Abstract
The mucociliary clearance system is comprised of three components, ion transport activities controlling the height of airway surface liquid (ASL), mucin secretion, and ciliary activity. These activities in humans are controlled principally by local agonists, extracellular nucleotides and nucleosides released from the epithelium. Importantly, mechanical stresses stimulate goblet cell mucin secretion, ciliary beating, and Cl− and fluid secretion through mechanically-induced nucleotide release. Emerging evidence also implicates co-secretion of nucleotides and mucin from goblet cells as a source of extracellular agonist. At rest, ATP is released onto airway surfaces at ∼370 fmoles/min cm2, but only ∼3% of released ATP is recovered in ASL. Secreted UTP meets with a similar fate. A wide variety of hydrolytic and trans-phosphorylating ecto-enzymes convert the triphosphate nucleotides into ADP, AMP, and adenosine, UDP, UMP, and uridine. Of these, ATP, adenosine, UTP, and UDP act as agonists at apical P2Y2 (ATP, UTP), P2Y6 (UDP), and A2B (adenosine) receptors on ciliated and/or goblet cells to regulate mucociliary clearance.
1.0 Introduction
The mucociliary clearance (MCC) system in the airways, crucial for innate lung defense, is comprised of three principal components: [i] mucins, secreted by goblet cells or from submucosal glands, which maturate into mucus, [ii] cilia which propel the mucus toward the mouth, and [iii] ion transport elements in the epithelium which maintain a proper, enabling aqueous environment on the airway surface. Component failures may lead to airway inflammatory diseases. Cystic fibrosis, for example, results from a failure in epithelial Cl− and fluid secretion, primary ciliary dyskinesia results from structural failures in the ciliary axoneme which negatively affect ciliary activity, and chronic bronchitis and asthma, in part, result from mucin hypersecretion (Salathe et al. 1996; Wanner et al. 1996; Knowles et al. 2002; Boucher 2004). Since ATP was first shown to stimulate Cl− secretion in the airways in the early 1990s, all three components of the MCC system in healthy human lungs have been shown to be regulated in powerful ways by purinergic signaling (Mason et al. 1991; Knowles et al. 1991; Stutts et al. 1992). Early in this period, the source of ATP on airway surfaces was uncertain, but ultimately it has been shown to emanate from the airway epithelium itself (Grygorczyk et al. 1997; Taylor et al. 1998; Donaldson et al. 2000), likely in response to mechanical stimuli (Hansen et al. 1993; Grygorczyk et al. 1997; Homolya et al. 2000; Douillet et al. 2005; Button et al. 2007).
In recent years, mechanical forces related to shear stresses imparted by perfusion and other stimuli have been shown to stimulate airway epithelial ion transport and ciliary activity (e.g., see Sanderson et al. 1986; Leuba et al. 1996). That these effects of shear stresses on measured epithelial activities might relate to the release of ATP and/or other nucleotides has been demonstrated only recently. Changes in ion transport from the application of shear stress, for instance, was blocked by a combination of apyrase to hydrolyze ATP and 8-SPT to block adenosine signaling (Tarran et al. 2005, and see companion review, Button et al. 2008). Similarly, flow related shear stresses that stimulate ciliary beating in mouse trachea were blocked by apyrase, and were significantly reduced in the P2Y2-R null mouse (Winters et al. 2007). The sensitivity of mucin secretion by airway epithelial goblet cells to shear stresses has not been formally demonstrated; however, problems with secretion assays from human bronchial epithelial (HBE) cell cultures have been indicated, that likely result from inadvertent mechanical stimulation during experiments (Conway et al. 2003). Additionally, we have observed that application of shear stresses to HBE cultures amounting to 0.02 – 0.1 dyne/cm2, forces that have minimal effects on luminal bulk [ATP], have major effects on mucin secretion (Figure 1). Hence, it appears that release of purinergic agonists from the airway epithelium in response to a variety of mechanical stresses has major effects on all three components of the MCC system. In this review we will discuss the mechanisms by which ATP and other nucleotides/nucleosides are released into the airway surface liquid (ASL) in response to mechanical stresses, the ecto-metabolism of these compounds on airway surfaces, and the purinergic receptor-based signaling systems responsible for the cellular responses to mechanical perturbations observed experimentally.
Figure 1.
Effects of rotational shear stresses on HBE culture luminal ATP and mucin secretion. Cultures were rotated horizontally in a start-stop action with the indicated average shear forces and luminal ATP levels determined after 60 min by luminometry (data courtesy of Dr. Brian Button). Mucins secretion was determined in other cultures subjected to either 0.02 or 0.1 dyne/cm2 shear force, and in static cultures exposed to 100 μM ATPγS, or not (inset). Note that levels of shear having minimal effects on bulk [ATP] had near-maximal effects on mucin secretion.
2.0 Luminal Pathways for Purinergic Metabolism
Nucleotides are essential components of living cells. They are the building blocks of nucleic acid chains, the energy source of metabolism and biosynthetic processes, and participate as co-factors or activators of numerous enzymatic reactions and guanine nucleotide binding protein (G protein) signaling. In addition, nucleotides are released from cells in a regulated manner to accomplish autocrine and paracrine roles via activation of cell surface purinergic receptors.
Two groups of purinergic receptors, P2X and P2Y receptors, transduce the presence of nucleotides in the extracellular milieu into cellular responses (Burnstock 2006). The P2X receptor family, constituted by seven molecularly defined species (P2X1-P2X7), are ligand-gated anion channels activated exclusively by ATP (North 2002). The P2Y receptor family is comprised by eight G protein-coupled receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14) that are activated by ATP, ADP, UTP, UDP, and UDP-sugars in the extracellular milieu (Burnstock 2006). In addition, the nucleoside, adenosine, the final product of ATP breakdown, activates a separate family of G protein-coupled receptors, initially named P1 receptors. The adenosine receptor family is constituted by four species currently designed as A1, A2a, A2b, and A3 adenosine receptors (Fredholm et al. 2001). The agonist selectivity and signaling properties of all purinergic receptors are illustrated in Table 1.
Table 1.
Purinergic receptors: agonist selectivity and signaling properties.
| Agonist | Signaling | |
|---|---|---|
| P2X receptors | ||
| P2X1-P2X7 | ATP | ATP-gated ion channel |
| P2Y receptors | ||
| P2Y1 | ADP | Gq/PLCβ → Ca2+ and PKC |
| P2Y2 | ATP & UTP | Gq/PLCβ → Ca2+ and PKC |
| P2Y4 | UTP | Gq/PLCβ → Ca2+ and PKC |
| P2Y6 | UDP | Gq/PLCβ → Ca2+ and PKC |
| P2Y11 | ATP | Gq/PLCβ → Ca2+ and PKC Gs/AC → ↑cAMP |
| P2Y12 | ADP | Gi/AC → ↓cAMP |
| P2Y13 | ADP | Gi/AC → ↓cAMP |
| P2Y14 | UDP-glucose | Gi/AC → ↓cAMP |
|
Adenosine receptors |
||
| A1, A3 | Adenosine | Gi/AC → ↓cAMP |
| A2a, A2b | Adenosine | Gs/AC → ↑cAMP |
Purinergic receptor-mediated responses include cell proliferation, migration, differentiation and death, embryological development, wound healing, restenosis, atherosclerosis, ischaemia, cell turnover of epithelial cells in skin and visceral organs, airway MCC, inflammation, neuroprotection and cancer (Burnstock 2006). The Gq/phospholipase C-coupled P2Y2 and P2Y6 receptors, the Gs/adenylyl cyclase-coupled A2b receptor, and possibly P2X4 receptors are expressed on the apical surface of human airway epithelial cells (Mason et al. 1991; Lazarowski et al. 1992; Parr et al. 1994; Lazarowski et al. 1996; Lazarowski et al. 1997b; Morse et al. 2001; Cobb et al. 2002; Cobb et al. 2003; Szkotak et al. 2003; Zsembery et al. 2004). Physiological, pharmacological, and genetic studies suggest that A2b and P2Y2 receptors are major transducers of nucleotide/nucleoside-regulated ciliary beating and ion and water transport (Mason et al. 1991; Lazarowski et al. 1997b; Morse et al. 2001; Cobb et al. 2002; Cobb et al. 2003; Szkotak et al. 2003; Zsembery et al. 2004). P2Y2 receptors, but not A2b receptors, appear to regulate mucin secretion in the airway epithelia (Conway et al. 2003; Davis et al. 2007, and see below).
2.1 Nucleotide release from epithelial cells
The superficial human airway epithelium that mediates MCC is composed mainly of ciliated cells and, to a lesser extent, mucin-secreting goblet cells. Airway surface liquid (ASL) volume regulation by ciliated cells and gel-forming mucin secretion from goblet cells are exquisitely coordinated to maintain physiologic viscoelastic properties of the two ASL layers, i.e., the periciliary and mucus layers. Depletion of ASL in cystic fibrosis leads to mucus dehydration and cilia collapse and, ultimately, impaired MCC and infection. Thus, an essential component of MCC function involves the regulation of ASL volume by electrolyte transport.
ATP and other nucleotides have been measured in liquids perfused over nasal turbinates (Donaldson et al. 2000) and recovered bronchoalveolar lavage liquids (Rice et al. 1989; Rich et al. 2003; Douillet et al. 2005; Esther et al. 2008), implying release from airway epithelial surfaces. More direct evidence for nucleotide release from the airway epithelium has come from in vitro studies using HBE cell cultures (Grygorczyk 1997; Watt et al. 1998; Donaldson et al. 2000; Homolya et al. 2000; Lazarowski et al. 2004; Okada et al. 2006; and see below). It is quite likely that ATP is secreted into the airway lumen from ciliated cells (Okada, S. F. et al. 2006); however, the cellular mechanism of release and its regulation are unknown. Interestingly, ATP is known to be co-packaged in synaptic vesicles of neurons, as well as granules of many secretory cells, from which it is released by regulated exocytosis. In the airway, we have found that an important secretory pathway for ATP release may be with mucins, from goblet cells.
Mucin, the principal polymeric species in the mucus matrix, is condensed inside secretory granules and is secreted without involving ion and water secretion from goblet cells (Verdugo 1991; Davis et al. 1992). Effective mucin dispersion in the ASL requires coordinated ion and water channel activities (mainly Na+ absorption and Cl− secretion) to provide adequate water secretion onto the mucosal surface (Boucher 2007). While it is recognized that mucus hydration and other MCC activities are regulated in part by signals generated within the ASL (Tarran et al. 2006), the mechanisms by which ion/water secretion and mucin secretion rates are coordinated are poorly understood. All naturally occurring nucleotides species known to be potent agonist on purinergic receptors are present in physiologically relevant concentrations in ASL both in vivo and in vitro (Lazarowski et al. 1997a; Watt et al. 1998; Donaldson et al. 2000; Lazarowski et al. 2000; Lazarowski et al. 2003; Lazarowski et al. 2004). Functional and biochemical evidence indicate that release and metabolism of nucleotides into ASL contribute to purinergic receptor-promoted activation of electrolyte transport and ASL volume homeostasis. For example, (i) in resting airway epithelia, enzymatic removal of endogenous adenosine in ASL or blockade of the A2b receptor results in reduced cyclic AMP formation and impaired Cl− secretion via the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) channel, leading to ASL volume depletion (Lazarowski et al. 2004), and (ii) shear-stress promotes apyrase-sensitive Ca2+-dependent ion transport and ASL production in both normal and CF airway epithelia (Tarran et al. 2005; Tarran et al. 2006). Recent studies with mucin secreting airway epithelial cell models suggest that nucleotide released to ASL is, at least in part, associated with mucin exocytosis. For example, in SPOC1 cells, a well-defined rat airway goblet cell line, which secrete gel forming-mucins in response to P2Y2 receptor stimulation (Abdullah et al. 1996), P2Y2 receptor activation resulted in enhanced mucin secretion which was accompanied by release of ATP (Kreda et al. 2007). Using polarized cultures of Calu-3 cells, an airway epithelial cell line composed by a mixed population of non-mucous cells and gel forming-mucin secreting goblet-like cells, it was illustrated that Ca2+-promoted mucin secretion was accompanied by luminal ATP release. Like mucin secretion, ATP release from Calu-3 cells was inhibited by maneuvers that disrupted vesicle exocytosis. Moreover, when Calu-3 cells were loaded with acridine orange or quinacrine, two fluorescent dyes known to label storage granules (and other acidic compartments) in secretory cells, strong fluorescence was associated with granules that were both competent for Ca2+-triggered exocytosis and resembled mucin granules in size, sub-cellular localization, and susceptibility to pharmacological inhibitors (Kreda et al. 2007). Thus, an attractive although not formally tested hypothesis is that nucleotide release from goblet cells is mechanistically associated with mucin secretion, perhaps, as co-cargo molecules within mucin granules. Such a scenario would provide paracrine information for ASL volume production and cilia beating during the discharge of mucins onto the airways.
2.2 Extracellular metabolism of nucleotides multi-signaling pathways
Although resting airway epithelial cells release ATP at a rate of ∼370 fmoles/min.cm2, ecto-ATPase activities hydrolyze extracellular ATP at about the same rate (Lazarowski et al. 2004). The resulting, relatively rapid clearance of ATP from the airway surface maintains ASL ATP levels (1-10 nM) below threshold values for activating the P2Y2 receptor [EC50 = 230 nM (Lazarowski et al. 1996)]. However, ATP release and metabolism provides a source of adenosine. Since adenosine removal from ASL occurs with a k value [0.08 min−1; Lazarowski, E. R. et al. 2004] 10 fold smaller than that for ATP, adenosine accumulation on resting epithelia may be physiologically relevant. Indeed, this prediction was verified by direct measurement of purines in ASL. By combining ASL microsampling and high performance liquid chromatography analysis of fluorescent 1,N6-ethenoadenine derivatives, adenosine, AMP, ADP, and ATP concentrations in ASL could be quantified simultaneously, with nanomolar sensitivity. While ATP concentration on resting epithelia represented only a ∼3% of the total ASL adenyl purine pool, adenosine levels (100-200 nM) were in range of activating A2b receptor (Lazarowski et al. 2004). The physiological role of adenosine was established by illustrating that adenosine removal or inhibition of adenosine receptors in resting primary cultures of human bronchial epitleial cells impaired ASL production (Lazarowski et al. 2004).
While adenosine acting via A2b receptors appears to be the major input to ion channel activities in resting epithelia, ASL ATP levels arise sharply upon shear stress, reaching levels capable of promoting P2Y2 receptor-mediated Ca2+-dependent Cl− secretory responses (Tarran et al. 2005).
Levels of ATP and adenosine in ASL, and consequently airway epithelial P2Y2 and A2b receptor activation, are controlled by a complex array of nucleotide metabolizing enzymes that include ecto-nucleotidases, non-specific phosphatases, and transphosphorylating activities (Donaldson et al. 2000; Lazarowski et al. 2000; Picher et al. 2003). Lastly, adenosine deaminase converts adenosine into pharmacologically inactive inosine, and nucleoside transporters incorporate adenosine, inosine, and other nucleosides back into cells (Hirsh et al. 2007).
Thus, airway epithelial MCC functions reflect a multi-signaling system in which release, metabolism, and inter-conversion of purinergic agonists on the epithelial cell surface play a crucial role. Major nucleotide hydrolyzing activities expressed on the lumen of the airway epithelia include (i) ecto-ATPase (most likely NTPDase3, CD39L3), which removes the terminal phosphate from nucleoside triphosphates (NTP) and diphosphates (NDP) with a 3:1 preference for NTP, (ii) E-NPP, which catalyzes the breakdown of NTP to nucleoside monophosphate (NMP) plus pyrophosphate, and (iii) non-specific alkaline phosphatase (NSAP), which sequentially removes phosphate from NTP, NDP, and NMP (Picher et al. 2001; Picher et al. 2004; Tarran et al. 2005). Ecto-5′nucleotidase (ecto-5′NT, CD73) de-phosphorylates AMP and other NMPs, generating the corresponding nucleoside. The relatively low Km value towards AMP displayed by ecto-5′NT (14 ± 3 μM) over NSAP (717 ± 49 μM) (Picher et al. 2004) suggests that ecto-5′NT activity generates most of ASL adenosine when AMP supply is in the submicromolar concentration range, as observed under various physiological conditions (Lazarowski et al. 2004). Indeed, in bronchial cultures and tissues, ecto 5′-NT accounted for >80% of total activity toward micromolar concentrations of exogenous AMP (Picher et al. 2004). Intriguing, the two AMP hydrolyzing enzymes, i.e., NSAP and ecto-5′NT, presented opposite airway distributions, ecto 5′-NT and NS AP mRNA dominating in higher and lower airways, respectively. While inhibition of ecto-5′NT activity reduced A2b receptor-promoted CFTR-mediated Cl− secretion in epithelial culture models, the potential role of NSAP on adenosine formation and airway epithelial A2b receptor signaling is not known (Huang et al. 2001; Lazarowski et al. 2004).
In addition to the above-mentioned nucleotidase activities, ASL nucleotides are substrate of transphosphorylating activities, i.e., nucleoside diphosphokinase (NDPK) and adenyly kinase (AK). NDPK reversibly phosphorylates ADP and UDP to their respective NTP (e. g., ADP + UTP ↔ ATP + UDP), and AK converts two molecules of ADP to ATP plus AMP (2ADP ↔ ATP + AMP). The reason for the occurrence of ASL NDPK and AK is not well understood. However, although it is widely assumed that nucleotides are released from epithelial cells in their triphosphate forms (e.g., ATP and UTP), this has not been established unambiguously. UDP and ADP are abundantly present in the lumen of the secretory pathway and they may be released together with ATP via vesicular exocytosis (Lazarowski et al. 2003a). Once in the extracellular medium, ADP is susceptible to conversion to ATP via either NDPK or AK. Similarly, UDP may become acceptor substrate in the NDPK-catalyzed formation of UTP. Since the activities of both NDPK and AK in ASL surmount that of ecto-nucleotidases under a number of conditions (Lazarowski, E. R. et al. 2000; Picher, M. et al. 2003), these ecto enzymes may play an important role by propagating P2Y2 receptor mediated signaling.
In summary, ASL nucleotide levels reflect a balance between rates of nucleotide release and metabolism. P2Y2 and A2b receptor-mediated Ca2+ and cyclic AMP-regulated ion channel activities in ciliated cells greatly depend on such a balance. Nucleotide release occurring simultaneously with mucin secretion provides a signaling mechanism for ion and water transport necessary for mucin hydration and dispersion into the ASL, and for its cilia-driven transport.
3.0 Purinergic Regulation of Ciliary Activity
Ciliated cells dominate the superficial epithelium in the airways of healthy lungs, each cell possessing some 100-200 cilia beating synchronously (for recent reviews, see Salathe 2007; Nakahari 2007; Satir et al. 2007). Ciliary function was originally estimated to be regulated primarily by β-adrenergic agonists (e.g., see Wanner et al. 1996). ATP was found to have major, P2-mediated effects on alveolar type II cell secretion in 1986 (Rice et al. 1986), and on airway ion transport functions in the early 1990s, and it was subsequently found to also stimulate ciliary activity to a greater degree than β-adrenergic agonists. (Wong et al. 1992; Korngreen et al. 1994). β-adrenergic receptors couple to Gs and stimulate adenylate cyclase-mediated cyclic AMP production, whereas ATP in the airways activates P2Y2 receptors which couple to Gq, activate phospholipase C, and ultimately mobilize Ca2+ (for detailed treatment of cellular messenger function in ciliary activity, see Salathe, M. 2007, or companion review, Braiman et al. 2008). The recent finding, however, that adenosine, acting via A2B receptors, stimulates ciliary activity to approximately the same degree as ATP suggested it as the primary cyclic AMP-related agonist (Wong et al. 1992; Morse 2001; Salathe 2007). Interestingly, ATP and UTP both activate P2Y2 receptors, but elicit responses in ciliary activity with different time courses. As discussed below, the difference between the two P2 agonists appears to be due to the ecto-metabolism of ATP to adenosine.
Stimulation of ciliary activity by ATP, UTP, and adenosine indicates clearly that these agonists potentially serve a physiological coupling function in the airways between mechanical stimuli and elevated ciliary function. The recent findings of Winters, et al., that shear stresses simulate ciliary activity, apparently through the P2Y2 and A2B receptor systems, are consistent with this notion (Winters et al. 2007). Experimentally, the mechanosensitivity of ciliated epithelia signals a clear warning that extreme care needs to be taken in experimental design and implementation, to avoid artifacts in measurement of ciliary activity caused by high perfusion flow rates, mechanical vibration, etc. The ciliary literature is rife with examples of disparate data from different laboratories (for an excellent discussion of this problem, see reference Nakahari 2007). In addition to problems arising from the use of different tissues (e.g., proximal and distal airways), experiments done at different temperatures or on different species, authors need to also ensure their data are not affected inadvertently by mechanical stresses.
3.1 Regulation of Mucin Secretion from Goblet Cells
In the healthy lung, goblet cells comprise some 20% of columnar epithelial cells; they expand hyperplastically or metaplastically in proximal or distal airways, respectively, in all of the airway inflammatory diseases. Mucins are released from the goblet cell regulated exocytic pathway following cell activation by agonists. In contrast to submucosal glands, which are innervated richly and regulated primarily by acetylcholine and possibly other neurotransmitters acting at the basolateral aspect of the cells, goblet cells of the superficial epithelium receive sparse innervation and are regulated primarily by local mediators acting from the airway lumen, at their apical membranes (see Davis 1997; Davis et al. 2007). The current body of data indicate strongly that ATP and UTP are the predominant goblet cell mucin secretagogues in the airways. ATP was initially shown to stimulate mucin secretion in cultures of hamster tracheal epithelial cells and in explanted canine tracheal epithelium (Kim et al. 1991; Davis et al. 1992). Later, we found that UTP was an equally effective mucin secretagogue in explanted nasal turbinate epithelium (Lethem et al. 1993). These findings have been repeated in several other goblet cell models, and in the airways in vivo, suggesting that ATP and UTP are universally important airway goblet cell secretagogues (e.g., Abdullah et al. 1996; Davis et al. 1997; Shin et al. 2000; Conway et al. 2003; Evans et al. 2004). Given that the P2Y2 receptor is expressed, at least at the mRNA level, in two goblet cell models (Abdullah et al. 1996; Kim et al. 1996), and since the P2Y2 receptor sorts to the apical membrane (Wolff et al. 2005), it is quite likely that this purinoceptor is responsible for mediating the effects of both agonists. However, since the human P2Y4 receptor is also activated by UTP, couples to PLC, and sorts to the apical membrane (Wolff et al. 2005) we cannot formally rule out the possibility that it mediates some of the secretory response to UTP. More information on purinergic signaling in goblet cells is provided in the next section.
Both the P2Y2 and P2Y4 purinoceptors couple to phospholipase C, implicating diacylglycerol, IP3 and Ca2+ as cellular messengers in regulated mucin secretion. Existing data, in fact, suggest these messenger molecules as the only ones involved in mucin secretion (for detailed reviews, see Davis 1997; Davis 2002; Kim et al. 2003; Davis et al. 2007).
3.2 Comparative Ciliated and Goblet Cell Purinoceptor Pharmacology
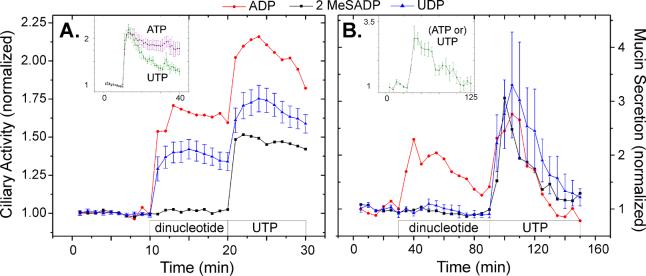
Mucociliary clearance is driven by the coordinated behavior of ciliated cells and goblet cells, with the former providing salt and water homeostasis and the propulsive force and the later providing the mucins necessary to form mucus (with contributions of materials from submucosal glands). Purinergic agonists appear to provide the necessary coordination between the two cells types. Interestingly, ciliated and goblet cells exhibit different patterns of response to these agonists (Figure 2). As the figure shows, the two cell types also respond differently to diphosphate nucleotides (Morse et al. 2001; Conway et al. 2003).
Figure 2.
Responses of ciliated cells (A) and goblet cells (B) elicited by diphosphate nucleotides. As indicated by the boxes at the bottom of each figure, baseline ciliary activity (ciliary beat frequency) or mucin secretion was determined for human bronchial epithelial cell cultures, following which they were exposed to a dinucleotide agonist, and then to UTP. Agonist concentrations were 100 μM, except for ADP in the ciliary activity experiments where it was 3 μM. For clarity, SEs are shown only for UDP, which are typical of the other datasets. Insets: Responses of the cells to 100 μM ATP and/or UTP. 2 MeSADP = 2-methlythio ADP. Graphs redrawn from data presented in references Morse et al. 2001 and Conway et al. 2003.
Goblet cell mucin secretory responses to maximal doses ATP and UTP are virtually identical, with time courses that overlie one another statistically (Figure 2B; Abdullah et al. 1996; Conway et al. 2003), whereas ciliated cells have distinctly different time courses of increased ciliary activity (Figure 2A; Morse et al. 2001). Significantly, the ciliated cell response to ATP is prolonged, relative to that for UTP, and the difference is due to ectometabolism of ATP to adenosine: stimulation with the poorly metabolizable ATP analog, ATPγS, or with ATP in the presence of the adenosine receptor inhibitor, 8-SPT, yields time courses of increased ciliary activity that overlie that of UTP (Morse et al. 2001). Goblet cells, in contrast, are resistant to stimulation by luminal adenosine (Davis et al. 1992; Abdullah et al. 1996; Conway et al. 2003).
Both ciliated and goblet cells respond to ADP (Figure 2). Neither cell type, however, responds to 2-methlythio ADP (2MeSADP), a P2Y1 agonist, the ADP purinoceptor that couples to Gq and phospholipase C (Table 1). The likely explanation for the stimulation by ADP in each case is that it is metabolized to a different, active form. For ciliated cells, ADP appears to be metabolized to adenosine, as the ADP stimulation of ciliary activity disappears in the presence of 8-SPT. For goblet cells, it is likely that the stimulatory effects of ADP are due to its metabolism to ATP by adenylate kinase, which is abundant on airway surfaces (Donaldson et al. 2000; Picher et al. 2003).
Ciliated cells, but not goblet cells, response to UDP, indicating the likely involvement of the P2Y6 purinoceptor in the regulation of ciliary activity (Table 1). Although the increase in ciliary activity is only about half the response to UTP, should a ciliary-specific therapy to stimulate mucus clearance be desired, UDP may be a good candidate.
4.0 Summary
It is clear that mechanical stresses in the airways result in the release of nucleotides and/or nucleosides into the lumen. Released into the near membrane environment, these purinergic compounds dynamically achieve sufficiently high effective concentrations as to be active signaling molecules that stimulate several physiologic activities. For mucociliary clearance, it is clear that ATP, UTP, and adenosine, and UDP to a lesser degree, stimulate ciliary activity (and ion transport), and that ATP and UTP stimulate the secretion of mucins from goblet cells. In the absence of major innervation of the superficial epithelium in human airways, these compounds coordinate this most important aspect of lung innate immunity.
Acknowledgements
The authors thank their many colleagues for their efforts and other valuable contributions made to better understand the regulation of mucociliary clearance. We also acknowledge the receipt of funding for studies leading to many of the references cited from the North American Cystic Fibrosis Foundation, Inspire Pharmaceuticals, Inc., and the National Institutes of Health (CWD: HL063756 and HL034322; ERL: HL 034322).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah LH, Davis SW, Burch L, Yamauchi M, Randell SH, Nettesheim P, Davis CW. P2u purinoceptor regulation of mucin secretion in SPOC1 cells, a goblet cell line from the airways. Biochem. J. 1996;316(Pt 3):943–951. doi: 10.1042/bj3160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. Relationship of airway epithelial ion transport to chronic bronchitis. Proc. Am. Thorac. Soc. 2004;1:66–70. doi: 10.1513/pats.2306018. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol. Med. 2007;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Braiman A, Priel Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Resp. Physiol. Neurobiol. 2008 doi: 10.1016/j.resp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling. Br. J. Pharmacol. 2006;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Resp. Physiol. Neurobiol. 2008 doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J. Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BR, Fan L, Kovacs TE, Sorscher EJ, Clancy JP. Adenosine receptors and phosphodiesterase inhibitors stimulate Cl- secretion in Calu-3 cells. Am. J. Respir. Cell Mol. Biol. 2003;29:410–418. doi: 10.1165/rcmb.2002-0247OC. [DOI] [PubMed] [Google Scholar]
- Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JP. A(2) adenosine receptors regulate CFTR through PKA and PLA(2) Am. J. Physiol Lung Cell Mol. Physiol. 2002;282:L12–L25. doi: 10.1152/ajplung.2002.282.1.L12. [DOI] [PubMed] [Google Scholar]
- Conway JD, Bartolotta T, Abdullah LH, Davis CW. Regulation of mucin secretion from human bronchial epithelial cells grown in murine hosted xenografts. Am. J. Physiol Lung Cell Mol. Physiol. 2003;284:L945–L954. doi: 10.1152/ajplung.00410.2002. [DOI] [PubMed] [Google Scholar]
- Davis CW. Goblet cells: physiology and pharmacology. In: Rogers DF, Lethem MI, editors. Airway Mucus: Basic Mechanisms and Clinical Perspectives. Berkhauser; Basel: 1997. pp. 150–177. [Google Scholar]
- Davis CW. Regulation of mucin secretion from in vitro cellular models. Novartis. Found. Symp. 2002;248:113–125. [PubMed] [Google Scholar]
- Davis CW, Abdullah LH. In vitro models for airways mucin secretion. Pulm. Pharmacol. Ther. 1997;10:145–155. doi: 10.1006/pupt.1997.0088. [DOI] [PubMed] [Google Scholar]
- Davis CW, Dickey BF. Regulated Airway Goblet Cell Mucin Secretion. Annu. Rev. Physiol. 2007 doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- Davis CW, Dowell ML, Lethem M, Van Scott M. Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am. J. Physiol. 1992;262:C1313–C1323. doi: 10.1152/ajpcell.1992.262.5.C1313. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol. Med. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- Douillet CD, Robinson WP, III, Zarzaur BL, Lazarowski ER, Boucher RC, Rich PB. Mechanical ventilation alters airway nucleotides and purinoceptors in lung and extrapulmonary organs. Am. J. Respir. Cell Mol. Biol. 2005;32:52–58. doi: 10.1165/rcmb.2004-0177OC. [DOI] [PubMed] [Google Scholar]
- Esther CR, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Pedrosa Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular Purines are Biomarkers of Neutrophilic Airway Inflammation. Eur. Respir. J. 2008 doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, I, Jzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am. J. Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J. Cell Sci. 1993;106(Pt 4):995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, Picher M. Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: implications for inflammatory lung diseases. Biochemistry. 2007;46:10373–10383. doi: 10.1021/bi7009647. [DOI] [PubMed] [Google Scholar]
- Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J. Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Hisatsune A, Kim d.J., Miyata T. Pharmacology of airway goblet cell mucin release. J. Pharmacol. Sci. 2003;92:301–307. doi: 10.1254/jphs.92.301. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee BC. P2 purinoceptor regulation of mucin release by airway goblet cells in primary culture. Br. J. Pharmacol. 1991;103:1053–1056. doi: 10.1111/j.1476-5381.1991.tb12299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Park HR, Shin CY, Akiyama T, Ko KH. Nucleotide-induced mucin release from primary hamster tracheal surface epithelial cells involves the P2u purinoceptor. Eur. Respir. J. 1996;9:542–548. doi: 10.1183/09031936.96.09030542. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Clarke LL, Boucher RC. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Priel Z. Simultaneous measurement of ciliary beating and intracellular calcium. Biophys. J. 1994;67:377–380. doi: 10.1016/S0006-3495(94)80492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O'neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J. Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J. Biol. Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Homolya L, Boucher RC, Harden TK. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J. Biol. Chem. 1997a;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Mason SJ, Clarke L, Harden TK, Boucher RC. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br. J. Pharmacol. 1992;106:774–782. doi: 10.1111/j.1476-5381.1992.tb14412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc. Natl. Acad. Sci. U. S. A. 1997b;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol. Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J. Biol. Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Watt WC, Stutts MJ, Brown HA, Boucher RC, Harden TK. Enzymatic synthesis of UTP gamma S, a potent hydrolysis resistant agonist of P2U-purinoceptors. Br. J. Pharmacol. 1996;117:203–209. doi: 10.1111/j.1476-5381.1996.tb15175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethem MI, Dowell ML, Van Scott M, Yankaskas JR, Egan T, Boucher RC, Davis CW. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 1993;9:315–322. doi: 10.1165/ajrcmb/9.3.315. [DOI] [PubMed] [Google Scholar]
- Leuba D, De RY, Kucera P. Ion transport, ciliary activity, and mechanosensitivity of sinusal mucosa: an in vitro study. Am. J. Physiol. 1996;271:L349–L358. doi: 10.1152/ajplung.1996.271.3.L349. [DOI] [PubMed] [Google Scholar]
- Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br. J. Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am. J. Physiol Cell Physiol. 2001;280:C1485–C1497. doi: 10.1152/ajpcell.2001.280.6.C1485. [DOI] [PubMed] [Google Scholar]
- Nakahari T. Regulation of ciliary beat frequency in airways: shear stress, ATP action, and its modulation. Am. J. Physiol Lung Cell Mol. Physiol. 2007;292:L612–L613. doi: 10.1152/ajplung.00433.2006. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J. Biol. Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. U. S. A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picher M, Boucher RC. Metabolism of extracellular nucleotides in human airways by a multienzyme system. Drug Dev. Res. 2001;52:52–75. [Google Scholar]
- Picher M, Boucher RC. Human airway ecto-adenylate kinase. A mechanism to propagate ATP signaling on airway surfaces. J. Biol. Chem. 2003;278:11256–11264. doi: 10.1074/jbc.M208071200. [DOI] [PubMed] [Google Scholar]
- Burch LH, Boucher RC. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J. Biol. Chem. 2004;279:20234–20241. doi: 10.1074/jbc.M400305200. [DOI] [PubMed] [Google Scholar]
- Priel Z. Something about regulating cbf by Ca2+ Resp. Physiol. Neurobiol. 2008 [Google Scholar]
- Rice WR, Burhans M, Wispe JR. Effect of oxygen exposure on ATP content of rat bronchoalveolar lavage. Pediatr. Res. 1989;25:396–398. doi: 10.1203/00006450-198904000-00018. [DOI] [PubMed] [Google Scholar]
- Rice WR, Singleton FM. P2-purinoceptors regulate surfactant secretion from rat isolated alveolar type II cells. Br. J. Pharmacol. 1986;89:485–491. doi: 10.1111/j.1476-5381.1986.tb11148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PB, Douillet CD, Mahler SA, Husain SA, Boucher RC. Adenosine triphosphate is released during injurious mechanical ventilation and contributes to lung edema. J. Trauma. 2003;55:290–297. doi: 10.1097/01.TA.0000078882.11919.AF. [DOI] [PubMed] [Google Scholar]
- Salathe M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- Salathe M, O'Riordan TG, Wanner A. Treatment of mucociliary dysfunction. Chest. 1996;110:1048–1057. doi: 10.1378/chest.110.4.1048. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ, Dirksen ER. Mechanosensitivity of cultured ciliated cells from the mammalian respiratory tract: implications for the regulation of mucociliary transport. Proc. Natl. Acad. Sci. U. S. A. 1986;83:7302–7306. doi: 10.1073/pnas.83.19.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Shin CY, Kim KC, Lee WJ, Jo MJ, Park KH, Dalby R, Ko KH. Inhaled ATP causes mucin release from goblet cells of intact rats. Exp. Lung Res. 2000;26:1–11. doi: 10.1080/019021400269925. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Chinet TC, Mason SJ, Fullton JM, Clarke LL, Boucher RC. Regulation of Cl- channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkotak AJ, Ng AM, Man SF, Baldwin SA, Cass CE, Young JD, Duszyk M. Coupling of CFTR-mediated anion secretion to nucleoside transporters and adenosine homeostasis in Calu-3 cells. J. Membr. Biol. 2003;192:169–179. doi: 10.1007/s00232-002-1073-x. [DOI] [PubMed] [Google Scholar]
- Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu. Rev. Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J. Biol. Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. Am. J. Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Verdugo P. Mucin exocytosis. Am. Rev. Respir. Dis. 1991;144:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am. J. Respir. Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J. Biol. Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- Winters SL, Davis CW, Boucher RC. Mechanosensitivity of mouse tracheal ciliary beat frequency: roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am. J. Physiol Lung Cell Mol. Physiol. 2007;292:L614–L624. doi: 10.1152/ajplung.00288.2005. [DOI] [PubMed] [Google Scholar]
- Wolff SC, Qi AD, Harden TK, Nicholas RA. Polarized expression of human P2Y receptors in epithelial cells from kidney, lung, and colon. Am. J. Physiol Cell Physiol. 2005;288:C624–C632. doi: 10.1152/ajpcell.00338.2004. [DOI] [PubMed] [Google Scholar]
- Wong LB, Yeates DB. Luminal purinergic regulatory mechanisms of tracheal ciliary beat frequency. Am. J. Respir. Cell Mol. Biol. 1992;7:447–454. doi: 10.1165/ajrcmb/7.4.447. [DOI] [PubMed] [Google Scholar]
- Zsembery A, Fortenberry JA, Liang L, Bebok Z, Tucker TA, Boyce AT, Braunstein GM, Welty E, Bell PD, Sorscher EJ, Clancy JP, Schwiebert EM. Extracellular zinc and ATP restore chloride secretion across cystic fibrosis airway epithelia by triggering calcium entry. J. Biol. Chem. 2004;279:10720–10729. doi: 10.1074/jbc.M313391200. [DOI] [PubMed] [Google Scholar]