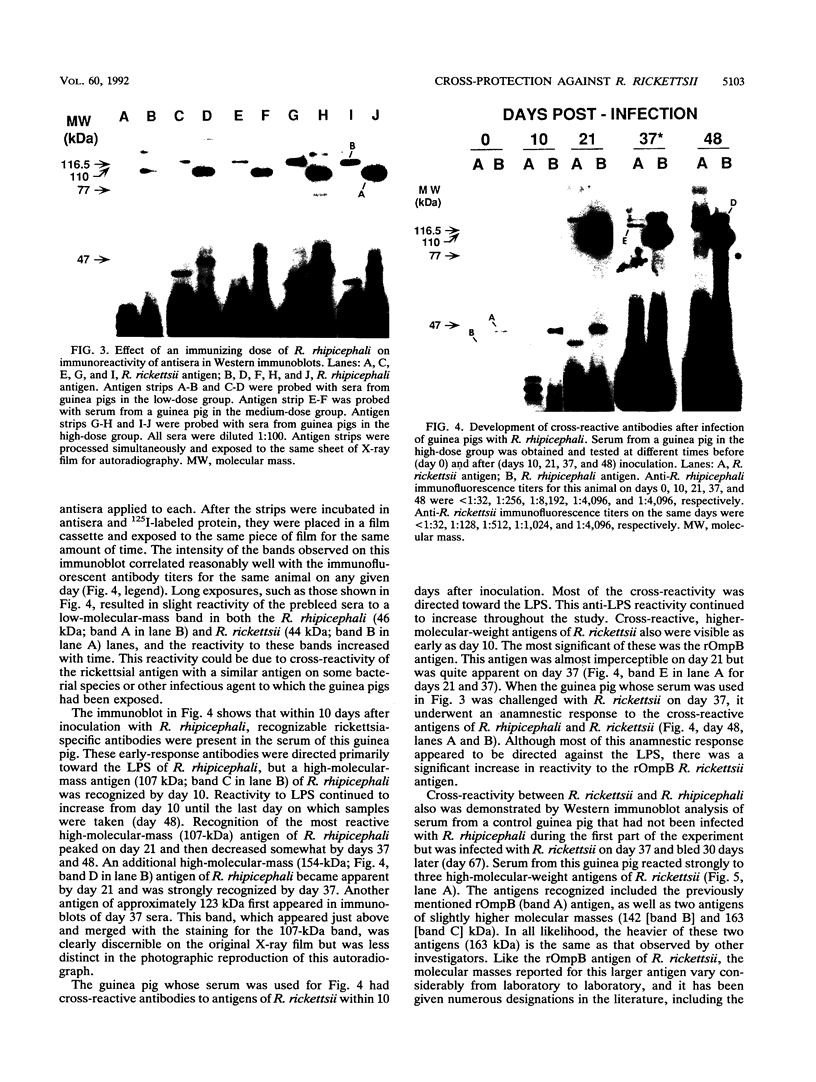
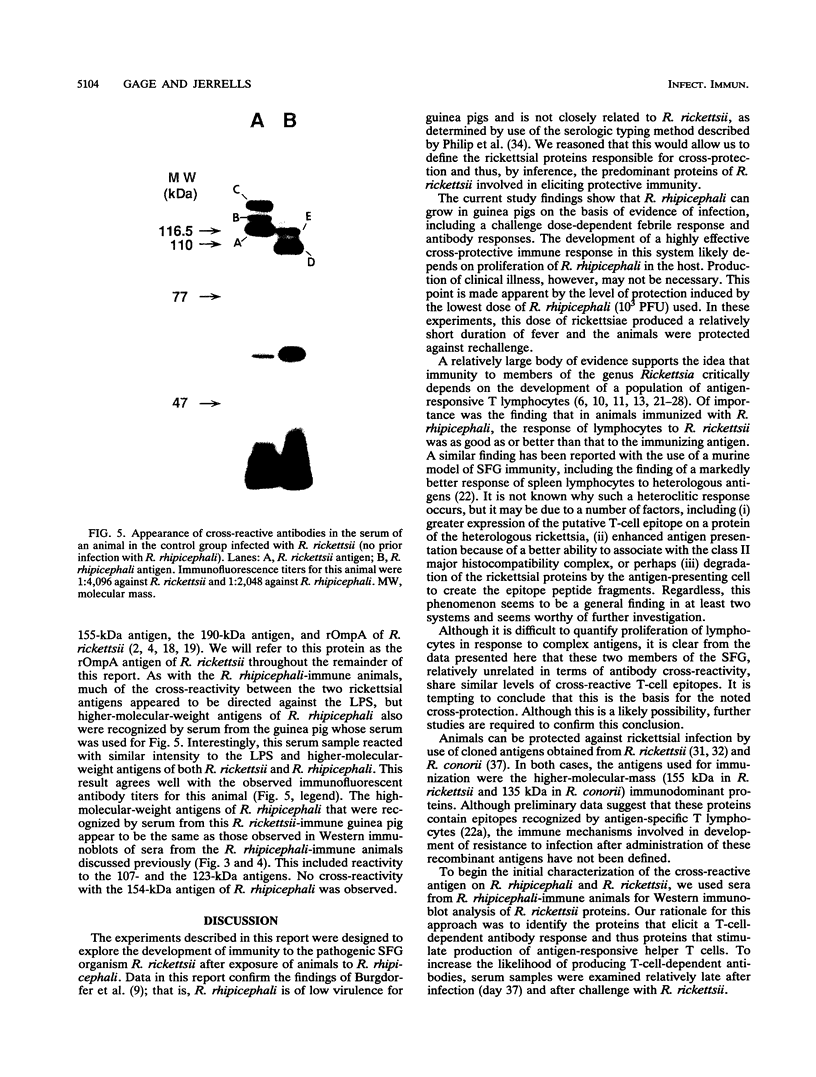
Abstract
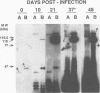
The relatively unrelated spotted fever group rickettsia Rickettsia rhipicephali conferred on guinea pigs protective immunity against challenge with virulent R. rickettsii. Immunity was conferred at all doses of R. rhipicephali used in the study. Because of the serologic unrelatedness of these two rickettsiae, determined by the use of microimmunofluorescence and other serological assays, further studies were performed to define the nature of the immune response elicited by R. rhipicephali and the characteristics of the rickettsial antigens that evoke cross-reactive antibody responses. Animals immune to R. rhipicephali tested at the time of challenge showed a complete cross-reactive lymphocyte proliferative response to rickettsial antigens prepared from each species. In fact, spleen cells from R. rhipicephali-immune animals responded better to R. rickettsii antigens than to homologous immunizing antigens. Serum samples were obtained from R. rhipicephali-infected animals at various times after infection and tested by the use of Western immunoblot assay for antibodies that were cross-reactive with antigens of R. rickettsii. By 10 days after infection with R. rhipicephali, antibodies to antigens of both species were noted, and by 37 days after infection, sera from immune animals showed strong reactivity to antigens of R. rhipicephali with apparent molecular masses of 107 and 151 kDa. The cross-reactive antibody response to antigens of R. rickettsii was relatively strong and involved predominantly the rOmpB protein and the rickettsial lipopolysaccharide. These findings establish the presence of T-cell-dependent epitopes associated with antigens of R. rhipicephali, which confer protective immunity against challenge with R. rickettsii. Results of Western immunoblot assays support the contention that the R. rickettsii rOmpB surface antigen contains important protective epitopes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., List R. H., Mann R. E., Hayes S. F., Thomas L. A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985 Jun;151(6):1052–1060. doi: 10.1093/infdis/151.6.1052. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., Mann R. E., Gonzales C. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J Clin Microbiol. 1987 Jan;25(1):167–171. doi: 10.1128/jcm.25.1.167-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., McDonald G. A., List R. H., Mann R. E. Neutralizing activity of monoclonal antibodies to heat-sensitive and heat-resistant epitopes of Rickettsia rickettsii surface proteins. Infect Immun. 1987 Mar;55(3):825–827. doi: 10.1128/iai.55.3.825-827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. E., McDonald G. A., Jones D. C., Regnery R. L. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990 Sep;58(9):2760–2769. doi: 10.1128/iai.58.9.2760-2769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bourgeois A. L., Dasch G. A., Strong D. M. In vitro stimulation of human peripheral blood lymphocytes by soluble and membrane fractions of renografin-purified typhus group rickettsiae. Infect Immun. 1980 Feb;27(2):483–491. doi: 10.1128/iai.27.2.483-491.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Sexton D. J., Gerloff R. K., Anacker R. L., Philip R. N., Thomas L. A. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect Immun. 1975 Jul;12(1):205–210. doi: 10.1128/iai.12.1.205-210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl M., Dasch G. A. The importance of the crystalline surface layer protein antigens of rickettsiae in T-cell immunity. J Autoimmun. 1989 Jun;2 (Suppl):81–91. doi: 10.1016/0896-8411(89)90119-4. [DOI] [PubMed] [Google Scholar]
- Catanzaro P. J., Shiral A., Agniel L. D., Jr, Osterman J. V. Host defenses in experimental scrub typhus: role of spleen and peritoneal exudate lymphocytes in cellular immunity. Infect Immun. 1977 Oct;18(1):118–123. doi: 10.1128/iai.18.1.118-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Dasch G. A., Carl M., Dobson M. E. Structural analyses of the 120-kDa serotype protein antigens of typhus group rickettsiae. Comparison with other S-layer proteins. Ann N Y Acad Sci. 1990;590:334–351. doi: 10.1111/j.1749-6632.1990.tb42241.x. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D., Shepard C. C. Lymphocyte transformation in rickettsioses. J Immunol. 1971 Jan;106(1):209–216. [PubMed] [Google Scholar]
- Cory J., Yunker C. E., Ormsbee R. A., Peacock M., Meibos H., Tallent G. Plaque assay of rickettsiae in a mammalian cell line. Appl Microbiol. 1974 Jun;27(6):1157–1161. doi: 10.1128/am.27.6.1157-1161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W. C., Waner J. L. Serological cross-reaction and cross-protection in guinea pigs infected with Rickettsia rickettsii and Rickettsia montana. Infect Immun. 1980 May;28(2):627–629. doi: 10.1128/iai.28.2.627-629.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R. D., Jr, Cieplak W., Jr, Policastro P. F., Hackstadt T. The 120 kilodalton outer membrane protein (rOmp B) of Rickettsia rickettsii is encoded by an unusually long open reading frame: evidence for protein processing from a large precursor. Mol Microbiol. 1991 Oct;5(10):2361–2370. doi: 10.1111/j.1365-2958.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Hackstadt T., Messer R., Cieplak W., Peacock M. G. Evidence for proteolytic cleavage of the 120-kilodalton outer membrane protein of rickettsiae: identification of an avirulent mutant deficient in processing. Infect Immun. 1992 Jan;60(1):159–165. doi: 10.1128/iai.60.1.159-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphres R. C., Hinrichs D. J. Role of antibody in Coxiella burnetii infection. Infect Immun. 1981 Feb;31(2):641–645. doi: 10.1128/iai.31.2.641-645.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Eisemann C. S. Role of T-lymphocytes in production of antibody to antigens of Rickettsia tsutsugamushi and other Rickettsia species. Infect Immun. 1983 Aug;41(2):666–674. doi: 10.1128/iai.41.2.666-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Jarboe D. L., Eisemann C. S. Cross-reactive lymphocyte responses and protective immunity against other spotted fever group rickettsiae in mice immunized with Rickettsia conorii. Infect Immun. 1986 Mar;51(3):832–837. doi: 10.1128/iai.51.3.832-837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983 Apr;40(1):147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: delayed-type hypersensitivity responses of inbred mice. Infect Immun. 1982 Jan;35(1):117–123. doi: 10.1128/iai.35.1.117-123.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Ascher M. S., Kishimoto R. A., Pedersen C. E., Jr In vitro guinea pig leukocyte reactions to Rickettsia rickettsii. Infect Immun. 1977 Dec;18(3):840–846. doi: 10.1128/iai.18.3.840-846.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Pedersen C. E., Jr Immune responses to Rickettsia akari infection in congenitally athymic nude mice. Infect Immun. 1980 May;28(2):310–313. doi: 10.1128/iai.28.2.310-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokorin I. N., Kabanova E. A., Shirokova E. M., Abrosimova G. E., Rybkina N. N., Pushkareva V. i. Role of T lymphocytes in Rickettsia conorii infection. Acta Virol. 1982 Jan;26(1-2):91–97. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDonald G. A., Anacker R. L., Garjian K. Cloned gene of Rickettsia rickettsii surface antigen: candidate vaccine for Rocky Mountain spotted fever. Science. 1987 Jan 2;235(4784):83–85. doi: 10.1126/science.3099387. [DOI] [PubMed] [Google Scholar]
- McDonald G. A., Anacker R. L., Mann R. E., Milch L. J. Protection of guinea pigs from experimental Rocky Mountain spotted fever with a cloned antigen of Rickettsia rickettsii. J Infect Dis. 1988 Jul;158(1):228–231. doi: 10.1093/infdis/158.1.228. [DOI] [PubMed] [Google Scholar]
- Murphy J. R., Wisseman C. L., Jr, Fiset P. Mechanisms of immunity in typhus infection: analysis of immunity to Rickettsia mooseri infection of guinea pigs. Infect Immun. 1980 Mar;27(3):730–738. doi: 10.1128/iai.27.3.730-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., McDonald G. A., Watkins N. G. A recombinant Rickettsia conorii vaccine protects guinea pigs from experimental boutonneuse fever and Rocky Mountain spotted fever. Infect Immun. 1990 Mar;58(3):646–653. doi: 10.1128/iai.58.3.646-653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]