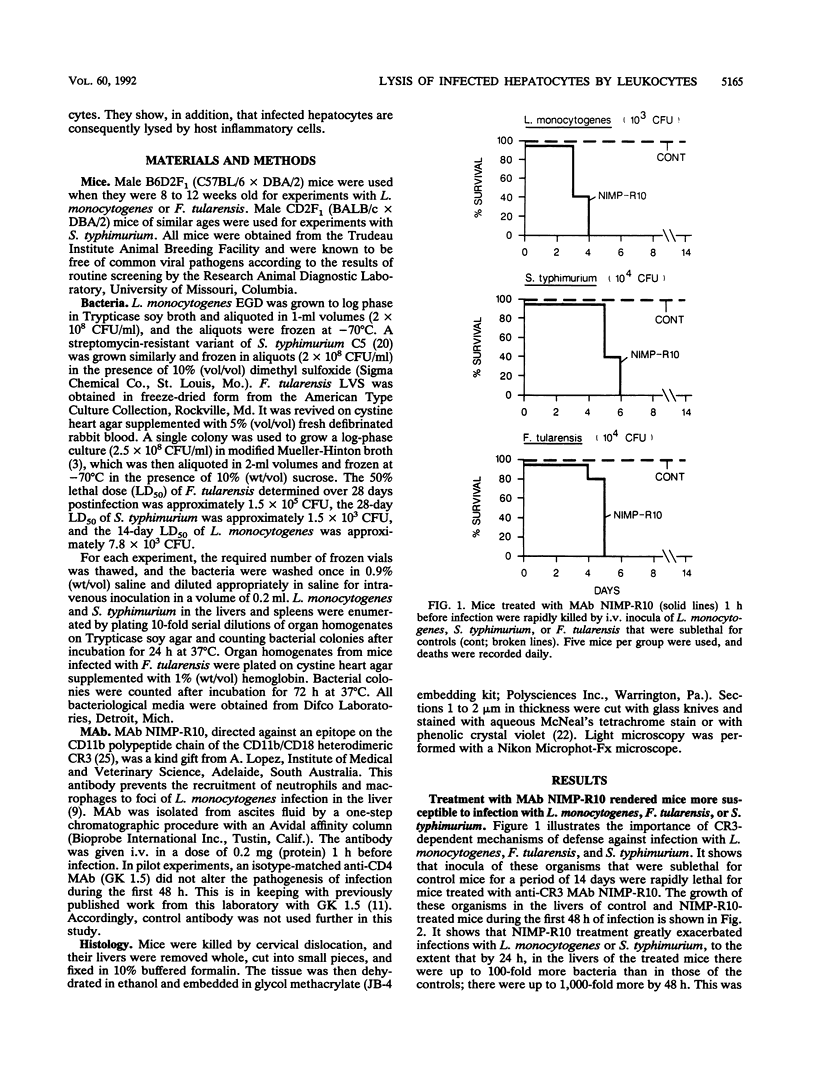
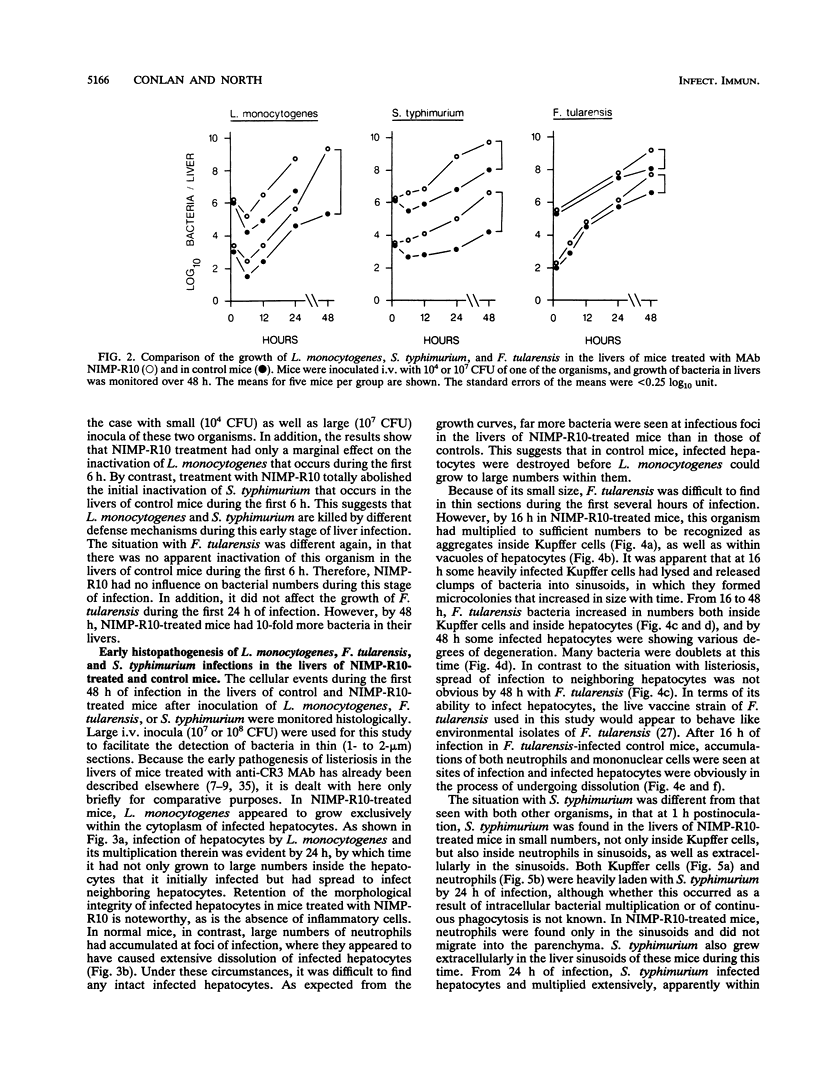
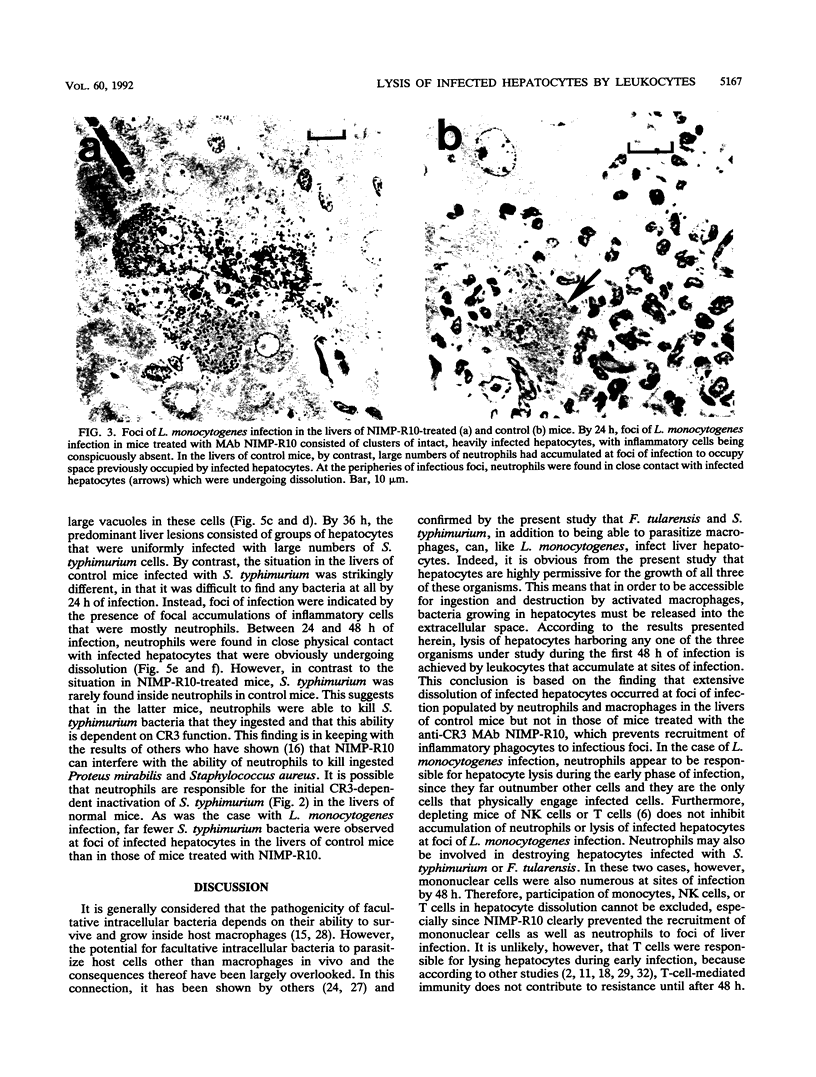
Abstract
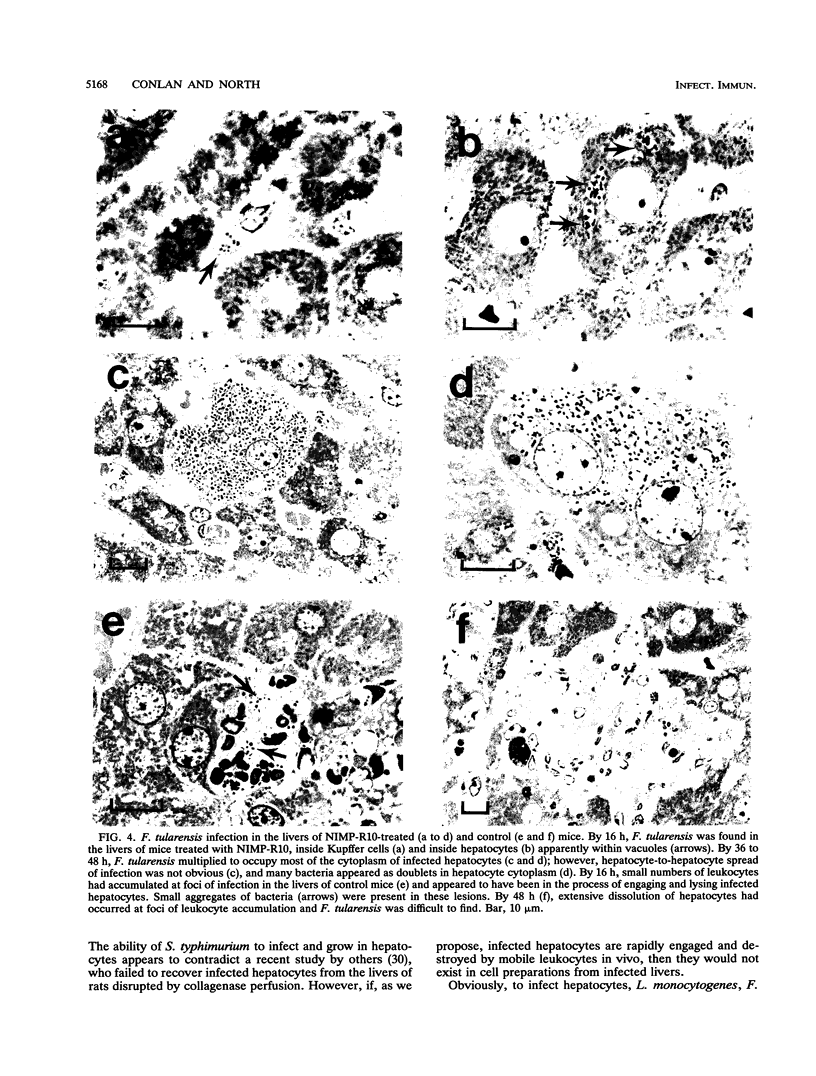
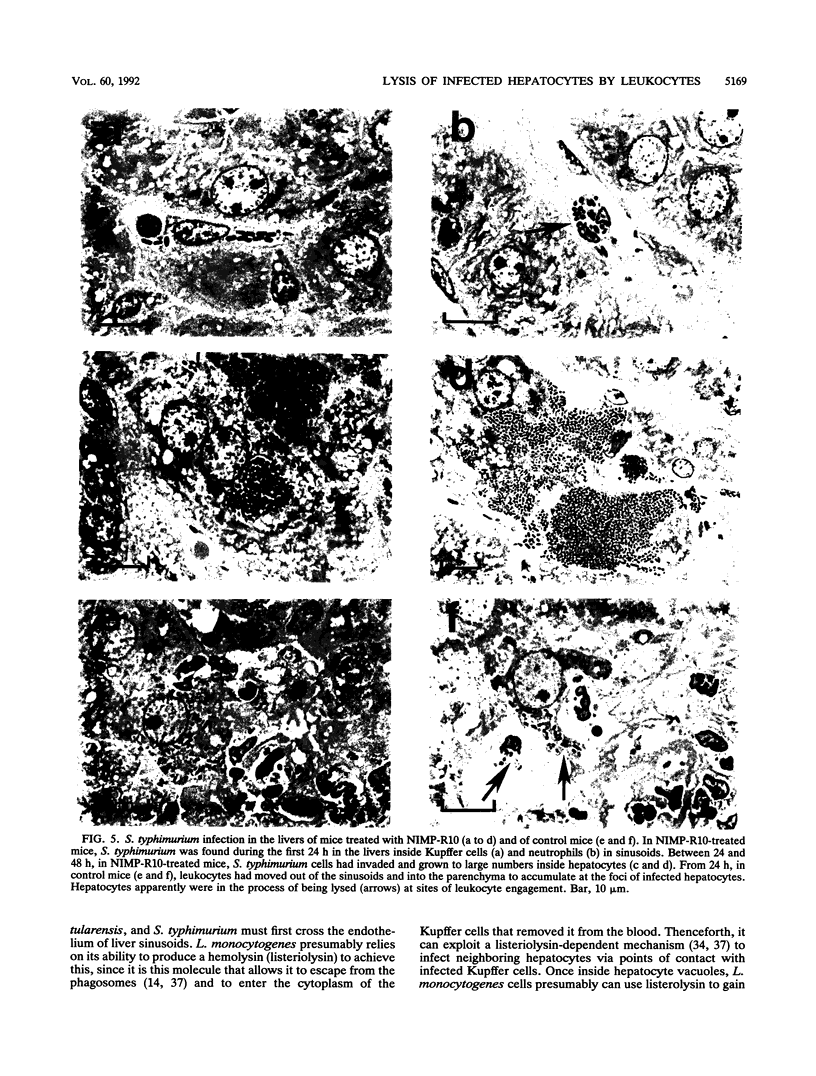
The results show that Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium are facultative intracellular bacteria with a capacity to invade and grow in nonphagocytic cells in vivo. In the liver, all of these pathogens were seen to invade and to multiply extensively in hepatocytes. In all three cases, inflammatory phagocytes were rapidly marshalled to foci of infection where they appeared to cause the destruction of infected hepatocytes, thereby releasing bacteria into the extracellular space, in which presumably they could be ingested and destroyed by the phagocytes. If phagocytic cells were prevented from accumulating at foci of liver infection by treatment of the mice with a monoclonal antibody (NIMP-R10) directed against the type 3 complement receptor of myelomonocytic cells, then lysis of hepatocytes failed to occur and bacteria proliferated unrestrictedly within them. Under these circumstances, otherwise sublethal infections became rapidly lethal. These findings strongly suggest that lysis of infected hepatocytes by phagocytic cells is an important general early-defense strategy against liver infection with at least three different intracellular bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony L. D., Burke R. D., Nano F. E. Growth of Francisella spp. in rodent macrophages. Infect Immun. 1991 Sep;59(9):3291–3296. doi: 10.1128/iai.59.9.3291-3296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony L. S., Kongshavn P. A. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987 Jan;2(1):3–14. doi: 10.1016/0882-4010(87)90110-0. [DOI] [PubMed] [Google Scholar]
- Baker C. N., Hollis D. G., Thornsberry C. Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller-Hinton broth. J Clin Microbiol. 1985 Aug;22(2):212–215. doi: 10.1128/jcm.22.2.212-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991 Jul;59(7):2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Monoclonal antibody NIMP-R10 directed against the CD11b chain of the type 3 complement receptor can substitute for monoclonal antibody 5C6 to exacerbate listeriosis by preventing the focusing of myelomonocytic cells at infectious foci in the liver. J Leukoc Biol. 1992 Jul;52(1):130–132. doi: 10.1002/jlb.52.1.130. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991 Sep 1;174(3):741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Roles of Listeria monocytogenes virulence factors in survival: virulence factors distinct from listeriolysin are needed for the organism to survive an early neutrophil-mediated host defense mechanism. Infect Immun. 1992 Mar;60(3):951–957. doi: 10.1128/iai.60.3.951-957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Resolution of primary murine listeriosis and acquired resistance to lethal secondary infection can be mediated predominantly by Thy-1+ CD4- CD8- cells. J Infect Dis. 1991 Nov;164(5):869–877. doi: 10.1093/infdis/164.5.869. [DOI] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. The importance of route of infection in determining the extent of exacerbation of listeriosis by anti-interferon-gamma monoclonal antibody. J Interferon Res. 1991 Oct;11(5):291–295. doi: 10.1089/jir.1991.11.291. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Fortier A. H., Polsinelli T., Green S. J., Nacy C. A. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992 Mar;60(3):817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Nikoloutsopoulos A., Lopez A. F., Vadas M. A., McDonald P. J., Finlay-Jones J. J. Role of cell surface receptors in the regulation of intracellular killing of bacteria by murine peritoneal exudate neutrophils. Infect Immun. 1986 Apr;52(1):245–251. doi: 10.1128/iai.52.1.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Hormaeche C. E., Mastroeni P., Arena A., Uddin J., Joysey H. S. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology. 1990 Jun;70(2):247–250. [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- Kagaya K., Watanabe K., Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989 Feb;57(2):609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECHTMAN M. D., BARTHOLOMEW J. W., PHILLIPS A., RUSSO M. RAPID METHODS OF STAINING BACTERIAL SPORES AT ROOM TEMPERATURE. J Bacteriol. 1965 Mar;89:848–854. doi: 10.1128/jb.89.3.848-854.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby D. A., Fortier A. H., Crawford R. M., Schreiber R. D., Nacy C. A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992 Jan;60(1):84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Wang X. M., Hsu H. S., Mumaw V. R., Nakoneczna I. Electron microscopic studies on the location of bacterial proliferation in the liver in murine salmonellosis. Br J Exp Pathol. 1987 Aug;68(4):539–550. [PMC free article] [PubMed] [Google Scholar]
- López A. F., Burns G. F., Stanley I. J. Epitope diversity of monoclonal antibodies revealed by cross-species reactivity. Mol Immunol. 1984 May;21(5):371–374. doi: 10.1016/0161-5890(84)90033-6. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková D., Blazek K., Danielová V., Holubová J., Lavicková M., Marhoul Z., Schramlová J. Some diagnostic, biologic and morphologic characteristics of Francisella tularensis strains isolated from the ticks Ixodes ricinus (L.) in the Prague agglomeration. Folia Parasitol (Praha) 1986;33(1):87–95. [PubMed] [Google Scholar]
- Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992 Feb;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A., Shnyra A., Hultenby K., Lindberg A. A. Salmonella choleraesuis and Salmonella typhimurium associated with liver cells after intravenous inoculation of rats are localized mainly in Kupffer cells and multiply intracellularly. Infect Immun. 1992 Jul;60(7):2758–2768. doi: 10.1128/iai.60.7.2758-2768.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- Portnoy D. A., Schreiber R. D., Connelly P., Tilney L. G. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989 Dec 1;170(6):2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gordon S., North R. J. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989 Jul 1;170(1):27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz P., Tenner K., Mérö E. Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental listeria infection. Lab Invest. 1972 Jun;26(6):694–700. [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]