Abstract
It is becoming increasingly clear that protein phosphatases are important modulators of cellular function and that disruption of these proteins are involved in neurodegenerative disease processes. Serine/threonine protein phosphatases (PP) such as protein phosphatase PP1, PP2A, and calcineurin are involved in hyperphosphorylation of τ- as well as β-amyloid-induced cell death. We have previously shown serine/threonine protein phosphatases to be involved in estrogen-mediated neuroprotection. The purpose of this study was to delineate the role of PP1, PP2A, and calcineurin in the mechanism of estrogen mediated neuroprotection against oxidative stress and excitotoxicity. Treatment with protein phosphatases inhibitor II, endothall, or cyclosporin A, which are specific inhibitors of PP1, PP2A, and calcineurin, respectively, did not have an effect on cell viability. However, in combination, these inhibitors adversely affected cell survival, which suggests the importance of serine/threonine protein phosphatases in maintenance of cellular function. Inhibitors of PP1, PP2A, and calcineurin attenuated the protective effects of estrogen against glutamate-induced -neurotoxicity but did not completely abrogate the estrogen-mediated protection. The attenuation of estrogen-induced neuroprotection was achieved through decrease in the activity of theses serine/threonine phosphatases without the concomitant decrease in protein expression. In an animal model, transient middle cerebral artery occlusion caused a 50% decrease in levels of PP1, PP2A, and PP2B ipsilateral to the lesion in a manner that was prevented by estradiol pretreatment. Therefore, we conclude that in the face of cytotoxic challenges in vitro and in vivo, estrogens maintain the function of PP1, PP2A, and calcineurin.
NEURODEGENERATIVE DISEASES, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), involve multifaceted processes that lead to neuronal dysfunction and ultimately death. Much experimental data show neurodegenerative diseases exhibit increased oxidative and excitotoxic stress (1,2,3,4,5), ubinquintin-proteosome system dysfunction (6,7,8), mitochondrial dysfunction (9,10), synaptic failure, and abnormal phosphorylation/dephosphorylation (11,12,13,14,15) among other system failures. The question of cause and effect is yet unresolved, but these dysfunctional processes are unifying pathologies in many slow, progressive neurodegenerative diseases. It is clear, however, that phosphorylation and dephosphorylation processes are important in not only general maintenance of neuronal function but also diseased states.
Aberrant neuronal expression of phosphorylated ERK1/2 and other MAPKs in AD patients’ brains is association with markers of oxidative stress (16). MAPK phosphorylation was also noted in a variety of sporadic and familial neurodegenerative diseases characterized by τ-deposits (11,13). Phospho-ERK1/2 is increased in substantia nigra neurons of patients with PD and other Lewy body diseases, and the midbrains of these patients show elevated ERK activity (13). In addition to chronic neurodegenerative diseases, increased ERK1/2 phosphorylation has been noted in the vulnerable penumbra after acute ischemic stroke in humans (17). Also, hyperphosphorylation of τ is one of the major hallmarks of AD. In addition, the activities of various serine/threonine protein phosphatases (PP) have been shown to be decreased in AD brains, compared to age-matched controls (18). Interestingly, τ and various MAPKs are substrates of serine/threonine phosphatases, such as PP1, PP2A, and calcineurin.
Experimental and epidemiological studies have demonstrated the beneficial effects of estrogens against neuronal dysfunction and memory loss (19,20,21,22,23). We and others have shown that estrogens and estrogen analogs are potent neuroprotectants in vitro against a variety of toxicities, including serum deprivation, oxidative stress, β-amyloid-induced toxicity, and excitotoxicity (24,25,26,27,28,29,30). In vivo studies have also demonstrated the neuroprotective effects of estrogens in animal models of transient and permanent middle cerebral artery occlusion (31,32,33), global forebrain ischemia (34), photothrombotic focal ischemia (35), glutamate-induced focal cerebral ischemia (36), and subarachnoid hemorrhage (37).
The mechanisms of estrogen-induced neuroprotection are unclear. However, we have recently shown that inhibition of serine/threonine phosphatases completely abrogated the neuroprotective effects of 17β-estradiol (38). In the present study, we examined the specific protein phosphatases that are involved in the neuroprotective effects of estrogens.
Materials and Methods
Chemicals
17β-Estradiol was purchased from Steraloids, Inc. (Wilton, NH) and dissolved in dimethyl sulfoxide at a concentration of 10 mm and diluted to appropriate concentration in culture media. Calcein AM was purchased from Molecular Probes, Inc. (Eugene, OR). Okadaic acid, l-glutamate, and dimethyl sulfoxide were purchased from Sigma (Paris, KY). Protein phosphatase inhibitor II (PPI2), endothall, and cyclosporin A were purchased from Calbiochem (San Diego, CA). Anti-PP1, anti-PP2A, and anti-PP2B were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell culture
HT-22 and C-6 glioma cells were cultured in DMEM supplemented with 10% charcoal-stripped fetal bovine serum (HyClone, Logan, UT) and gentamicin (50 μg/ml) at 37 C in an atmosphere containing 5% CO2 and 95% air. HT-22 cells were obtained from David Schubert (Salk Institute, San Diego, CA). C6-gilioma cells were obtained from American Type Culture Collection (Manassas, VA). HT-22 and C6-glioma cultures were maintained at 50% and 100% confluency, respectively, in monolayers in plastic 75-cm2 flasks. For viability assays, HT-22 and C6-glioma cells were seeded in 96-well plates at a density of 3,500 cells/well, and for immunoblot analysis, the cells were seeded in 100-mm dishes at a density of 250,000 cells/ml.
Culture of primary cortical neurons
Cerebral cortex rat embryos (18 d) were dissected and harvested in preparation medium (DMEM, glucose 4.5 g/liter, penicillin 100 μ/ml, streptomycin 100 μg/ml). Individual cells were isolated by mechanical trituration using three different sizes of fire polished Pasteur pipettes. The cells were harvested in seeding medium (DMEM, glucose 4.5 g/liter, penicillin 100 μg/ml, streptomycin 100 μg/ml, glutamine 2 mm, 5% horse serum) and filtered through a 40-μm filter. Cerebral cortical cells were seeded in various poly-l-lysine-treated dishes at a density of 500,000 cells/ml and 96-well plates at 25,000 cells/well. The cells were incubated in neurobasal medium (DMEM, glucose 4.5 g/liter, penicillin 100 μ/ml, streptomycin 100 μg/ml, glutamine 2 mm) supplemented with 2% B-27 containing antioxidants in normal cell culture condition of 37 C in a humid atmosphere of 5% CO2. Media were changed every third day, and experiments were performed after 14 d culture in vitro. Two hours before treatment with various inhibitors, glutamate, and/or 17β-estradiol, the media were replaced with neurobasal medium supplemented with B-27 minus antioxidants.
Calcein-AM viability assay
Primary cortical neurons, HT-22, and C6-glioma cells were exposed to various treatments and then were rinsed with PBS, and cell viability was measured using the membrane-permeant calcein AM dye (Molecular Probes). Calcein AM is a fluorogenic esterase substrate that is hydrolyzed to a fluorescent product in cells having esterase activity and intact membranes. Cells were incubated in a solution of 2.5 μm calcein AM in PBS. Twenty minutes later, fluorescence was determined using a Bio-Tek FL600 microplate reader (Winooski, VT) with an excitation/emission filter set of 485/530 nm. Cell culture wells treated with methanol served as blanks. The results, obtained in relative fluorescent units, are expressed as the percentage of untreated or vehicle-treated control values.
Immunoblot analysis
Protein from whole-cell lysates (25 μg) was separated by SDS-PAGE and transferred to Immunobilon-P polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Membranes were rinsed in Tris-buffered saline [10 mm Tris-base (pH 8.0), 100 mm NaCl] containing 0.2% Tween 20 and then blocked with 3% BSA. Blots were then incubated with primary antibodies overnight at 4 C, rinsed, and incubated in the appropriate secondary antibody before detection using enhanced chemiluminescence (Pierce Biotechnology, Inc., Rockford, IL). Enhanced chemiluminescence results were digitized and quantified using a bioimaging system (UVP, Upland, CA). Protein concentration was determined by Bio-Rad protein assay using BSA as the standard (Bio-Rad Laboratories, Hercules, CA). Immunoblots were normalized to β-actin and graphs are represented as percent nontreated controls.
Serine/threonine protein phosphatase activity assay
Calcineurin activity was quantitated using a nonradioactive assay system (Promega, Madison, WI), according to the supplier’s instructions with a minor modification (39,40,41). Briefly, primary neurons were lysed on ice using 250 μl of storage buffer containing 50 mm Tris-HCl (pH 7.5), 1 mm EDTA, 0.1% β-mercaptoethanol, 0.1 mm leupeptin, and 75 μm pepstatin A. After centrifugation (100,000 × g at 4 C for 1 h), the supernatant solution was applied to a Sephadex G-25 resin column and centrifuged at 600 × g at 4 C for 5 min. The sample lysate in storage buffer was obtained. The sample lysate (5 μg) was added to the reaction premix containing phospho-threonylpeptide in 5 μl of phosphate-free water, 10 μl protein phosphatase 2B 5× buffer [250 mm imidazole (pH 7.2), 1 mm EGTA, 5 mm NiCl2, 250 μg/ml calmodulin, 0.1% β-mercaptoethanol, 100 μg/ml BSA, 200 μm sodium vanadate, and 500 nm okadaic acid], and 30 μl storage buffer in the well of a 96-well plate. After incubation for 30 min, 50 μl of molybdate dye/additive mixture were added to stop the reaction. The OD of the samples was obtained using a microplate reader with a 630-nm filter. The calcineurin activity in each sample was calculated using a standard curve for free phosphate generated by a phosphate standard solution. After the calculation, phosphatase activity was divided by the protein content in each sample as measured by a protein assay system (Bio-Rad Laboratories). All experiments were performed in triplicate.
PP2A activity was measured in the manner described above; however, instead of the PPTase-2B 5× buffer, 10 μl protein phosphatase 2A 5× buffer [250 mm imidazole (pH 7.2), 1 mm EGTA, 0.1% β-mercaptoethanol, 500 μg/ml BSA] was used.
Experimental animals
Female Sprague Dawley rats (weight, 250 g; Charles River, Wilmington, MA) were acclimatized for 3 d before surgery. Bilateral ovariectomy was performed 2 wk before transient middle cerebral artery occlusion (tMCAO). Ischemic stroke was induced by tMCAO as previously described (42). For the 17β-estradiol treatment group, 17β-estradiol was first dissolved in 100% ethanol; then corn oil was added and the ethanol was allowed to evaporate. 17β-Estradiol was administered at the dose of 100 μg/kg body weight via sc injection between the shoulder blades 2 h before the onset of stroke. Control group of animals received injection of equal volume of corn oil. Briefly, animals were anesthetized by ip injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). Rectal temperature was maintained at 37.5 ± 0.5 C during the procedure. The left femoral artery was canalized and connected to a blood pressure monitor for mean arterial blood pressure monitoring. The left middle cerebral artery was occluded by a 3–0 monofilament suture introduced via the internal carotid artery. After 1 h, the suture was withdrawn for reperfusion. Animals were decapitated after 23 h of reperfusion. Cerebral cortex of the ipsilateral and the contralateral hemispheres were harvested and homogenized for protein analysis. All animal procedures were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Dose and sampling time
17β-Estradiol was used at a concentration of 100 nm, which was shown to be potently neuroprotective (43,44) and to preserve protein phosphatase activity against glutamate toxicity (44). Glutamate (50 μm) and okadaic acid (50 nm) concentrations were used to produce 50% cell death in primary cortical cultures (38). All samples were collected 24 h after treatment with glutamate, okadaic acid, estrogens, and/or kinase inhibitors because we have shown in our previous studies that okadaic acid abolishes estrogen-mediated neuroprotection against glutamate when sampled at 24 h (38,44).
Statistical analysis
Statistical significance was determined by one-way ANOVA followed by a Tukey’s multiple comparison test. P < 0.05 was considered significant for all experiments. Each set of data represents three or more independent assays. For viability assays, 10 independent experiments, each containing two replicate wells, were performed. For activity assays, six independent experiments, each containing three replicate wells, were performed. The values are reported as the mean ± sem.
Results
Effects of PPI2, endothall, and/or cyclosporine A on cell viability in primary cortical neurons
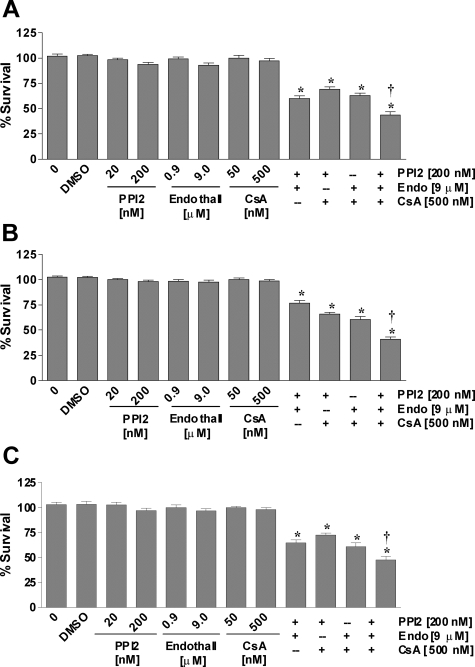
Our previous studies showed that nonspecific inhibition of serine/threonine protein phosphatases with okadaic acid or calyculin A was neurotoxic in primary cortical neurons, HT-22 cells, and C6-glioma cells (38). To determine the effects of individual protein phosphatase inhibition on cell viability, we examined the effects of specific protein phosphatase inhibitors on the viability of neurons and glia. Protein phosphatase inhibitor-2 specifically and potently inhibits the catalytic subunit of PP1 with an IC50 of 2 nm. Endothall is a cell-permeable, specific inhibitor of protein phosphatase 2A (IC50 = 90 nm), which exhibits a weak effect on PP1 (IC50 = 5 μm). Cyclosporin A forms a complex with cyclophilin to inhibit calcineurin with nanomolar affinity. Specific inhibition of PP1, PP2A, or calcineurin had no effect on cell viability in HT-22 cells (Fig. 1A), C6-glia (Fig. 1B), or primary neurons (Fig. 1C). Interestingly, any combination of two inhibitors of PP1, PP2A, or calcineurin caused a decrease in cell viability by approximately 25–35%, and inhibiting all three phosphatases caused 50% reduction in cell survival.
Figure 1.
Effects of PPI2, endothall, and cyclosporine A (CsA) on cell survival. HT-22 cells and C6-glioma were seeded into 96-well plates at a density of 3500 cells/well. Primary cortical neurons were seeded into 96-well plates at a density of 25,000 cells/well. A, HT-22 cells were treated with varying concentrations of PPI2, endothall, and/or cyclosporine A. B, C-6 cells were treated with varying concentrations of PPI2, endothall, and/or cyclosporine A. C, Primary cortical neurons were treated with varying concentrations of PPI2, endothall, and/or cyclosporine A. Cell viability was determined by calcein AM assay (Molecular Probes) after 24 h exposure to the various compounds. All data were normalized to percent survival of nontreated control. Depicted are mean ± sem for 10 independent experiments with two replicates per experiment. DMSO, Dimethyl sulfoxide. *, P < 0.05 vs. vehicle control; †, P < 0.05 vs. two combination treatment.
Effects of PPI2, endothall, and/or cyclosporine A on estrogen-mediated neuroprotection against glutamate induced cytotoxicity in primary cortical neurons
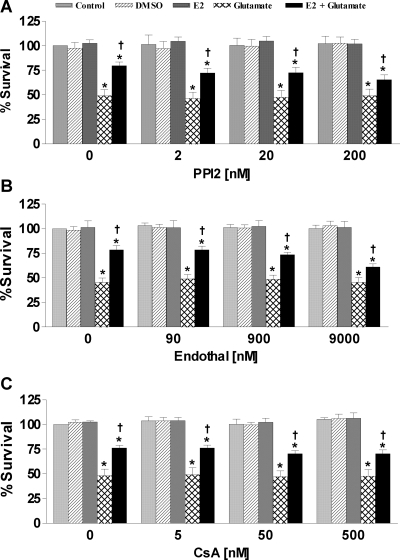
Nontoxic concentrations of okadaic acid have been shown to abrogate the estrogen-mediated neuroprotection against oxidative stress and excitotoxicity (38). Therefore, we wanted to assess whether individually inhibiting PP1, PP2A, or calcineurin would have a similar effect. Glutamate exposure caused approximately 50% cell death in primary neurons. Simultaneous treatment with 17β-estradiol protected approximately 60% of the cells exposed to glutamate. In addition, PP1, PP2A, or calcineurin inhibition resulted in attenuating the estrogen effect by approximately 70% (Fig. 2). Although individual inhibition of PP1, PP2A, or calcineurin did not have any effect on cell viability, inhibition of PP1, PP2A, or calcineurin attenuated but did not completely block the estrogen-mediated neuroprotection against glutamate-induced neurotoxicity in primary cortical neurons, which suggests that inhibition of just one of these serine/threonine phosphatases are not sufficient to block the estrogen effect.
Figure 2.
Effects of PPI2, endothall, or cyclosporine A on 17β-estradiol-mediated neuroprotection in primary cortical neurons. Primary cortical neurons were seeded into 96-well plates at a density of 25,000 cells/well. A, Cells were treated simultaneously with 200 nm PPI2, 50 μm glutamate, and/or 100 nm 17β-estradiol. B, Cells were treated simultaneously with 9 μm endothall, 50 μm glutamate, and/or 100 nm 17β-estradiol. C, Cells were treated simultaneously with 500 nm CsA, 50 μm glutamate, and/or 100 nm 17β-estradiol. Cell viability was determined by calcein AM assay (Molecular Probes) after 24 h exposure to the various compounds. All data were normalized to percent survival of nontreated control. Depicted are mean ± sem for 10 independent experiments with two replicates per experiment. *, P < 0.05 vs. vehicle control; †, P < 0.05 vs. glutamate-treated group.
PP1, PP2A, and calcineurin protein expression in estrogen-mediated neuroprotection against glutamate in the presence of specific inhibitor of PP1, PP2A, or calcineurin in primary cortical neurons
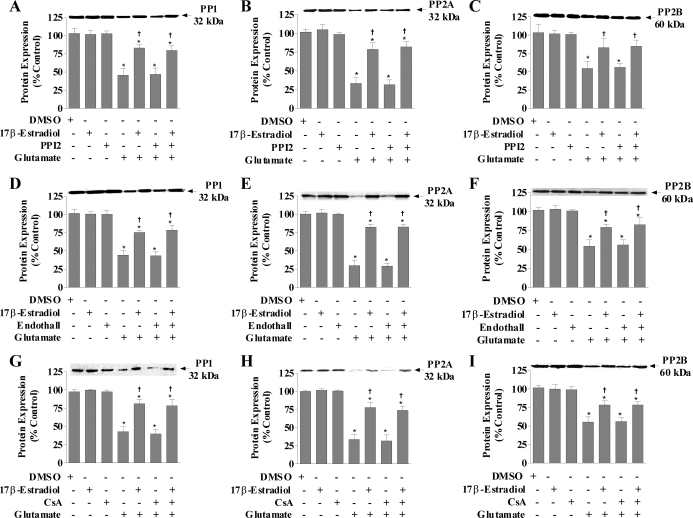
Next, we examined the protein expressions of PP1, PP2A, and calcineurin in response to treatment with glutamate and/or 17β-estradiol in the presence of the specific inhibitors in primary cortical neurons. Twenty-four hours of treatment with 17β-estradiol or PPI2 alone did not significantly alter the expression of PP1, PP2A, or calcineurin compared with nontreated controls (Fig. 3A–C, respectively) and normalized to β-actin (not shown). Glutamate treatment caused approximately 50% reduction in PP1 protein levels. The presence of 17β-estradiol prevented this glutamate-mediated decrease in PP1, PP2A, and calcineurin protein expressions; however, inhibition of PP1 with PPI2 did not block the decreases in PP1, PP2A, and calcineurin expressions caused by glutamate toxicity. In addition, the presence of PPI2 did not alter the 17β-estradiol attenuation of the glutamate-induced decreases in PP1, PP2A, and calcineurin protein expressions. PP2A inhibition with endothall (Fig. 3, D–F) or calcineurin inhibition with cyclosporine A (Fig. 3, G–I) also had no effect on the 17β-estradiol-mediated attenuation of decreased protein contents of PP1, PP2A, or calcineurin caused by glutamate toxicity.
Figure 3.
PP1, PP2A, and calcineurin protein expression in response to glutamate and/or 17β-estradiol treatment in the presence of specific inhibitors of PP1, PP2A, or calcineurin in primary cortical neu. Primary cortical neurons were seeded in 100-mm dishes at a density of 500,000 cells/ml. Cells were treated simultaneously with 200 nm PPI2, 9 μm endothall, 500 nm cyclosporine A (CsA), 50 μm glutamate, and/or 100 nm 17β-estradiol. Cells were harvested after 24 h of treatment for Western blot analysis of PP1 (A, D, and G), PP2A (B, E, and H), and calcineurin (C, F, and I). Immunoblots were normalized to β-actin and graphs are represented as percent nontreated controls. Depicted are mean ± sem for six independent experiments. DMSO, Dimethyl sulfoxide. *, P < 0.05 vs. control; †, P < 0.05 vs. glutamate-treated group.
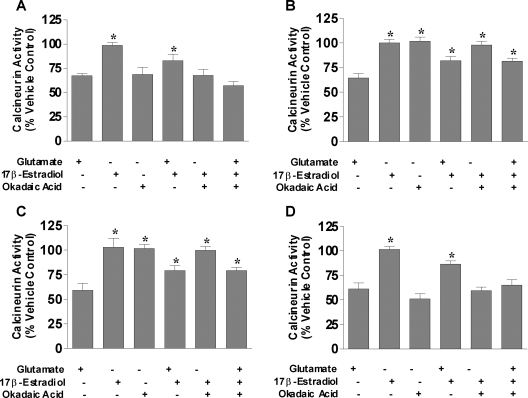
PP2A and calcineurin activity in estrogen-mediated neuroprotection against glutamate-induced cell death in primary cortical neurons
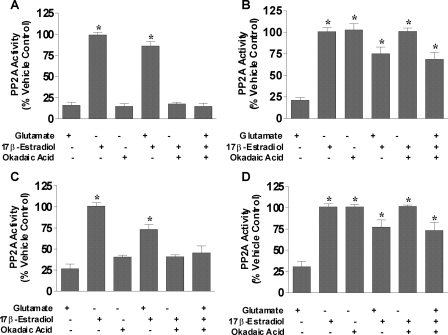
We show that estrogens protect neurons and other types of cells via maintenance of serine/threonine phosphatase expression at control levels during oxidative and excitotoxic stress; however, protein expression does not always translate equally to activity. Therefore, the activities of PP2A and calcineurin were measured in primary cortical neurons to determine whether estrogen-mediated neuroprotection required the activity of serine/threonine phosphatases. The effects of glutamate, 17β-estradiol, and various specific inhibitors of protein phosphatases on the activity of PP2A and calcineurin in primary cortical neurons were measured by simultaneously treating with glutamate, okadaic acid, 17β-estradiol, PPI2, endothall, and/or cyclosporin A. Figures 4 and 5 show the PP2A and calcineurin activities in cortical neurons, respectively. Glutamate, okadaic acid, and endothall treatment caused decreases in PP2A activity, whereas PPI2 and cyclosporine A had no effect (Fig. 4). The decreased PP2A activity mediated by glutamate was attenuated in the presence 17β-estradiol; however, 17β-estradiol was unable block the okadaic acid or endothall-mediated decrease in PP2A activity (Fig. 4, A and C). In fact, the presence of okadaic acid or endothall prevented the 17β-estradiol-mediated attenuation of the decreased PP2A activity caused by glutamate. Inhibition of PP1 with PPI2 or calcineurin with cyclosporin A had no effect on PP2A activity either in the presence or absence of glutamate and/or 17β-estradiol (Fig. 4, B and D). Calcineurin activity was also measured using the same treatment paradigm (Fig. 5). Glutamate or okadaic acid treatment of neurons reduced calcineurin activity by only approximately 30–40% contrasted to the approximately 75–80% reduction seen in PP2A activity. Cyclosporine A inhibited calcineurin activity to approximately 50% of nontreated control (Fig. 5D), whereas neither PP1 nor PP2A inhibitors had no effect on calcineurin activity (Fig. 5, B and C, respectively). Estrogen attenuated the glutamate-mediated decrease in calcineurin activity, but neither the okadaic acid- nor cyclosporine A-induced reduction in calcineurin activity was blocked by 17β-estradiol. Although glutamate treatment induced decreases in PP2A and calcineurin activities, the extent to which this reduction occurs was much greater for PP2A than calcineurin, suggesting that PP2A activity is more prominent than calcineurin activity in neurons in response to oxidative and excitotoxic stresses.
Figure 4.
PP2A activity in primary cortical neurons after treatment with glutamate and/or 17β-estradiol in the presence of specific inhibitors of PP1, PP2A, or calcineurin. Primary cortical neurons were seeded in 100-mm dishes at a density of 500,000 cells/ml. A, Cells were treated simultaneously with 50 nm okadaic acid, 50 μm glutamate, and/or varying 100 nm 17β-estradiol. B, Cells were treated simultaneously with 200 nm PPI2, 50 μm glutamate, and/or 100 nm 17β-estradiol. C, Cells were treated simultaneously with 9 μm endothall, 50 μm glutamate, and/or 100 nm 17β-estradiol. D, Cells were treated simultaneously with 500 nm cyclosporine A (CsA), 50 μm glutamate, and/or 100 nm 17β-estradiol. PP2A activity was determined using a serine/threonine phosphatase activity assay (Promega) after 24 h exposure to the various compounds. All data were normalized to percent survival of vehicle-treated control. Depicted are mean ± sem for six independent experiments with triplicates per experiment. *, P < 0.05 vs. glutamate-treated group.
Figure 5.
Calcineurin activity in primary cortical neurons after treatment with glutamate and/or 17β-estradiol in the presence of specific inhibitors of PP1, PP2A, or calcineurin. Primary cortical neurons were seeded in 100-mm dishes at a density of 500,000 cells/ml. A, Cells were treated simultaneously with 50 nm okadaic acid, 50 μm glutamate, and/or varying 100 nm 17β-estradiol. B, Cells were treated simultaneously with 200 nm PPI2, 50 μm glutamate, and/or 100 nm 17β-estradiol. C, Cells were treated simultaneously with 9 μm endothall, 50 μm glutamate, and/or 100 nm 17β-estradiol. D, Cells were treated simultaneously with 500 nm cyclosporine A (CsA), 50 μm glutamate, and/or 100 nm 17β-estradiol. Calcineurin activity was determined using a serine/threonine phosphatase activity assay (Promega) after 24 h exposure to the various compounds. All data were normalized to percent survival of vehicle-treated control. Depicted are mean ± sem for six independent experiments with triplicates per experiment. *, P < 0.05 vs. glutamate-treated group.
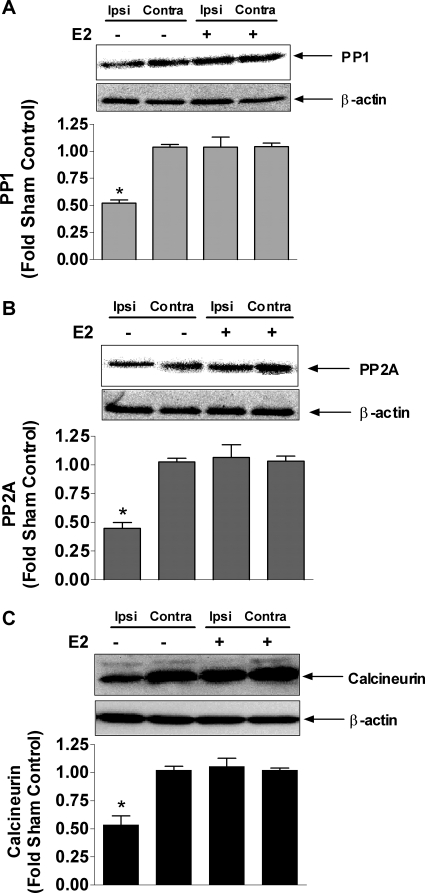
PP1, PP2A, and calcineurin protein expression in estrogen-mediated neuroprotection in stroke
To determine the in vivo relevance of estrogen-mediated changes in phosphatase expression and activity, we examined PP1, PP2A, and calcineurin levels in an animal model of stroke. Female ovariectomized rats underwent tMCAO, and some were pretreated with 17β-estradiol (100 μg/kg, sc) 2 h before the onset of tMCAO. PP1, PP2A, and calcineurin levels were decrease by approximately 50% in the ipsilateral cortex, compared with the contralateral cortex. Estrogen pretreatment prevented the stroke-mediated decrease in PP1, PP2A, and calcineurin (Fig. 6).
Figure 6.
PP1, PP2A, and calcineurin protein expressions after tMCAO in female rats. Ovariectomized female rats were pretreated with vehicle or 17β-estradiol (100 μg/kg, sc) 2 h before the tMCAO. Brains were harvested after 23 h reperfusion after a 1-h occlusion period. Western blot analysis of PP1 (A), PP2A (B), and calcineurin (C) are shown for ipsilateral (ipsi, stroked) and contralateral (contra, nonstroked) with or without 17β-estradiol treatment. Immunoblots were normalized to β-actin and graphs are represented as percent sham controls. Depicted are mean ± sem for six animals per treatment group. *, P < 0.05 vs. contralateral nonstroked side.
Discussion
These observations are important in view of the Women’s Health Initiative Memory Study results that indicate chronic postmenopausal treatment of older women with estrogens (45,46,47,48) not only does not protect the brain from dementia but also appears to exacerbate the condition. The Women’s Health Initiative Memory Study results are inconsistent with numerous experimental, epidemiological, and clinical data that show the beneficial effect of estrogen replacement therapy on cognition and delayed onset of neurodegenerative diseases such as AD (23,49,50,51,52,53). As the elderly population grows in magnitude, it is necessary to devise a treatment paradigm to cure, prevent, or delay the progression of neurodegenerative diseases, such as AD and PD. Yet despite major efforts, the mechanisms responsible for neurodegeneration and estrogen-mediated neuroprotection remain unclear. The present study shows that maintenance of serine/threonine protein phosphatase activity is an important aspect of the estrogen-mediated neuroprotection against oxidative stress and excitotoxicity.
We have previously reported that estrogens and nonfeminizing analogs of estrogens are protective against a variety of cytotoxic insults (24,25,26,27) as well as ischemic conditions caused by middle cerebral artery occlusion in the rat (33,54,55). The protective effects of estrogens against glutamate toxicity were partially antagonized by specific protein phosphatase inhibitors, PPI2, endothall, and cyclosporine A. In addition, we show that estrogens persistently antagonize the reduction in PP1, PP2A, and PP2B protein expression induced by oxidative and excitotoxic stresses, an effect that is not modified by specific inhibitors. The activities of PP2A and calcineurin are also maintained in cells treated with 17β-estradiol during oxidative and excitotoxic stress induced by glutamate. Collectively, these data support the hypothesis that serine/threonine phosphatase regulation is a major component of estrogen-mediated neuroprotection.
This is an important observation because AD-affected brains have significantly decreased activities of PP1, PP2A, and calcineurin (18). With this down-regulation of phosphatase activities and up-regulation of protein kinase activities, it is likely that hyperphosphorylation of τ and activation of other pathological events involved in neurodegenerative diseases are triggered. Further, these phosphatases are directly involved in synaptic plasticity and memory formation, which is affected in neurodegenerative diseases. The observation that estrogen-mediated neuroprotection involves maintaining the activities of these important phosphatases can lead to a more clinically relevant postmenopausal hormone replacement regimen that would minimize the adverse effects of estrogens and maximize the beneficial effects. Indeed, in our tMCAO animal model, we completely prevented the stroke-induced decrease in PP1, PP2A, and PP2B.
The inability of individual protein phosphatase inhibitors to prevent cell death after toxic treatment with glutamate in primary neurons cannot be explained by incomplete inhibition of the target protein phosphatase. The inhibitor concentrations used in this experiment were 10- to 100-fold higher than the reported IC50s of each inhibitor. PPI2 has been shown to have an IC50 of 2 nm (56,57). The IC50 of endothall has been reported to be approximately 90 nm (58,59,60). Various reports have shown that cyclosporin A inhibits 50% of calcineurin in concentrations ranging from 2 to 8 nm (61,62). Therefore, it is unlikely that there is incomplete inhibition of the various serine/threonine phosphatases used in this study.
The mechanism of estrogen-mediated attenuation of the decline in protein phosphatase levels and activity during oxidative and excitotoxic stress is not clear. There are no previous experimental data suggesting a direct interaction between estrogens and protein phosphatase. Whereas PP2A has been shown to regulate estrogen receptor (ER)-α by mRNA stabilization (63) as well as direct interaction with ERα in the absence of estrogen (64), our data indicate that effects of estrogens on protein phosphatases occur without ER interactions because non-ER active estrogen analogs exert similar effects (44). The absence of effects of estrogens alone and the broad range of protein phosphatases affected by estrogens suggests that estrogens reduce the clearance of protein phosphatases that is activated by oxidative/excitotoxic insult, rather than causing expression of new protein. Our laboratory recently reported that estrogens attenuate ischemia-induced increases in activities of matrix metalloproteinase-2 and -9, which belong to a class of proteases (65). We have also shown that transient cerebral ischemia causes neurofibrillary tangle like tauopathy involving cyclin-dependent kinase (cdk)-5 (66). This study showed that calpain II, a cytoplasmic cysteine protease, caused the cleavage of p35 to p25, which are coactivators of cdk5, and that the inhibition of calpain by MDL 28170 attenuated this cleavage, which prevented the activation of cdk5. It has also been shown that estrogen induced expression of secretory leukocyte protease inhibitor in the rat uterus (67). Because estrogens have been shown to modify the expression or functions of various proteases, it is likely that 17β-estradiol protects cells by blocking the ubinquitination and/or degradation of protein phosphatases caused by oxidative or excitotoxic stresses.
The present study provides a potential ER-independent mechanism for the neuroprotective effects of estrogens through the prevention of insult-induced decrease in phosphatase activity and the resulting neurotoxic, persistent hyperphosphorylation of proteins in multiple signaling pathways that are detrimental to cell survival. Inhibition of individual phosphatases does not abolish estrogen-mediated neuroprotection as seen with general inhibition with okadaic acid or calyculin A (38). In light of the data that show AD brains have decreased activities of protein phosphatases and our results that demonstrate estrogens maintain phosphatase activities during oxidative stress, it seems likely that clinical studies that show improved cognition and delayed progression of AD with estrogen treatment may be due to this action of estrogens.
Footnotes
This work was supported by Grants AG10485 and AG22550 from the National Institutes of Health.
Disclosure Statement: J.W.S. has patents related to the use of estrogens for neuroprotection. K.D.Y. has nothing to declare.
First Published Online July 19, 2008
Abbreviations: AD, Alzheimer’s disease; cdk, cyclin-dependent kinase; ER, estrogen receptor; PD, Parkinson’s disease; PP, protein phosphatase; PPI2, protein phosphatase inhibitor II; tMCAO, transient middle cerebral artery occlusion.
References
- Butterfield DA 2006 Oxidative stress in neurodegenerative disorders. Antioxid Redox Signal 8:1971–1973 [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W 2006 The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta 1762:1068–1082 [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW 2006 The pathogenesis of cell death in Parkinson’s disease. Neurology 66:S24–S36 [DOI] [PubMed] [Google Scholar]
- Liu Q, Xie F, Rolston R, Moreira PI, Nunomura A, Zhu X, Smith MA, Perry G 2007 Prevention and treatment of Alzheimer disease and aging: antioxidants. Mini Rev Med Chem 7:171–180 [DOI] [PubMed] [Google Scholar]
- Perry G, Cash AD, Smith MA 2002 Alzheimer disease and oxidative stress. J Biomed Biotechnol 2:120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P 2001 Failure of the ubiquitin-proteasome system in Parkinson’s disease. Nat Rev Neurosci 2:589–594 [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR 2000 Impaired proteasome function in Alzheimer’s disease. J Neurochem 75:436–439 [DOI] [PubMed] [Google Scholar]
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L 2004 Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem 279:13256–13264 [DOI] [PubMed] [Google Scholar]
- Reddy PH, Beal MF 2005 Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Brain Res Rev 49:618–632 [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Song C, Gluck M, Ehrhart J 2005 Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration. Antioxid Redox Signal 7:1117–1139 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Barrachina M, Tolnay M, Rey MJ, Vidal N, Carmona M, Blanco R, Puig B 2003 Phosphorylated protein kinases associated with neuronal and glial tau deposits in argyrophilic grain disease. Brain Pathol 13:62–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV 1999 MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA 96:12866–12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JH, Kulich SM, Oury TD, Chu CT 2002 Cytoplasmic aggregates of phosphorylated extracellular signal-regulated protein kinases in Lewy body diseases. Am J Pathol 161:2087–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Q, Ladner CJ, Magnuson D, Lee JM 2001 Selective changes of calcineurin (protein phosphatase 2B) activity in Alzheimer’s disease cerebral cortex. Exp Neurol 167:158–165 [DOI] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM 2001 PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol 168:402–412 [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee HG, Raina AK, Perry G, Smith MA 2002 The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals 11:270–281 [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Slowik A, Rubio F, Szczudlik A, Gaffney J 2000 Activation of MAP kinase (ERK-1/ERK-2), tyrosine kinase and VEGF in the human brain following acute ischaemic stroke. Neuroreport 11:2759–2764 [DOI] [PubMed] [Google Scholar]
- Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K 1993 Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 61:921–927 [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N 1994 Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocr J 41:361–371 [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Ross RK, Henderson BE 1988 Postmenopausal oestrogen treatment and stroke: a prospective study. BMJ 297:519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW 1994 Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol 140:256–261 [DOI] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R 1996 Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 348:429–432 [DOI] [PubMed] [Google Scholar]
- Henderson VW, Buckwalter JG 1994 Cognitive deficits of men and women with Alzheimer’s disease. Neurology 44:90–96 [DOI] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW 1996 Estradiol protects against β-amyloid (25–35)-induced toxicity in SK-N-SH human neuroblastoma cells. Neurosci Lett 218:165–168 [DOI] [PubMed] [Google Scholar]
- Green PS, Gordon K, Simpkins JW 1997 Phenolic A ring requirement for the neuroprotective effects of steroids. J Steroid Biochem Mol Biol 63:229–235 [DOI] [PubMed] [Google Scholar]
- Green PS, Bishop J, Simpkins JW 1997 17α-Estradiol exerts neuroprotective effects on SK-N-SH cells. J Neurosci 17:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW 1998 Nuclear estrogen receptor-independent neuroprotection by estratrienes: a novel interaction with glutathione. Neuroscience 84:7–10 [DOI] [PubMed] [Google Scholar]
- Gridley KE, Green PS, Simpkins JW 1997 Low concentrations of estradiol reduce β-amyloid (25–35)-induced toxicity, lipid peroxidation and glucose utilization in human SK-N-SH neuroblastoma cells. Brain Res 778:158–165 [DOI] [PubMed] [Google Scholar]
- Behl C, Widmann M, Trapp T, Holsboer F 1995 17β-Estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun 216:473–482 [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS 2001 Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol 41:569–591 [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD 1998 Gender-linked brain injury in experimental stroke. Stroke 29:159–165; discussion 166 [DOI] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM 1998 Estradiol protects against ischemic injury. J Cereb Blood Flow Metab 18:1253–1258 [DOI] [PubMed] [Google Scholar]
- Yang SH, Shi J, Day AL, Simpkins JW 2000 Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke 31:745–749; discussion 749–750 [DOI] [PubMed] [Google Scholar]
- Sudo S, Wen TC, Desaki J, Matsuda S, Tanaka J, Arai T, Maeda N, Sakanaka M 1997 β-Estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neurosci Res 29:345–354 [DOI] [PubMed] [Google Scholar]
- Fukuda K, Yao H, Ibayashi S, Nakahara T, Uchimura H, Fujishima M, Hall ED 2000 Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke 31:155–160 [DOI] [PubMed] [Google Scholar]
- Mendelowitsch A, Ritz MF, Ros J, Langemann H, Gratzl O 2001 17β-Estradiol reduces cortical lesion size in the glutamate excitotoxicity model by enhancing extracellular lactate: a new neuroprotective pathway. Brain Res 901:230–236 [DOI] [PubMed] [Google Scholar]
- Yang SH, He Z, Wu SS, He YJ, Cutright J, Millard WJ, Day AL, Simpkins JW 2001 17β-Estradiol can reduce secondary ischemic damage and mortality of subarachnoid hemorrhage. J Cereb Blood Flow Metab 21:174–181 [DOI] [PubMed] [Google Scholar]
- Yi KD, Chung J, Pang P, Simpkins JW 2005 Role of protein phosphatases in estrogen-mediated neuroprotection. J Neurosci 25:7191–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu JS, Omiecinski CJ 1997 An okadaic acid-sensitive pathway involved in the phenobarbital-mediated induction of CYP2B gene expression in primary rat hepatocyte cultures. J Pharmacol Exp Ther 282:1122–1129 [PubMed] [Google Scholar]
- Tian Y, Ke S, Thomas T, Meeker RJ, Gallo MA 1998 Transcriptional suppression of estrogen receptor gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J Steroid Biochem Mol Biol 67:17–24 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kawanishi-Tabata R, Haba A, Tabata M, Haruta Y, Tsai H, Seon BK 2001 Association of serum endoglin with metastasis in patients with colorectal, breast, and other solid tumors, and suppressive effect of chemotherapy on the serum endoglin. Clin Cancer Res 7:524–532 [PubMed] [Google Scholar]
- Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW 2001 The nonfeminizing enantiomer of 17β-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology 142:400–406 [DOI] [PubMed] [Google Scholar]
- Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW 2005 Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res 1038:216–222 [DOI] [PubMed] [Google Scholar]
- Yi KD, Cai ZY, Covey DF, Simpkins JW 2008 Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J Pharmacol Exp Ther 324:1188–1195 [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J 2004 Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D 2003 Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2663–2672 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones 3rd BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J 2003 Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH 2004 Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR 1999 Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 24:657–677 [DOI] [PubMed] [Google Scholar]
- Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J 1986 Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology 11:337–345 [DOI] [PubMed] [Google Scholar]
- Ditkoff EC, Crary WG, Cristo M, Lobo RA 1991 Estrogen improves psychological function in asymptomatic postmenopausal women. Obstet Gynecol 78:991–995 [PubMed] [Google Scholar]
- Rauramo L, Lagerspetz K, Engblom P, Punnonen R 1975 The effect of castration and peroral estrogen therapy on some psychological functions. Front Horm Res 3:94–104 [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E 1997 A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology 48:1517–1521 [DOI] [PubMed] [Google Scholar]
- Liu R, Yang SH, Perez E, Yi KD, Wu SS, Eberst K, Prokai L, Prokai-Tatrai K, Cai ZY, Covey DF, Day AL, Simpkins JW 2002 Neuroprotective effects of a novel non-receptor-binding estrogen analogue: in vitro and in vivo analysis. Stroke 33:2485–2491 [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL 1997 Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg 87:724–730 [DOI] [PubMed] [Google Scholar]
- Park IK, DePaoli-Roach AA 1994 Domains of phosphatase inhibitor-2 involved in the control of the ATP-Mg-dependent protein phosphatase. J Biol Chem 269:28919–28928 [PubMed] [Google Scholar]
- Cohen PT, Berndt N 1991 Reactivation of protein phosphatase 1 expressed at high levels from recombinant baculovirus. Methods Enzymol 201:408–414 [DOI] [PubMed] [Google Scholar]
- Li YM, Casida JE 1992 Cantharidin-binding protein: identification as protein phosphatase 2A. Proc Natl Acad Sci USA 89:11867–11870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Mackintosh C, Casida JE 1993 Protein phosphatase 2A and its [3H]cantharidin/[3H]endothall thioanhydride binding site. Inhibitor specificity of cantharidin and ATP analogues. Biochem Pharmacol 46:1435–1443 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Blazsek I, Legras S, Marion S, Reynes M, Anjo A, Adam R, Misset JL 1999 Hepatocellular carcinoma cell lines from diethylnitrosamine phenobarbital-treated rats. Characterization and sensitivity to endothall, a protein serine/threonine phosphatase-2A inhibitor. Hepatology 29:1406–1417 [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Blume R, Oetjen E, Knepel W 1993 The immunosuppressive drugs cyclosporin A and FK506 inhibit calcineurin phosphatase activity and gene transcription mediated through the cAMP-responsive element in a nonimmune cell line. Naunyn Schmiedebergs Arch Pharmacol 348:541–545 [DOI] [PubMed] [Google Scholar]
- Kung L, Batiuk TD, Palomo-Pinon S, Noujaim J, Helms LM, Halloran PF 2001 Tissue distribution of calcineurin and its sensitivity to inhibition by cyclosporine. Am J Transplant 1:325–333 [DOI] [PubMed] [Google Scholar]
- Keen JC, Zhou Q, Park BH, Pettit C, Mack KM, Blair B, Brenner K, Davidson NE 2005 Protein phosphatase 2A regulates estrogen receptor (ER) α expression through modulation of ER mRNA stability. J Biol Chem 280:29519–29524 [DOI] [PubMed] [Google Scholar]
- Lu Q, Surks HK, Ebling H, Baur WE, Brown D, Pallas DC, Karas RH 2003 Regulation of estrogen receptor α-mediated transcription by a direct interaction with protein phosphatase 2A. J Biol Chem 278:4639–4645 [DOI] [PubMed] [Google Scholar]
- Liu H, Liu K, Bodenner DL 2005 Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor κB transactivation. Cytokine 31:251–257 [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang SH, Liu R, Perez EJ, Brun-Zinkernagel AM, Koulen P, Simpkins JW 2006 Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta 1772:473–483 [DOI] [PubMed] [Google Scholar]
- Chen D, Xu X, Cheon YP, Bagchi MK, Bagchi IC 2004 Estrogen induces expression of secretory leukocyte protease inhibitor in rat uterus. Biol Reprod 71:508–514 [DOI] [PubMed] [Google Scholar]