Abstract
Polychlorinated biphenyls (PCBs) are persistent and ubiquitous environmental chemicals that bioaccumulate and have hepatic tumor promoting activity in rodents. The present study examined the effect of deleting the p50 subunit of NF-κB on the hepatic tumor promoting activity of 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB-153) in mice. Both wild-type and p50−/− male mice were injected i.p. with diethylnitrosamine (DEN, 90 mg/kg) and then subsequently injected biweekly with 20 i.p. injections of PCB-153 (300 µmol/kg/injection). p50 deletion decreased the tumor incidence in both PCB- and vehicle-treated mice, whereas PCB-153 slightly (P = 0.09) increased the tumor incidence in wild-type and p50−/− mice. PCB-153 increased the total tumor volume in both wild-type and p50−/− mice, but the total tumor volume was not affected by p50 deletion in either PCB- or vehicle-treated mice. The volume of tumors that were positive for glutamine synthetase (GS), which is indicative of mutations in the beta-catenin gene, was increased in both wild-type and p50−/− mice administered PCB-153 compared to vehicle controls and inhibited in p50 −/− mice compared to wild-type mice (in both PCB- and vehicle-treated mice). The volume of tumors that were negative for GS was increased in p50 −/− mice compared to wild-type mice but was not affected by PCB-153. PCB-153 increased cell proliferation in normal hepatocytes in wild-type but not p50−/− mice; this increase was inhibited in p50 −/− mice. In hepatic tumors, the rate of cell proliferation was much higher than in normal hepatocytes, but was not affected by PCB treatment or p50 deletion. The rate of apoptosis, as measured by the TUNEL assay, was not affected by PCB-153 or p50 deletion in normal hepatocytes. In hepatic tumors, the rate of apoptosis was lower than in normal hepatocytes; PCB-153 slightly (P = 0.10) increased apoptosis in p50−/− but not wild-type mice; p50 deletion had no effect. Taken together, these data indicate that the absence of the NF-κB p50 subunit inhibits the promoting activity of PCB-153 and alters the proliferative and apoptotic changes in mouse liver in the response to PCBs.
Keywords: PCBs, NF-κB, carcinogenesis, cell proliferation
Introduction
Polychlorinated biphenyls (PCBs) were commercially manufactured in the United States from 1930 to 1970 for use as dielectrics in transformers and capacitors, and as cooling fluids in hydraulic systems. PCBs have also been used in the formulation of lubricating and cutting oils, in pesticides and flame retardants, and as plasticizers in paints, copying paper, adhesives, sealants and plastics (World Health Organization, 1976). The stability of these compounds, one of their commercial attributes, has led to their worldwide distribution in the environment, an observation which was first reported by Jensen in 1966 (Jensen, 1966). The production of PCBs peaked in 1970 and has steadily declined thereafter as many countries throughout the world have banned certain uses or limited their production. Nevertheless these compounds remain in use and in our environment today and represent a potential human health hazard (Robertson and Hansen, 2001).
Although most PCBs are not particularly acutely toxic, their persistence and lipophilicity as well as their propensity to accumulate in fatty tissues raise concerns over their long-term effects. In animals and humans, chronic exposure to PCBs produces a variety of effects including decreased body weight (wasting syndrome), chloracne, edema, liver hypertrophy, porphyria, estrogenic activity, immunosuppression and neurotoxicity (National Research Council, 1979; Robertson and Hansen, 2001). The carcinogenicity of PCBs in humans has been examined in several epidemiological studies, and associations have been noted in some studies between PCB exposure and cancers of the liver, biliary tract, and intestines, as well as malignant melanoma (Faroon et al., 2001). In the Yusho poisoning incident, a statistically significant increase in the mortality from liver cancer was observed in males (but not females) exposed to PCBs (Kuratsume et al., 1996).
The evidence to date indicates that mixtures of PCBs can induce preneoplastic lesions and hepatocellular carcinomas in animals when given at appropriate doses for extended periods of time (Silberhorn et al., 1990; Mayes et al., 1998). Although their potency varies, mixtures of halogenated biphenyls as well as many individual congeners have been reported to be promoters of carcinogenesis in various liver tumor models (Glauert et al., 2001). Generally those compounds which are inducers of cytochrome P-450 (such as the higher halogenated biphenyls) were more potent as promoters. This includes PCB congeners that activate the Ah receptor, the constitutive androstane receptor (CAR), and the pregnane X receptor (PXR) (Ludewig et al., 2007).
Although PCBs clearly have promoting activity in the liver, their mechanism of action is not known. A number of mechanisms have been proposed, including direct effects on signal transduction pathways, induction of oxidative stress, effects on vitamin A metabolism, and effects on intercellular communication (Glauert et al., 2001). One mechanism by which PCBs may promote hepatic tumors is by inducing oxidative damage in the liver. Forms of oxidative damage that may be important are the induction of lipid peroxidation, the induction of oxidative DNA damage, and the alteration of gene expression. The majority of studies have found that PCBs increase hepatic lipid peroxidation (Kamohara et al., 1984; Oda et al., 1987; Dogra et al., 1988; Pelissier et al., 1990; Saito, 1990; Fadhel et al., 2002). Certain congeneric PCBs administered for short time periods were found to increase the levels of oxidative DNA damage, using 8-hydroxyguanosine as the endpoint (Oakley et al., 1996).
Nuclear factor-κB (NF-κB) is a eukaryotic transcription factor family consisting of dimers of the following proteins: p50 (NF-κB1), p65 (RelA), p52 (NF-κB2), c-Rel, and RelB. It is normally found in the cytoplasm as an inactive dimer bound to an inhibitory subunit, IκB, which also has several family members, including IκBα, IκBβ, IκBγ, and IκBε (Karin and Lin, 2002). Upon activation, NF-κB is released from IκB and translocates to the nucleus, where it increases the transcription of specific genes. There are two main pathways: the classical pathway, in which the p50:p65 heterodimer is predominate; and an alternative pathway, in which the p52:RelB dimer is activated (Senftleben et al., 2001; Karin and Lin, 2002). These processes require the phosphorylation of IκB, followed by the subsequent degradation via an ubiquitin-mediated 26S proteosome pathway (Karin and Lin, 2002). A 900 kDa complex, termed the IκB kinase (IKK) complex has been identified and consists of two kinase subunits of IKK, IKKα and IKKβ, and a regulatory subunit, IKKγ (Zandi et al., 1997; Karin and Delhase, 2000). These two kinase subunits form homo- or hetero-dimers that phosphorylate IκB molecules, leading to their degradation. NF-κB has been shown to be important in the activation of genes that regulate cell proliferation and apoptosis (Beg et al., 1995; Fitzgerald et al., 1995).
Several studies have used genetically modified mice to examine the role of NF-κB subunits on cell proliferation and apoptosis in the liver and other tissues. A clear role for NF-κB in inhibiting apoptosis by TNF-α or other apoptosis inducers has been demonstrated in several cell types, in studies in which NF-κB activity has been inhibited by the deletion of one of its subunits, the inhibition of its translocation, or the expression of a dominant negative form of IκB (Beg and Baltimore, 1996; Vanantwerp et al., 1996; Wang et al., 1996; Xu et al., 1998a; Schoemaker et al., 2002). However, DNA synthesis and liver regeneration were not affected by the absence of the p50 subunit following partial hepatectomy or carbon tetrachloride treatment (Deangelis et al., 2001). Similarly, the hepatic-specific expression of a truncated IκBα super-repressor did not affect DNA synthesis, apoptosis, or liver regeneration following partial hepatectomy, but led to increased apoptosis after treatment with TNF-α (Chaisson et al., 2002). Also, the hepatic inflammatory response after ischemia/reperfusion was not altered in p50 −/− mice (Kato et al., 2002). In addition, B cells lacking p50, RelB, or c-Rel (but not p52 or p65) have decreased proliferation in response to LPS (Kontgen et al., 1995; Sha et al., 1995; Snapper et al., 1996a; Snapper et al., 1996b; Horwitz et al., 1999). Overall, whether specific NF-κB subunits are essential for cell proliferation depends on the tissue and the stimulus for DNA synthesis.
One mechanism by which NF-κB may be activated is by increased oxidative stress. NF-κB can be activated in vitro by H2O2, and its activation can be inhibited by antioxidants, such as vitamin E or N-acetyl cysteine (NAC), or by increased expression of antioxidant enzymes (Staal et al., 1990; Schreck et al., 1991; Schreck et al., 1992; Meyer et al., 1993; Nilakantan et al., 1998; Li et al., 2000; Calfee-Mason et al., 2004). In addition, agents that activate NF-κB frequently also increase oxidative stress (Schmidt et al., 1995). However, Hayakawa et al. (2003) found that NAC inhibits NF-κB activation independently of its antioxidant function.
PCBs also can activate NF-κB. PCB-77 was found to activate NF-κB in rats after long-term treatment but not after a single dose (Tharappel et al., 2002; Lu et al., 2003; Glauert et al., 2005). PCB-153, in contrast, was found to activate NF-κB in rats after a single dose (Lu et al., 2003), but only activated NF-κB after multiple doses in one of three studies (Tharappel et al., 2002; Lu et al., 2004; Glauert et al., 2005). We subsequently found that cell proliferation induced by PCB-153 could be inhibited in mice lacking the p50 subunit of NF-κB (p50 −/− mice) (Lu et al., 2004).
In this study we have examined if the deletion of the p50 subunit of NF-κB would inhibit the tumor promoting activity of PCB-153. Wild-type and p50 −/− mice were first administered diethylnitrosamine (DEN) as a tumor initiator and then were exposed to PCB-153. The induction of hepatic tumors as well as effects on cell proliferation and apoptosis were quantified.
Materials and Methods
Chemicals
PCB-153 (2,2’,4,4’,5,5’-hexachlorobiphenyl) was synthesized and characterized as described previously; its purity was greater than 99%, as assayed by gas-chromatography (Schramm et al., 1985). Diethylnitrosamine was obtained from Sigma Chemical Co., St. Louis, MO.
Experimental Design
Mice homozygous for p50−/− deletion and B6129SF2/J age-matched wild type controls were obtained from our breeding colony. Founders of this strain had been obtained from The Jackson Laboratory (Bar Harbor, ME). After weaning, mice were fed an unrefined diet (Harlan Teklad 2018 Global 18% protein rodent diet) and water ad libitum. When mice of both strains were 9 weeks old, they received an i.p. injection of DEN (90 mg/kg). After a two-week recovery period, all mice were injected i.p. with 300 µmol/kg of PCB-153 or corn oil every 14 days. Mice received 20 PCB-153 injections and then were maintained for an additional 15 weeks. PCB-153 injections were stopped due to increased mortality and loss of body weight in these mice. Six days before euthanasia, all mice were given bromodeoxyuridine (BrdU, 0.5 mg/ml) in the drinking water. Mice were euthanized by overexposure to carbon dioxide gas. Liver tissue was then collected and part of it was frozen in liquid nitrogen and the remaining was fixed in formalin for pathological analysis and immunohistochemistry
Immunohistochemical Staining
The tissues processed for paraffin sections were made into 5-µm serial sections on glass slides. One section was used for double immunostaining, which used an anti-BrdU antibody to label the nuclei that took up BrdU and an anti-glutamine synthetase (GS) antibody using a Vectastain ABC kit for labeling GS-positive and -negative tumors. Another section was used for double immunostaining for GS and for TUNEL staining using the Apotag kit from Intergen (Purchase, NY), which allows for direct immunoperoxidase detection of digoxigenin-labeled genomic DNA.
Image Analysis
The volumes of GS-positive and GS-negative foci were measured using a computer digitizing system developed at University of Wisconsin (Campbell et al., 1982; Campbell et al., 1986; Xu et al., 1998b). The images were captured using a Nikon Eclipse E800 microscope equipped with MACRO 0.5x and 1.0x lenses. The volume fraction was quantified using the Delesse method.
Labeling Indexes
The cell proliferation and apoptosis labeling indexes were quantified in both normal hepatocytes and in tumors. At least 3000 nuclei from normal hepatocytes were randomly counted per slide (>1000 in each of three lobes) and the labeling indexes were expressed as the percentage of number of labeled nuclei out of the total number of nuclei counted.
PCB extraction from liver samples
PCB extraction and clean-up were performed as described previously (Bunaciu et al., 2007; Kania-Korwel et al., 2007). In short, liver samples pooled from 2 to 3 animals (0.46–0.78 g) were mixed thoroughly with 2 g of diatomaceous earth (Dionex, Sunnyvale, CA). Approximately one third of the mixture was placed in an extraction cell containing 10 g of Florisil (60–100 mesh, Fisher Sci., Pittsburg, PA). The cell was spiked with a surrogate standard containing 2,3,5,6-tetrachlorobiphenyl (PCB 65, 2.1µg/ml) and 2,3,4,4’,5,6-hexachlorobiphenyl (PCB 166, 2.0 µg/ml) and extracted with hexane-acetone (1:1, v/v) using an Accelerated Solvent Extractor ASE 200 (Dionex, Sunnyvale, CA). The following extraction conditions were used: temperature 100°C, pressure 1500 psi, 6 min heating, one 5 min static cycle, 60% of cell flush volume. The extract was concentrated under a gentle stream of nitrogen and an internal standard containing 2,4,6-trichlorobiphenyl (PCB 30, 0.5 µg/ml) and 2,2’,3,4,4’,5,6,6’-octachlorobiphenyl (PCB 204, 0.5 µg/ml) was added. An additional clean-up step was preformed by shaking each extract with 2 ml of 2-propanol and 2 ml of tetrabutylammonium sulfite (2 ml) and re-shaking with 5 ml of ultra-pure water (Kania-Korwel et al., 2005; Kania-Korwel et al., 2007). The organic phase was separated and allowed to stand overnight over concentrated sulfuric acid (2 ml) before gas chromatographic analysis.
Gas chromatographic PCB analysis
The samples were analyzed on an Agilent 6890N gas chromatograph equipped with a DB-5 MS column (60 mx0.25 mmx0.25 µm, Supelco, St. Louis, MO) and a 63Ni µ-ECD detector (Kania-Korwel et al., 2005). The following temperature program was used: 80°C for 1 min, then increased 25°/min from 80°C to 280°C, hold for 10 min. The injector temperature was 250°C and the detector temperature was 300°C. An aliquot of extract (2 µl) was injected and the PCB congener concentration was determined using the internal standard method. The detector was linear over the entire concentration range used in the study.
Quality assurance
The limit of detection (LOD), calculated based on method blanks as LOD = xb + k sb (xb is mean of the 7 blank measures, k is Student’s t-value for n-1 degrees of freedom at 99% confidence level, and sb is standard deviation of the blank measures) (Kania-Korwel et al., 2007), was equal to 1.9 ng for PCB 153. The limit of quantification (LOQ) was conservatively calculated as LOQ = 10 ·LOD and equaled 19 ng for PCB 153. The recovery rates were 98±5 % and 106±7 % for PCB 65 and PCB 166, respectively. PCB 153 levels were corrected when the recovery rates of PCB 166 were < 100%.
Lipids extraction from liver samples
The remaining sample-diatomaceous earth mixtures were used to determine the lipid content as described earlier (Kania-Korwel et al., 2007). In short, samples were placed in extraction cells and extracted with Accelerated Solvent Extractor ASE 200 (Dionex, Sunnyvale, CA) using chloroform-methanol (2:1, v/v). The following extraction conditions were used: temperature 100°C, pressure 1500 psi, 6 min heating, two 5 min static cycle, 100% of cell flush volume. The extract was concentrated and the lipid content was determined gravimetrically. The non-volatile residue in solvent blanks was 0.45±0.30 mg (n=7) and was significantly lower than the average lipid amount in all tissues samples (23±7 mg, n=11).
Statistical analyses
Tumor incidence data were analyzed by χ2 analysis. Other data were analyzed by two-way ANOVA. If a significant interaction was observed in the two-way ANOVA, individual differences between means were determined using Tukey’s posthoc test. The results are expressed as means ± standard error of the mean (SEM). The results were considered significant at p<0.05.
Results
In this study, we examined whether the p50 subunit of NF-κB is necessary for the promoting activities of PCB-153. Following DEN administration, PCB-153 was administered every other week for 20 injections followed by 15 weeks of no further treatment. At the conclusion of the study, body weights were lower in p50 −/− mice compared to wild-type mice (in both PCB- and vehicle-treated). PCB-153 administration decreased weight gain in wild-type mice (Table 1); the p50 −/− mice not administered PCB-153 gained slightly less weight than wild-type mice (P = 0.077). Liver weights were increased by PCB-153 in both wild-type and p50 −/− mice; p50 deletion decreased the liver weight in mice administered PCB-153 but not in vehicle controls.
Table 1.
Effect of p50 deletion and PCB-153 on body weight, liver weight, and tumor incidence
| Groups | # of mice per group | % Mortality | Body Weight at Start of PCB Injections (g) | Final Body Weight (g) | Weight Gain (g) | Liver Weight (g) | Liver Weight/Body Weight Ratio (%) | Tumor incidence (%) |
|---|---|---|---|---|---|---|---|---|
| Corn Oil | ||||||||
| Wild-type | 17 | 12 | 27.2 ± 0.6 | 39.7 ± 1.8 | 12.31 ± 1.67 | 2.50 ± 0.19 | 6.38 ± 0.48 | 11/15 (73) |
| p50 −/− | 14 | 29 | 27.0 ± 0.5 | 33.1 ± 1.8# | 6.51 ± 1.77 | 2.10 ± 0.32 | 5.42 ± 0.13 | 5/11 (45)# |
| PCB-153 | ||||||||
| Wild-type | 14 | 57 | 32.6 ± 1.4* | 36.7 ± 1.0 | 5.24 ± 1.50* | 8.54 ± 1.59* | 23.37 ± 2.15* | 7/7 (100) |
| p50 −/− | 14 | 43 | 26.5 ± 0.6# | 30.5 ± 1.3# | 3.99 ± 1.58 | 3.63 ± 0.32*,# | 11.93 ± 1.08*,# | 6/9 (67)# |
Data are means ± standard errors
Significant effect in group receiving PCB-153, compared to corresponding vehicle control
Significant effect in p50 −/− mice, compared to corresponding wild-type control
The incidence of hepatic tumors is shown in Table 1. p50 deletion significantly decreased the tumor incidence in both PCB- and vehicle-treated mice. PCB treatment did not significantly affect the tumor incidence, although mice treated with PCB-153 had a slightly higher incidence (P = 0.09). The mouse livers showed “neoplastic nodules” (Squire and Levitt, 1975) and most of those nodules could be classified as hepatocellular carcinomas according to currently recommended nomenclature for hepatoproliferative lesions in rats (Maronpot et al., 1986; Goodman et al., 1994; Narama et al., 2003). None of those nodules showed obvious hallmarks of malignancy such as invasion and metastasis but most of them showed marked cellular or histological atypia and numerous mitotic figures. No additional characteristics were observed in tumors from the p50 −/− mice.
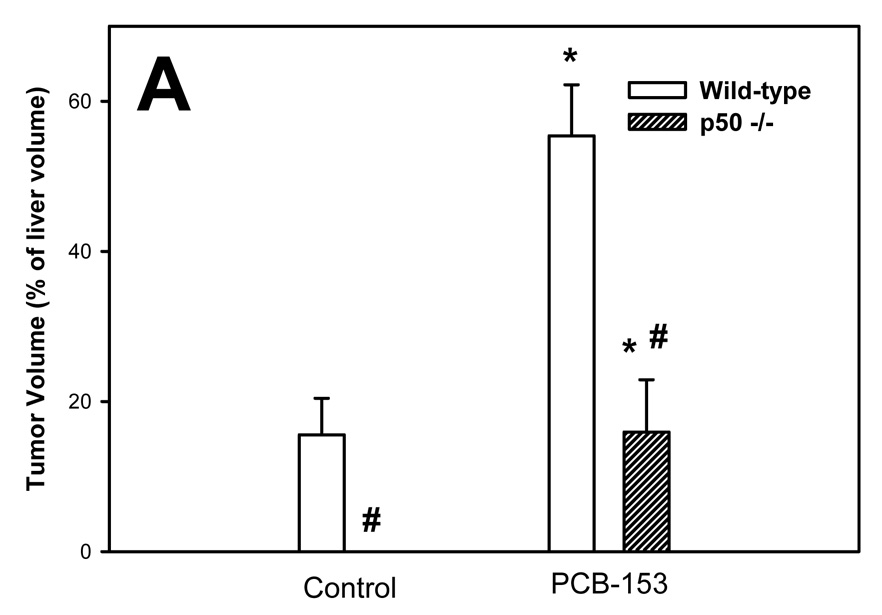
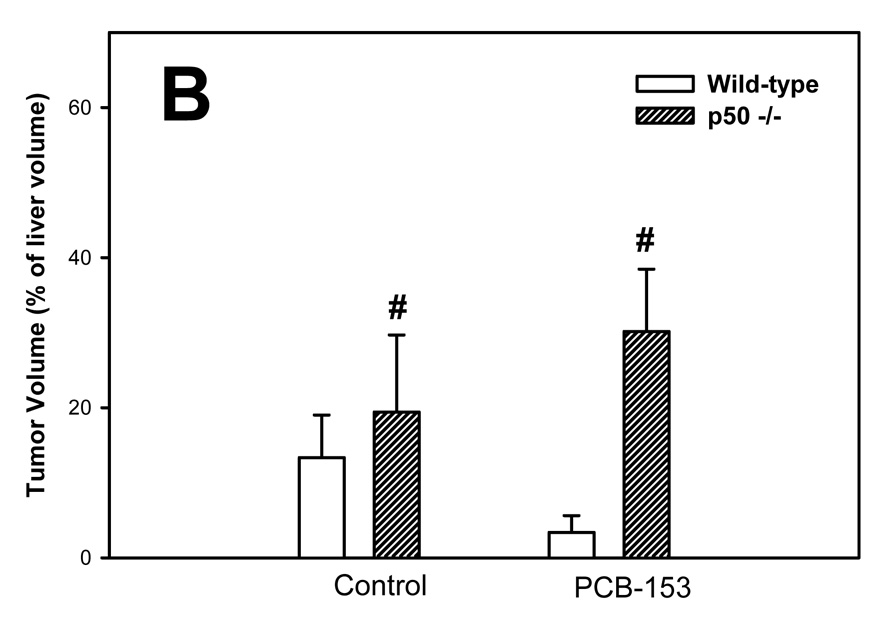
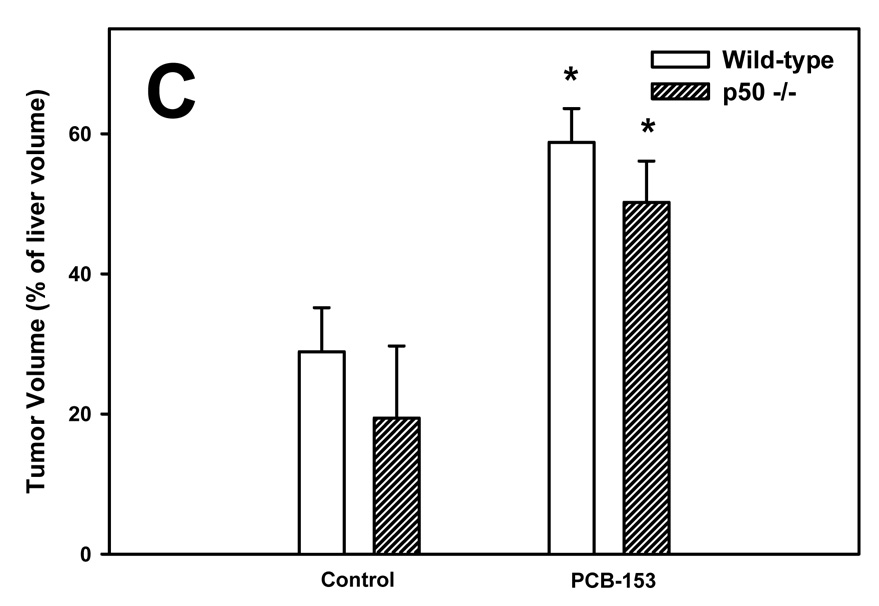
The volume of tumors was quantified using glutamine synthetase (GS) as a marker; both GS-positive and GS-negative tumors were identified (Figure 1). The volumes of total and GS-positive tumors were increased by PCB-153 in both wild-type and p50 −/− mice. The total tumor volume was not affected by p50 deletion, but the volume of GS-positive tumors was decreased by p50 deletion in both control and PCB-153 treated mice. The volume of GS-negative tumors was significantly increased by p50 deletion in both PCB- and vehicle-treated mice, but was not affected by PCB-153.
Figure 1. Effect of PCB-153 on the volume of tumors in p50 −/− and wild-type (WT) mice. A. GS-Positive Tumors. B. GS-Negative Tumors. C. All Tumors.
Mice were administered diethylnitrosamine (DEN) and then administered PCB-153 or vehicle. Histological sections for the liver were immunohistochemically stained for GS, and the volume fraction of GS-positive and GS-negative tumors was determined. Data are means ± standard errors.
*Significant effect in group receiving PCB-153, compared to corresponding vehicle control
#Significant effect in p50 −/− mice, compared to corresponding wild-type control
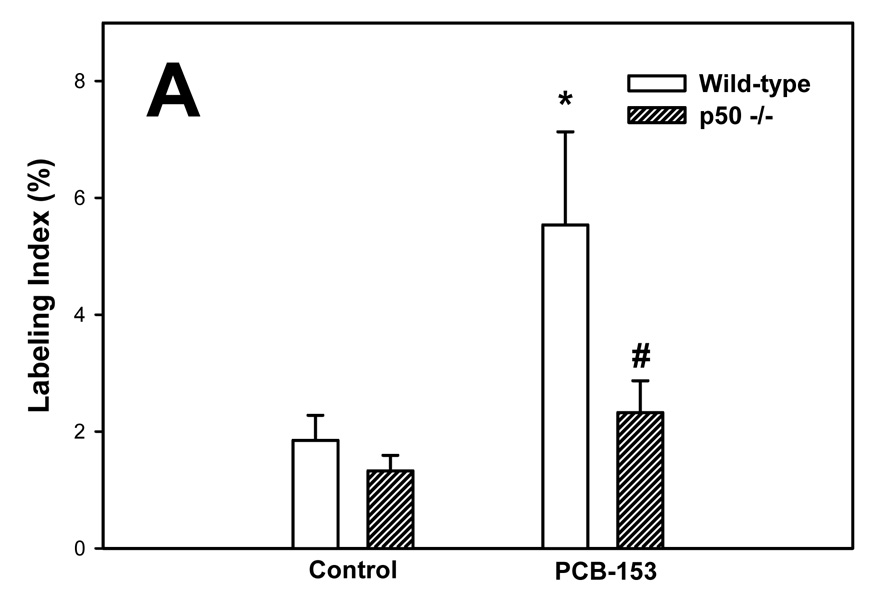
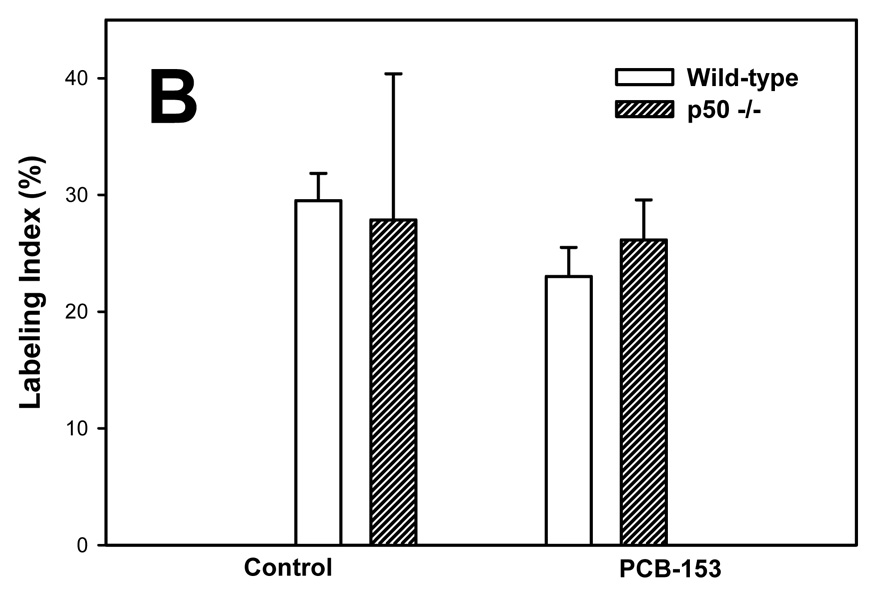
PCBs in previous studies have been found to increase hepatic cell proliferation (Tharappel et al., 2002; Lu et al., 2003; Lu et al., 2004). We therefore examined cell proliferation in normal hepatocytes and hepatic tumors by administering BrdU in the drinking water for 6 days before euthanasia and then double-immunostaining the liver sections for both BrdU and GS. Labeling indexes were much higher in the tumors in all groups (Figure 2). In normal hepatocytes, PCB-153 increased the labeling index in wild-type mice but not p50 −/− mice; this increase was inhibited in the p50 −/− mice. The labeling index in hepatic tumors was not significantly affected by either PCB-153 administration or p50 deletion.
Figure 2. Effect of PCB-153 on hepatocyte proliferation in p50 −/− and wild-type (WT) mice. A. Normal hepatocytes. B. Tumors.
Mice were administered DEN and then administered PCB-153 or vehicle. Six days before euthanasia, mice were administered drinking water containing bromodeoxyuridine (BrdU). Histological sections for the liver were immunohistochemically stained for BrdU and GS, and labeling indexes were determined in hepatocytes to determine the rate of DNA synthesis. Data are means ± standard errors.
*Significant effect in group receiving PCB-153, compared to corresponding vehicle control
#Significant effect in p50 −/− mice, compared to corresponding wild-type control
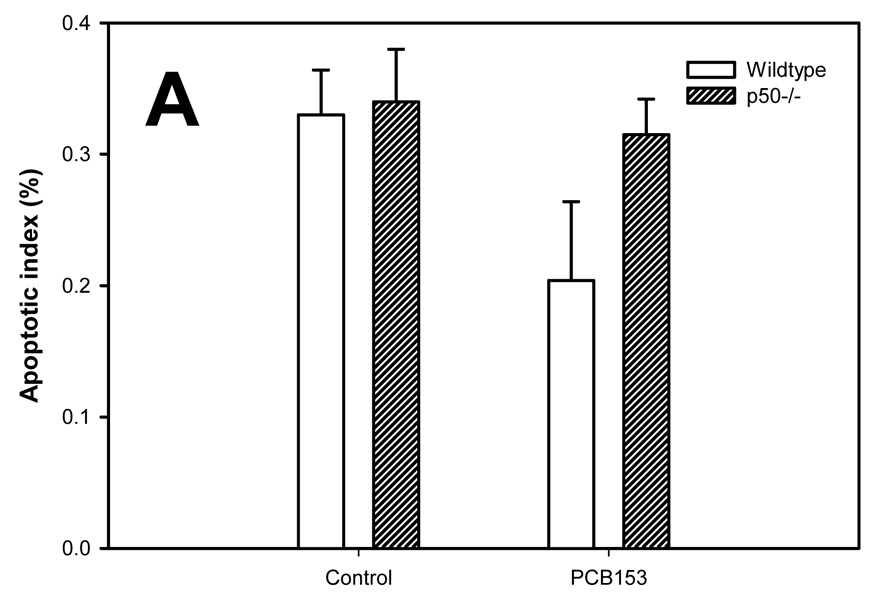
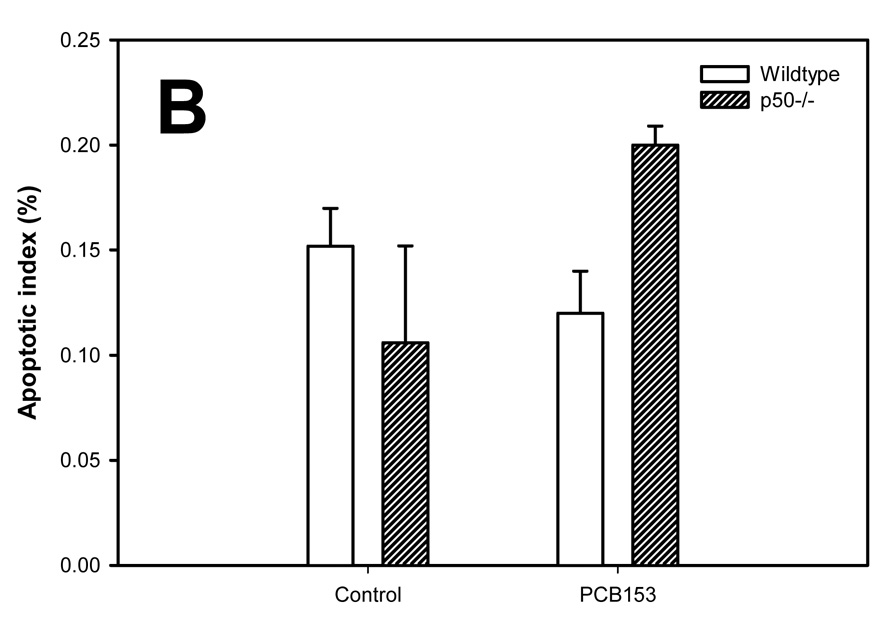
Apoptosis in the study was evaluated using the TUNEL assay (Figure 3). The apoptotic indexes were lower in the tumors compared to the normal hepatocytes in all groups. PCB-153 administration did not affect the apoptotic index in normal hepatocytes. In hepatic tumors, PCB-153 treatment slightly (P = 0.10) increased the apoptotic index in p50 −/− mice, but not in wild-type mice. p50 deletion did not significantly affect the apoptotic index in either normal hepatocytes or hepatic tumors.
Figure 3. Effect of PCB-153 on hepatocyte apoptosis in p50 −/− and wild-type (WT) mice. A. Normal hepatocytes. B. Tumors.
Mice were administered DEN, and then fed administered PCB-153 or vehicle. Histological sections for the liver were used for TUNEL staining, and apoptotic indexes were determined in hepatocytes to determine the rate of apoptosis. Data are means ± standard errors. No statistically significant effects (P < 0.05) were observed.
We quantified the PCB-153 levels in the liver to determine if the effects observed could have been due to differences in hepatic PCB concentrations. The administration of PCB-153 clearly increased hepatic PCB levels in PCB 153-treated animals compared to background levels in animals treated with corn oil alone (Table 2). PCB-153 concentrations were about 4 fold higher in p50 −/− mice receiving PCB-153 compared to wild-type mice receiving PCB-153.
Table 2.
Effect of p50 deletion and PCB-153 on hepatic PCB levels
| Groups | PCB-153 (ng/g liver) | PCB-153 (µg/g lipid) |
|---|---|---|
| Corn Oil | ||
| Wild-typea | 6.35 | 0.07 |
| p50 −/−b | 53.4 ± 49.9 | 0.95 ± 0.90 |
| PCB-153 | ||
| Wild-typec | 14,437 ± 1,643 | 281.9 ± 56.7 |
| p50 −/−b | 53,573 ± 16,772 | 1049.6 ± 541.9 |
Results from one pooled liver sample with the liver from three animals.
Results from two pooled liver samples with the liver from three animals per sample.
Results from two pooled liver samples with the liver from two animals per sample.
Data are means ± standard errors
Discussion
In this paper we have found that the deletion of the p50 subunit of NF-κB inhibits the tumor promoting activity of PCB-153. Both the tumor incidence and the volume of GS-positive tumors (the main tumor type) were decreased in p50 −/− mice. In addition the induction of cell proliferation by PCB-153 in normal hepatocytes was inhibited in p50 −/− mice. PCB-153 also slightly increased apoptosis in hepatic tumors in p50 −/− mice but not wild-type mice.
Overall the results show that p50 deletion inhibits the promotion of tumors by PCB-153,but the volume of GS-negative tumors was actually increased in p50 −/− mice. The mechanisms of this increase and the implications of this increase are not clear. When CAR activators such as PCB-153 or phenobarbital are used as the promoting agent, the predominant type of tumor observed in wild-type mice is GS-positive (Figure 1) (Loeppen et al., 2002; Strathmann et al., 2006). These GS-positive tumors contain mutations in the β-catenin gene (Loeppen et al., 2002; Strathmann et al., 2006). In GS-negative tumors observed in wild-type mice treated with phenobarbital or PCB-153, the type of mutation that was induced is unknown (Loeppen et al., 2002; Strathmann et al., 2006). It is possible that the deletion of the p50 subunit and the resulting changes in gene expression are leading to the selection of hepatocytes that have a particular type of mutation unrelated to β-catenin, but it is not clear at present what gene or genes this would be.
The deletion of the p50 subunit could be altering the promotion of carcinogenesis by PCB-153 by several molecular mechanisms. First, the deletion of p50 would lead to disruption of the classical pathway of NF-κB activation, since the formation of p65-p50 dimers would be prevented. The formation of p50 homodimers would also be prevented. Since p50 homodimers can translocate to the nucleus and act as repressors of transcription (Israel et al., 1989; Schmitz and Baeuerle, 1991; Brown et al., 1994), gene expression could clearly be altered in p50 −/− mice by this mechanism. It is also possible that some of these changes could be due to the deletion of the p50 precursor protein, p105. In addition to being processed to p50, p105 can directly affect signal transduction, such as the regulation of the MAP kinase pathway (Beinke and Ley, 2004).
This study supports the concept that inhibiting NF-κB activation inhibits experimental hepatocarcinogenesis. Two previous studies have observed that the inhibition of NF-κB activation leads to the inhibition of chemically-induced liver cancer in mice. Using the p50 −/− knockout model, Glauert et al. (2006) found that the promotion of hepatic tumors by the peroxisome proliferator Wy-14,643 was inhibited. Pikarksy et al. (2004) inhibited NF-κB activation using a hepatocyte-specific IκB-super-repressor transgene in Mdr2 knockout mice, which develop hepatocellular carcinoma spontaneously. They found that inhibiting NF-κB activation in the early stages of cancer development had no effect, but that inhibiting NF-κB activation at the later stages inhibited the development of hepatocellular carcinomas, likely by increasing apoptosis. Maeda et al. (2005), however, found that knocking out IκB kinase β (IKKβ) specifically in hepatocytes resulted in the induction of a higher incidence of hepatocellular carcinomas in mice injected with DEN. However, when IKKβ was also knocked out in hematopoietic cells (including Kupffer cells), this effect was negated, implying that increased Kupffer cell activity could have been the cause of the increased tumorigenesis in the mice lacking IKKβ in hepatocytes. Furthermore, in mice deficient in interleukin (IL)-6, an NF-κB regulated gene and a cytokine released by Kupffer cells, tumorigenesis by DEN is inhibited (Naugler et al., 2007). In the p50 −/− knockout model, NF-κB activation would be inhibited in Kupffer cells as well as in hepatocytes and this inhibition may have contributed to the decrease in tumorigenesis observed in the present and Glauert et al. (2006) studies.
The effects of PCB-153 and p50 deletion on tumorigenesis could be related to their effects on cell proliferation and apoptosis. In tumors, cell proliferation rates were higher and apoptosis rates were lower than in normal hepatocytes, both of which correlate with increased size and growth of the tumors. In normal hepatocytes (but not in tumors), PCB-153 increased cell proliferation in wild-type mice, but this increase was inhibited in p50 −/− mice. In tumors, PCB-153 increased apoptosis, but only in p50 −/− mice. Therefore the increase in tumor incidence and volume in PCB-153-treated mice and its inhibition in p50 −/− mice appear to be related to the changes in cell proliferation in normal hepatocytes and the changes in apoptosis in tumors.
The inhibitory effect of p50 deletion on the promotion of hepatocarcinogenesis by PCB-153 does not appear to be related to an alteration of hepatic PCB levels. PCB-153 levels were higher in p50 −/− mice, which would be expected to increase the induction of tumors, not decrease it. The mechanism of this effect is not clear. PCB-153 is poorly metabolized (Robertson and Hansen, 2001); after administration its concentration first increases in the liver and it then redistributes to the adipose tissue (Mühlebach and Bickel, 1981; Oberg et al., 2002). PCB-153 is transported by albumin and lipoproteins (Mühlebach et al., 1991). The higher concentrations observed in the p50 −/− mice may be related to differences in the hepatic lipid compostion and/or protein levels that alter the sequestration of PCB 153 into the liver, or to decreased redistribution to adipose tissue, but neither of these mechanisms has been demonstrated at this time. Hepatic PCB-153 concentrations were much higher in mice administered PCB-153, as expected.
In summary, we have demonstrated that the promotion of hepatic tumors in mice by PCB-153 is inhibited in mice lacking the p50 subunit of NF-κB. PCB-153 increased cell proliferation in normal hepatocytes in wild-type but not p50 −/− mice and increased apoptosis in hepatic tumors in p50 −/− mice but not wild-type mice, both of which correlates with the effects on tumorigenesis observed. Therefore it is likely that NF-κB target genes that regulate cell proliferation and apoptosis contribute to the promoting activity of PCB-153. The specific genes that are responsible for these effects, however, are currently unknown.
Acknowledgments
We thank Petruta Bunaciu, Jason Lu, Divinia Stemm, Jill Cholewa, and Amy Dugan for their assistance with the project. This study was funded by the National Institutes of Health (ES013661, ES07380, and ES012475) and by the Kentucky Agricultural Experiment Station. The study sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
There are no conflicts of interest.
References
- Beg AA, Baltimore D. An essential role for NF-kappa B in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Linhoff MW, Stein B, Wright KL, Baldwin AS, Jr, Basta PV, Ting JP. Function of NF-kappa B/Rel binding sites in the major histocompatibility complex class II invariant chain promoter is dependent on cell-specific binding of different NF-kappa B/Rel subunits. Mol. Cell. Biol. 1994;14:2926–2935. doi: 10.1128/mcb.14.5.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunaciu RP, Tharappel JC, Lehmler HJ, Kania-Korwel I, Robertson LW, Srinivasan C, Spear BT, Glauert HP. The effect of dietary glycine on the hepatic tumor promoting activity of polychlorinated biphenyls (PCBs) in rats. Toxicology. 2007;239:147–155. doi: 10.1016/j.tox.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee-Mason KG, Spear BT, Glauert HP. Effects of vitamin E on the NF-kB pathway in rats treated with the peroxisome proliferator, ciprofibrate. Toxicol. Appl. Pharmacol. 2004;199:1–9. doi: 10.1016/j.taap.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Campbell HA, Pitot HC, Potter VR, Laishes BA. Application of quantitative stereology to the evaluation of enzyme-altered foci in rat liver. Cancer Research. 1982;42:465–472. [PubMed] [Google Scholar]
- Campbell HA, Xu YD, Hanigan MH, Pitot HC. Application of quantitative stereology to the evaluation of phenotypically heterogeneous enzyme-altered foci in the rat liver. Journal of the National Cancer Institute. 1986;76:751–767. doi: 10.1093/jnci/76.4.751. [DOI] [PubMed] [Google Scholar]
- Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappa B leads to apoptosis after TNF treatment, but not after partial hepatectomy. J. Clin. Invest. 2002;110:193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deangelis RA, Kovalovich K, Cressman DE, Taub R. Normal liver regeneration in p50/nuclear factor kappa B1 knockout mice. Hepatology. 2001;33:915–924. doi: 10.1053/jhep.2001.23192. [DOI] [PubMed] [Google Scholar]
- Dogra S, Filser JG, Cojocel C, Greim H, Regel U, Oesch F, Robertson LW. Long-term effects of commercial and congeneric polychlorinated biphenyls on ethane production and malondialdehyde levels, indicators of in vivo lipid peroxidation. Archives of Toxicology. 1988;62:369–374. doi: 10.1007/BF00293625. [DOI] [PubMed] [Google Scholar]
- Fadhel Z, Lu Z, Robertson LW, Glauert HP. Effect of 3,3',4,4'-tetrachlorobiphenyl and 2,2',4,4',5,5'-hexachlorobiphenyl on the induction of hepatic lipid peroxidation and cytochrome P-450 associated enzyme activities in rats. Toxicology. 2002;175:15–25. doi: 10.1016/s0300-483x(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Faroon OM, Keith S, Jones D, de Rosa C. Carcinogenic effects of polychlorinated biphenyls. Toxicol Industrial Health. 2001;17:41–62. doi: 10.1191/0748233701th098oa. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417–427. [PubMed] [Google Scholar]
- Glauert HP, Eyigor A, Tharappel JC, Cooper S, Lee EY, Spear BT. Inhibition of hepatocarcinogenesis by the deletion of the p50 subunit of NF-kB in mice administered the peroxisome proliferator Wy-14,643. Toxicol Sci. 2006;90:331–336. doi: 10.1093/toxsci/kfj116. [DOI] [PubMed] [Google Scholar]
- Glauert HP, Kumar A, Lu Z, Patel S, Tharappel JC, Stemm DN, Bunaciu RP, Lee EY, Lehmler HJ, Robertson LW, Spear BT. Dietary vitamin E does not inhibit the promotion of liver carcinogenesis by polychlorinated biphenyls in rats. J. Nutr. 2005;135:283–286. doi: 10.1093/jn/135.2.283. [DOI] [PubMed] [Google Scholar]
- Glauert HP, Robertson LW, Silberhorn EM. PCBs and tumor promotion. In: Robertson LW, Hansen LG, editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: University Press of Kentucky; 2001. pp. 355–371. [Google Scholar]
- Goodman DG, Maronpot RR, Newberne PM, Popp JA, Squire RA. Guides for Toxicological Pathology. Washington, DC: STP/ARP/AFIP; 1994. Proliferative and selected other lesions in the liver of rats, GI-5. [Google Scholar]
- Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz BH, Zelazowski P, Shen Y, Wolcott KM, Scott ML, Baltimore D, Snapper CM. The p65 subunit of NF-kappa B is redundant with p50 during B cell proliferative responses, and is required for germline C-H transcription and class switching to IgG3. Journal of Immunology. 1999;162:1941–1946. [PubMed] [Google Scholar]
- Israel A, Le Bail O, Hatat D, Piette J, Kieran M, Logeat F, Wallach D, Fellous M, Kourilsky P. TNF stimulates expression of mouse MHC class I genes by inducing an NF kappa B-like enhancer binding activity which displaces constitutive factors. EMBO J. 1989;8:3793–3800. doi: 10.1002/j.1460-2075.1989.tb08556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. A new chemical hazard. New Scientist. 1966;32:612. [Google Scholar]
- Kamohara K, Yagi N, Itokawa Y. Mechanism of lipid peroxide formation in polychlorinated biphenyls (PCB) and dichlorodiphenyltrichloroethane (DDT)-poisoned rats. Environmental Research. 1984;34:18–23. doi: 10.1016/0013-9351(84)90071-9. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Hornbuckle KC, Peck A, Ludewig G, Robertson LW, Sulkowski WW, Espandiari P, Gairola CG, Lehmler H-J. Congener specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ. Sci. Technol. 2005;39:3513–3520. doi: 10.1021/es047987f. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler H-J. Enantioselective disposition of PCB 136 (2,2',3,3',6,6'-hexachlorobifenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality. 2007;19:56–66. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin.Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappa B at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kato A, Edwards MJ, Lentsch AB. Gene deletion of NF-kappa B p50 does not alter the hepatic inflammatory response to ischemia/reperfusion. J. Hepatol. 2002;37:48–55. doi: 10.1016/s0168-8278(02)00068-5. [DOI] [PubMed] [Google Scholar]
- Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Kuratsume M, Yoshimura H, Hori Y, Okumura M, Masuda Y. Yusho: A Human Disaster Caused by PCBs and Related Compounds. Fukuoka, Japan: Kyushu University Press; 1996. [Google Scholar]
- Li YX, Glauert HP, Spear BT. Activation of nuclear factor-kappa B by the peroxisome proliferator ciprofibrate in H4IIEC3 rat hepatoma cells and its inhibition by the antioxidants N-acetylcysteine and vitamin E. Biochemical Pharmacology. 2000;59:427–434. doi: 10.1016/s0006-2952(99)00339-1. [DOI] [PubMed] [Google Scholar]
- Loeppen S, Schneider D, Gaunitz F, Gebhardt R, Kurek R, Buchmann A, Schwarz M. Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002;62:5685–5688. [PubMed] [Google Scholar]
- Lu Z, Lee EY, Robertson LW, Glauert HP, Spear BT. Effect of 2,2',4,4',5,5'-hexachlorobiphenyl (PCB-153) on hepatocyte proliferation and apoptosis in mice deficient in the p50 subunit of the transcription factor NF-kB. Toxicol Sci. 2004;81:35–42. doi: 10.1093/toxsci/kfh193. [DOI] [PubMed] [Google Scholar]
- Lu Z, Tharappel JC, Lee EY, Robertson LW, Spear BT, Glauert HP. Effect of a single dose of polychlorinated biphenyls on hepatic cell proliferation and the DNA binding activity of NF-kappaB and AP-1 in rats. Mol. Carcinog. 2003;37:171–180. doi: 10.1002/mc.10135. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Esch H, Robertson LW. Polyhalogenierte bi- und terphenyle. In: Dunkelberg H, Gebel T, Hartwig A, editors. Handbuch der Lebensmitteltoxikologie. Germany: Wiley-VCH Weinheim; 2007. pp. 1031–1094. [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Montgomery CAJ, Boorman GA, McConnell EE. National toxicology program nomenclature for hepatoproliferative lesions of rats. Toxicological Pathology. 1986;14:263–273. doi: 10.1177/019262338601400217. [DOI] [PubMed] [Google Scholar]
- Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW, Brown JF, Menton RG, Moore JA. Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol Sci. 1998;41:62–76. doi: 10.1093/toxsci/41.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Schreck R, Baeuerle PA. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidantresponsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlebach S, Bickel MH. Pharmacokinetics in rats of 2,4,5,2',4',4'-hexachlorobiphenyl, an unmetabolizable lipophilic model compound. Xenobiotica. 1981;11:249–257. doi: 10.3109/00498258109045299. [DOI] [PubMed] [Google Scholar]
- Mühlebach S, Wyss PA, Bickel MH. The use of 2,4,5,2',4',5'-hexachlorobiphenyl (6-CB) as an unmetabolizable lipophilic model compound. Pharmacol. Toxicol. 1991;69:410–415. doi: 10.1111/j.1600-0773.1991.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Narama I, Imaida K, Iwata H, Nakae D, Nishikawa A, Harada T. A review of nomenclature and diagnostic criteria for proliferative lesions in the liver of rats by a Working Group of the Japanese Society of Toxicologic Pathology. J Toxicol Pathol. 2003;16:1–17. [Google Scholar]
- National Research Council. Polychlorinated Biphenyls. Washington, DC: National Academy of Sciences; 1979. [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Nilakantan V, Spear BT, Glauert HP. Liver-specific catalase expression in transgenic mice inhibits NF- kappaB activation and DNA synthesis induced by the peroxisome proliferator ciprofibrate. Carcinogenesis. 1998;19:631–637. doi: 10.1093/carcin/19.4.631. [DOI] [PubMed] [Google Scholar]
- Oakley GG, Devanaboyina U, Robertson LW, Gupta RC. Oxidative DNA damage induced by activation of polychlorinated biphenyls (PCBs): implications for PCB-induced oxidative stress in breast cancer. Chem Res Toxicol. 1996;9:1285–1292. doi: 10.1021/tx960103o. [DOI] [PubMed] [Google Scholar]
- Oberg M, Sjodin A, Casabona H, Nordgren I, KlassonWehler E, Hakansson H. Tissue distribution and half-lives of individual polychlorinated biphenyls and serum levels of 4-hydroxy- 2,3,3 ',4 ',5-pentachlorobiphenyl in the rat. Toxicol Sci. 2002;70:171–182. doi: 10.1093/toxsci/70.2.171. [DOI] [PubMed] [Google Scholar]
- Oda H, Yamashita K, Sasaki S, Horio F, Yoshida A. Long-term effects of dietary polychlorinated biphenyl and high level of vitamin E on ascorbic acid and lipid metabolism in rats. J. Nutr. 1987;117:1217–1223. doi: 10.1093/jn/117.7.1217. [DOI] [PubMed] [Google Scholar]
- Pelissier MA, Boisset M, Atteba S, Albrecht R. Lipid peroxidation of rat liver microsomes membranes related to a protein deficiency and/or a PCB treatment. Food Addit. Contam. 1990;7:S172–S177. doi: 10.1080/02652039009373875. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Hansen LG. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: University Press of Kentucky; 2001. [Google Scholar]
- Saito M. Polychlorinated biphenyls-induced lipid peroxidation as measured by thiobarbituric acid-reactive substances in liver subcellular fractions of rats. Biochimica et Biophysica Acta. 1990;1046:301–308. doi: 10.1016/0005-2760(90)90245-s. [DOI] [PubMed] [Google Scholar]
- Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Chem. Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Baeuerle PA. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker MH, Ros JE, Homan M, Trautwein C, Liston P, Poelstra K, vanGoor H, Jansen PLM, Moshage H. Cytokine regulation of pro- and anti-apoptotic genes in rat hepatocytes: NF-kappa B-regulated inhibitor of apoptosis protein 2 (CIAP2) prevents apoptosis. J. Hepatol. 2002;36:742–750. doi: 10.1016/s0168-8278(02)00063-6. [DOI] [PubMed] [Google Scholar]
- Schramm H, Robertson LW, Oesch F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochemical Pharmacology. 1985;34:3735–3739. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]
- Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic. Res. Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senftleben U, Cao YX, Xiao GT, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu YL, Fong A, Sun SC, Karin M. Activation by IKK alpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit. Rev. Toxicol. 1990;20:439–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Rosas FR, Zelazowski P, Moorman MA, Kehry MR, Bravo R, Weih F. B cells lacking RelB are defective in proliferative responses, but undergo normal B cell maturation to Ig secretion and Ig class switching. J. Exp. Med. 1996a;184:1537–1541. doi: 10.1084/jem.184.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper CM, Zelazowski P, Rosas FR, Kehry MR, Tian M, Baltimore D, Sha WC. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J Immunol. 1996b;156:183–191. [PubMed] [Google Scholar]
- Squire RA, Levitt MH. Report of a workshop on classification of specific hepatocellular lesions in rats. Cancer Research. 1975;35:3214–3223. [PubMed] [Google Scholar]
- Staal FJT, Roederer M, Herzenberg LA, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kB and transcription of human immunodeficiency virus. Proceedings of the National Academy of Sciences-USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathmann J, Schwarz M, Tharappel JC, Glauert HP, Spear BT, Robertson LW, Appel KE, Buchmann A. PCB 153, a non-dioxin-like tumor promoter, selects for beta-catenin (Catnb)-mutated mouse liver tumors. Toxicol Sci. 2006;93:34–40. doi: 10.1093/toxsci/kfl041. [DOI] [PubMed] [Google Scholar]
- Tharappel JC, Lee EY, Robertson LW, Spear BT, Glauert HP. Regulation of cell proliferation, apoptosis, and transcription factor activities during the promotion of liver carcinogenesis by polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 2002;179:172–184. doi: 10.1006/taap.2001.9360. [DOI] [PubMed] [Google Scholar]
- Vanantwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappa B. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS. TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-kappa B. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva, Switzerland: Environmental Health Criteria 2, WHO; Polychlorinated Biphenyls and Terphenyls. 1976
- Xu Y, Bialik S, Jones BE, Iimuro Y, Kitsis RN, Srinivasan A, Brenner DA, Czaja MJ. NF-kappa B inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Amer. J. Physiol. Cell. Physiol. 1998a;44:C1058–C1066. doi: 10.1152/ajpcell.1998.275.4.C1058. [DOI] [PubMed] [Google Scholar]
- Xu YH, Dragan YP, Campbell HA, Pitot HC. STEREO: A program on a PC-Windows 95 platform for recording and evaluating quantitative stereologic investigations of multistage hepatocarcinogenesis. Computer Methods and Programs in Biomedicine. 1998b;56:49–63. doi: 10.1016/s0169-2607(98)00010-8. [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkB kinase complex (IKK) contains two kinase subunits, IKK-alpha and IKK-beta, necessary for IkB phorphorylation and NF-kB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]