Abstract
The locus coeruleus (LC) provides the sole source of norepinephrine (NE) to the cortex for modulation of cortical synaptic activity in response to salient sensory information. NE has been shown to improve signal-to-noise ratios, sharpen receptive fields and function in learning, memory, and cognitive performance. Although LC-mediated effects on neurons have been addressed, involvement of astrocytes has thus far not been demonstrated in these neuromodulatory functions. Here we show for the 1st time in live mice, that astrocytes exhibit rapid Ca2+ increases in response to electrical stimulation of the LC. Additionally, robust peripheral stimulation known to result in phasic LC activity leads to Ca2+ responses in astrocytes throughout sensory cortex that are independent of sensory-driven glutamate-dependent pathways. Furthermore, the astrocytic Ca2+ transients are competitively modulated by α2-specific agonist/antagonist combinations known to impact LC output, are sensitive to the LC-specific neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine, and are inhibited locally by an α-adrenergic antagonist. Future investigations of LC function must therefore consider the possibility that LC neuromodulatory effects are in part derived from activation of astrocytes.
Keywords: calcium transients, footshock, somatosensory, LC, metabotropic glutamate receptor, neuromodulator
Introduction
The locus coeruleus (LC) is a pontine nucleus containing the sole source of noradrenergic neurons innervating the cerebral cortex. LC neurons are unmyelinated, highly branched axonal arbors with extensive numbers of varicosities allowing single neurons to release transmitter on a broad scale throughout the brain (Levitt and Moore 1978). Interestingly, less than 10% of LC projection neurons to the cortex form conventional synapses (Cohen et al. 1997) with the remainder forming open synapses where transmitter is released for diffusion across considerable distances. These have been shown to be closely apposed to blood vessels, axons, dendrites, and glial processes (Paspalas and Papadopoulos 1996; Cohen et al. 1997; Aoki et al. 1998; Latsari et al. 2002).
The LC–norepinephrine (NE) network constitutes 1 of the major neuromodulatory networks in the central nervous system that displays both tonic and phasic firing modes shown important in focus, attention, and performance (Berridge and Waterhouse 2003; Aston-Jones and Cohen 2005). Changes in tonic firing of the LC maintain long-term changes in sensory network characteristics associated with different states of arousal (behavioral/emotional state). Phasic (short bursts) firing of LC activity, via α- and β-adrenergic receptors on neurons (Devilbiss and Waterhouse 2000), directly alter neural networks by modulating signal-to-noise ratios and receptive fields for salient sensory information (Castro-Alamancos 2002; Hirata et al. 2006). In addition, neuromodulators can influence neural network plasticity in learning and memory associated with neuromodulator-mediated emotional states (Kirkwood et al. 1999; Dringenberg et al. 2006; Origlia et al. 2006; Yamada et al. 2006). Although the modulation of signal-to-noise ratios and receptive fields is mediated through effects on neurons, the longer timescale associated with synaptic modification may include neuromodulator effects on astrocytes.
Cortical astrocytes, with their individual microdomains and extensive gap junctional connectivity, span vast areas of the brain and cortex. They ensheath synapses and play an integral part in normal synaptic function and plasticity (Haydon 2001; Nedergaard et al. 2003). Astrocytes are also able to propagate Ca2+ waves over considerable distances (Dani et al. 1992; Arcuino et al. 2002) and have the capacity to integrate neural activity from multiple sources (Kang et al. 1998; Fellin and Carmignoto 2004; Perea and Araque 2005) with subsequent release of gliotransmitters (Cotrina et al. 1998; Volterra and Meldolesi 2005). As astrocytes are well positioned and fully capable of extending LC-network neuromodulatory functions, we examined the hypothesis that LC output directly stimulates cortical astrocyte Ca2+ transients throughout LC somatosensory projection areas. Using 2-photon imaging of cortical astrocytes loaded with a fluorescent Ca2+ indicator (Hirase et al. 2004; Wang et al. 2006), we find that direct LC stimulation results in robust astrocytic Ca2+ responses that are sensitive to treatment with an LC-specific neurotoxin. Furthermore, robust peripheral stimulation evoked increases in astrocytic Ca2+ across broad areas of cortex independent of sensory pathways, and were competitively modulated by α2-specific agonist/antagonist combinations known to impact LC output (Hayashi et al. 1995; Guo et al. 1996) and blocked by local application of an α-adrenergic antagonist.
Materials and Methods
Animal Preparation and Dye Loading
Male FVB mice (8–10 weeks) were initially anesthetized with an ip injection of ketamine (6 mg) and xylazine (0.12 mg) for intubation and ventilation with a ventilator (SAAR-830, CWE, Ardmore, PA) in series with an isoflurane vaporizer. Animals were transferred to isoflurane slowly beginning at 0.5%, 15–20 min after initial ketamine/xylazine (kx) injection increasing to a level of 1.5% where maintained until time of experiment (∼3 h after kx injection). A cranial window was prepared as previously described (Wang et al. 2006). Briefly, following establishment of ventilation and stable anesthesia, a cranial window was opened (2–3 mm diameter; skull removed with agarose and coverslip enclosing) centered over the left hindlimb (−0.5 mm anteroposterior [AP] and 1.5 mm mediolateral [ML] to bregma), trunk (−1.7 mm AP and 1.75 mm ML), forelimb (0.4 mm AP and 2.5 mm ML) or barrel field somatosensory cortex (-0.7 mm AP and 3.5 mm ML). For LC stimulation, a bipolar concentric electrode (FHC CBARC, Bowdoinham, ME or David Kopf SNE-100, Tujunga, CA) was lowered through a small hole above the cerebellum into the LC at 30° from vertical (LC coordinates from bregma: −5.4 mm AP, 0.8 mm ML and −3.75 mm dorsoventral [DV]) and cemented in place with dental acrylic. Fluo-4/am (0.5 mM; Invitrogen, Carlsbad, CA) loading was performed by topical application to the pial surface for ∼50 min (Wang et al. 2006). Specific astrocyte loading has previously been shown using histochemical and double loading techniques (Nimmerjahn et al. 2004; Wang et al. 2006). This technique occasionally results in loading of endothelial cells with dye. However, endothelial/blood vessel responses are easily discriminated from the robust astrocyte responses. Alexa 594 (Invitrogen) was added to the drug or ACSF pipette solutions to aid visualization of drug delivery or pipette location. All experiments were approved by the Institution of Animal Care and Use Committee of the University of Rochester.
In Vivo Two-Photon Imaging and Stimulation
A custom-built microscope attached to a Tsunami/Millenium laser (10 W, Spectra Physics, Mountain View, CA) and scan box (FV300 Fluoview Software, Olympus, Center Valley, PA) was used for 2-photon imaging through a 20× objective (0.9 NA, Olympus). Excitation wavelength was in the range of 800–820 nm. Emission wavelengths were split to detect fluo-4 and Alexafluor 594 signals as previously described (Wang et al. 2006). Images of astrocytic Ca2+ signaling were recorded every 2–3 s, which was sufficient to capture evoked responses while limiting laser-induced photodamage at a laser power of ≤30 mW. Prior to foot stimulation experiments, anesthesia was lightened from 1.5% to 0.5% isoflurane and animals were injected with 0.5 mg/kg D-tubocurarine to prevent small reflex movements that could distort imaging. Foot stimulation was applied through a pair of 30-gauge needles attached to a photoelectric stimulus isolation unit (PSIU6; Grass Telefactor, West Warwick, RI) connected to a square pulse stimulator (S88K; Grass Telefactor) controlled by a Master-8 (A.M.P.I, Jerusalem, Israel) and involved the delivery of a 60 pulse train of 10 mA/20 ms square pulses at 20 Hz (marked limb withdrawal similar to toe-pinch in animals not treated with d-tubocurarine). Direct LC stimulation was applied through a bipolar concentric electrode. Stimulation consisted of a single train (20–100 pulses, 100 Hz) of 50-1000 μA/0.5 ms square pulses.
Drug Delivery
For local agonist/antagonist experiments, pressure pulses (20 psi; 5–20 ms) were applied using a picospritzer III (Parker Instrumentation, Chicago, IL) externally controlled by a Master-8 in 3-s intervals beginning at 15 s prior to foot stimulation. 6-Methyl-2-(phenylethynyl)-pyridine (MPEP, 10 mg/kg; Tocris Cookson, Ellisville, MO), xylazine (6 mg/kg), and yohimbine (4.5 mg/kg) were administered ip. Xylazine and yohimbine effects on blood pressure were monitored from a femoral artery in a subset of animals using a pressure transducer (WPI, Sarasota, FL) and found to result in small changes less than 10% of mean pressures. Xylazine shows a small increase (n = 3) and yohimbine a very small decrease (n = 2) in blood pressure, which is counter to observed effects on astrocyte Ca2+. Experiments were performed 10–15 min after xylazine or yohimbine injection. Twenty to 30 min were allowed for MPEP to take effect. N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) treatment involved 2 ip injections of 50 mg/kg 10–12 and 6–8 days prior to experiments. Drugs were dissolved in ACSF for pressure-injection or 0.9% NaCl for ip injection. All chemicals were from Sigma-Aldrich (St Louis, MO) unless otherwise stated.
Immunohistochemistry
Mice were deeply anesthetized with isoflurane (2%) and perfused transcardially with heparinized saline followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). Whole brains were removed and placed in 4% PFA for 3 h prior to cutting 100 μm coronal sections on a vibratome (Vibratome 1000 series, Warner Instruments, Hamden, CT) through hindlimb and trunk somatosensory cortex or through the pons. For analysis of electrode placements in the LC, brain slices were visualized using a light microscope. Tip placement was determined as distance from the center of LC for each animal and found not to be significantly different between control and DSP-4 treated groups. Vibratome slices were washed for 2 h in phosphate buffered saline (PBS) at 4 °C before blocking with 5% normal donkey serum in PBS containing 0.3% TX-100 at room temperature for 30 min. Slices were labeled with mouse antityrosine hydroxylase (anti-TH; Chemicon, MAB318, 1:400) which labels both dopaminergic and noradrenergic axons and chicken antimicrotubule associated protein 2 (Map2; Abcam, Cambridge, MA, ab5392-25, 1:10 000) in PBS containing 1% normal donkey serum and 0.1% TX-100 at 4 °C overnight. Primary labeling was followed by 3 × 10 min washes in PBS before applying donkey anti-mouse cy3 and donkey anti-chicken cy2 (all from JacksonImmunoResearch, West Grove, PA, 1:500) secondary antibodies in PBS containing 1% normal donkey serum and 0.3% TX-100 at room temperature for 2 h. Slices were then washed 3 × 10 min in PBS, counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Carlsbad, CA, D-21490, 1:10 000) in PBS at room temperature for 10 min, washed again 2 × 5 min in PBS and mounted on slides using SlowFade (Molecular Probes) mounting media.
Results
Astrocyte Ca2+ Transients are Induced by Direct LC Stimulation
To determine whether cortical astrocytes respond to NE, we 1st pressure ejected the α-adrenergic agonist methoxamine (200 μM) or the β-adrenergic agonist isoproterenol (200 μM) through a glass electrode in layer I somatosensory cortex (Wang et al. 2006). Both agonists elicited Ca2+ waves in astrocytes (Fig. 1) suggesting that astrocytes express both α- and β-adrenergic receptors and therefore are targets for LC-mediated NE release. In agreement with these observations, direct LC stimulation resulted in rapid, monophasic astrocytic Ca2+ transients (N = 9) (Fig. 2) that peaked with an average delay of 5.0 ± 0.31 s (51 cells in 7 animals). To further verify the LC dependence of astrocytic Ca2+ responses, the LC-specific neurotoxin, DSP-4 (Dudley et al. 1990), was injected in a subset of animals (see Methods) prior to experimentation. TH immunostaining showed a dramatic decrease throughout the cortex in DSP-4 treated animals consistent with DSP-4 treatment resulting in a 70–90% reduction in LC projections (Dudley et al. 1990). Astrocytic Ca2+ responses were significantly reduced in DSP-4 treated animals despite using a 2-fold higher stimulation than in control animals (N = 6) (Fig. 2D,E).
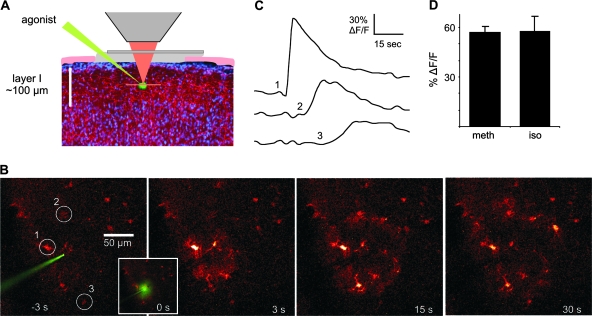
Figure 1.
Noradrenergic agonists elicit Ca2+ waves in cortical astrocytes. (A) The image at left illustrates the experimental setup with a cranial window over the somatosensory cortex and a glass electrode containing agonist positioned in the molecular layer (50–80 μm deep). (B) Series of images demonstrating a Ca2+ wave to pressure ejection of 200 μM methoxamine. (C) Changes in Ca2+ over time corresponding to numbered cells circled in (B) showing the time course of wave propagation. (D) Histogram showing that both α- (34 cells in 5 animals) and β-agonists (200 μM; 27 cells in 4 animals) are capable of eliciting Ca2+ responses in cortical astrocytes. meth, methoxamine; iso, isoproterenol.
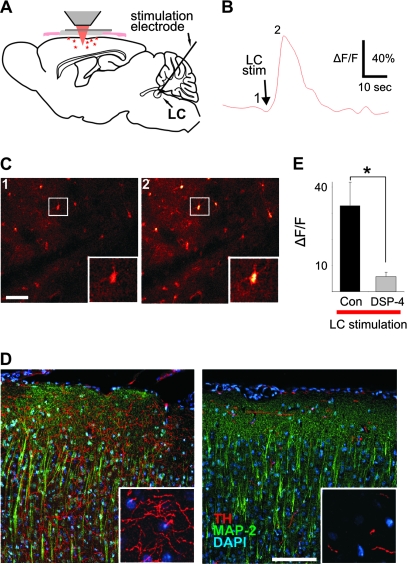
Figure 2.
Direct LC stimulation elicits Ca2+ transients in cortical astrocytes. (A) Schematic illustrating experimental setup. (B) Average fluorescence of the image field in (C) over time showing Ca2+ response to LC stimulation. (C) Fluo-4 labeled astrocytes taken at time points indicated by numbers in (B). Insets are blown-up view of boxed area in pictures. Scale bar 50 μm. (D) Images from coronal sections through somatosensory cortex illustrating the effect of DSP-4 on LC projection neurons as labeled by antibodies to TH and MAP-2 with DAPI labeling of nuclei for orientation. Scale bar 100 μm (20 μm inset). (E) Consistent with the significant reduction in LC projection neurons and despite almost twice the stimulation intensity (216 ± 33 vs. 400 ± 39 μA; P = 0.004, t-test), there is a significant reduction in the cortical Ca2+ response to LC stimulation in DSP-4 treated animals (P = 0.013, t-test).
Footshock-Induced Cortical Astrocyte Ca2+ Transients are Independent of Sensory Activity
In the awake state, salient sensory stimuli are known to result in LC discharge (Nieuwenhuis et al. 2005). However, only painful peripheral stimuli result in bilateral and robust activation of the LC (Tsuruoka et al. 2003) that is amenable to study in anesthetized animals. The experimental animal setup used to evaluate the role of LC discharges on astrocytic Ca2+ responses is illustrated in Figure 3. Footshock of the hindlimb results in activation of both sensory and c-fiber–mediated pathways that lead to increased LC activity (Hirata and Aston-Jones 1994). We found that footshock of either ipsi- or contralateral hindlimb triggers robust increases in astrocytic Ca2+ within the somatosensory cortex (16 ± 4.3% in 16 animals vs. 37 ± 4.3% in 26 animals, respectively) (Fig. 4A,B and Supplementary Video 1). In addition to detecting responses in hindlimb cortex (n = 9), we also observed robust Ca2+ transients in trunk (n = 8), forelimb (n = 6) and barrel sensory fields (n = 3). The Ca2+ responses (trunk sensory cortex) peaked within 6.4 ± 0.16 s of stimulation (126 cells in 8 animals). Grouping contralateral responses into hindlimb (including sensory components) and nonhindlimb areas for comparison demonstrated no dependence on stimulated sensory pathways (27.6 ± 3.35 in 9 animals vs. 41.2 ± 5.90 in 17 animals, respectively) (Fig. 4C). Thus, both ipsilateral and nonstimulated sensory area responses provide anatomical evidence that the astrocytic Ca2+ responses were not mediated by contralateral somatosensory glutamatergic projections.
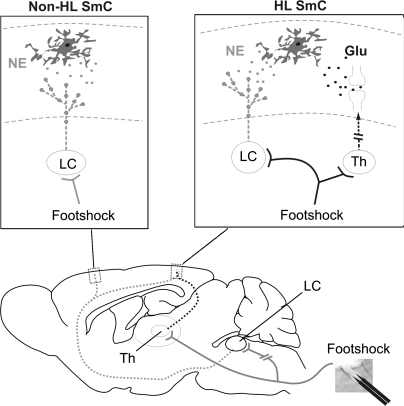
Figure 3.
Diagram outlining neural pathways activated in response to contralateral footshock or direct LC stimulation. Footshock triggers diffuse release of NE via LC activation in large areas of cortex (red), whereas sensory glutamatergic input is restricted to hindlimb sensory cortex. Insets: Astrocytes located in cortical layer I of hindlimb are activated by both NE and glutamate in response to footshock, whereas astrocytes in nonhindlimb areas only receive LC-mediated NE output. Glu, glutamate; SmC, somatosensory cortex; Th, thalamus.
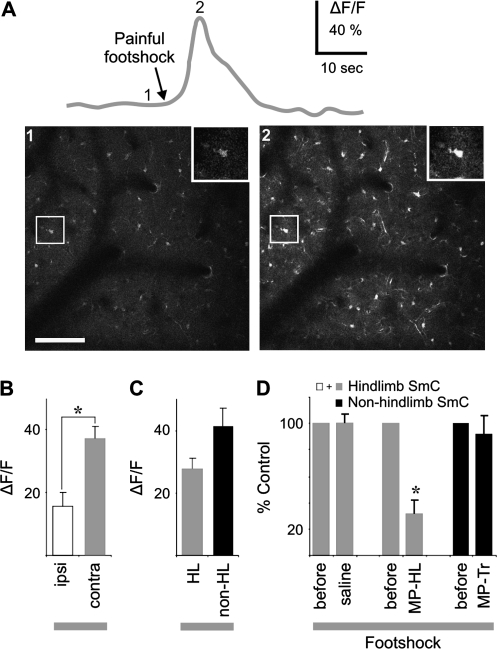
Figure 4.
Footshock stimulation elicits Ca2+ responses in cortical astrocytes independent of sensory activity. (A) Significant Ca2+ responses to contralateral foot stimulation with image of single cell response (inset) illustrated in top red trace. Images of time points indicated by numbers in top trace. (B) Comparison of bilateral Ca2+ responses (P = 0.002, t-test). (C) Contralateral hindlimb Ca2+ responses were subdivided into hindlimb and nonhindlimb sensory fields for further comparison (P = 0.140, t-test). Nonhindlimb areas include trunk, forelimb, and barrel sensory fields. (D) Effects of metabotropic glutamate antagonism in hindlimb versus nonhindlimb sensory fields (P = 0.043, paired t-test). MP-HL, MPEP with window over hindlimb; MP-Tr, MPEP over trunk. Scale bar is 100 μm.
To further substantiate the independence of the astrocyte Ca2+ response from glutamatergic sensory pathways, the metabotropic glutamate antagonist MPEP was administered intraperitoneally to suppress sensory evoked astrocytic Ca2+ transients (Zonta et al. 2003; Wang et al. 2006). Animals were subjected to 2 foot stimulations separated by 20–30 min with drug injection occurring immediately following the 1st response with the 2nd expressed as a percentage of the 1st. Responses to foot stimulation following administration of MPEP showed a significant 69 ± 10% reduction compared with saline when the cranial window was located over the hindlimb somatosensory cortex (N = 3; Fig. 4D). However, if the window was located over the trunk somatosensory cortex, MPEP had no significant effect on astrocytic Ca2+ increases (N = 4; 8 ± 14% reduction; Fig. 4D). Together, these data demonstrate that sensory glutamatergic pathways are not necessary for footshock-mediated astrocyte responses throughout sensory cortex.
Pharmacology Implicates LC–NE in Footshock-Induced Astrocyte Ca2+ Responses
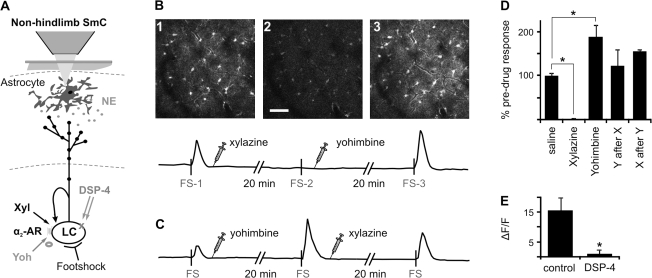
Noradrenergic α2-agonists are commonly used sedatives known to act in part by decreasing LC activity whereas α2-antagonists are used to rapidly reverse those actions (Hayashi et al. 1995; Guo et al. 1996). We used xylazine (α2-agonist) and yohimbine (α2-antagonist) to further analyze the involvement of the LC in astrocyte Ca2+ responses. Similar to the studies analyzing the effect of MPEP, the mice were subjected to 2 foot stimulations separated by 15–20 min (Fig. 5). Within 15 min of xylazine administration (5 mg/kg ip), the Ca2+ response to foot stimulation was completely blocked (Fig. 5B,D; 99 ± 2% reduction, N = 7) and required more than 2 h to recover (data not shown). However, yohimbine administration (4.5 mg/kg ip) reversed the block within 20 min (Fig. 5B,D; 123 ± 37% of control, N = 3). Furthermore, yohimbine administered alone potentiated astrocyte Ca2+ responses to footshock stimulation (228 ± 49%, N = 5), whereas subsequent administration of xylazine reduced the response (Fig. 5C,D; 155 ± 6%, N = 4). In addition to the transient systemic pharmacology discussed above, the LC-specific neurotoxin, DSP-4, significantly attenuates ipsilateral footshock-mediated astrocyte Ca2+ responses (Fig. 5E; 15.6 ± 4.32% in 16 animals vs. 1.04 ± 1.25% in 5 animals).
Figure 5.
Pharmacology implicates LC–NE in footshock-induced astrocyte Ca2+ responses. (A) Schematic illustrating LC pathway to cortex with proposed sites of intervention. (B), Example images of cortical astrocyte Ca2+ after contralateral foot stimulation corresponding to footshock (FS) displayed on timeline of fluo-4 fluorescence intensity of the whole field. The 2nd image is of a FS response 20 min after xylazine administration. Yohimbine reverses xylazine block after 20 min (>2 h typically). (C) Same as (B) except the drugs were given in reverse order to further demonstrate their competitive action at the α2-adrenergic receptor. (D) Histogram illustrating competitive action of the agonist/antagonist combinations on LC output. (E) The LC neurotoxin, DSP-4, dramatically reduces responses to ipsilateral footshock. N, numbers in parentheses. *P < 0.05 by t-test. Scale bar represents 50 μm. α2-AR, α2-adrenergic receptor; FS, footshock; Xyl and X, xylazine; Yoh and Y, yohimbine.
α-Adrenergic Receptors Mediate Footshock-Induced Astrocyte Ca2+ Responses
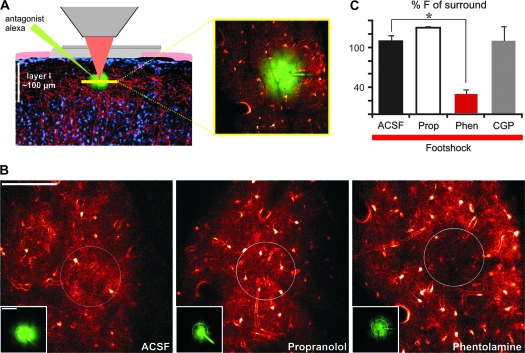
To demonstrate involvement of NE directly in responses of cortical astrocytes to peripheral footshock, NE antagonists were next applied locally in layer I of the sensory cortex by a pressure pulse through a glass electrode (Fig. 6). Local injection of the nonspecific α-adrenergic antagonist phentolamine (50 μM) significantly reduced the Ca2+ response to foot stimulation in the region surrounding the tip of the electrode to 30 ± 6.1% of the surrounding area (N = 9) (Fig. 6A,B; Supplementary Video 2). Subsequent stimulation demonstrated a partial recovery. Astrocytes located within the center of the field exhibited Ca2+ responses with an amplitude 65 ± 2.8% of their surroundings (N = 4, 15 min washout). Neither artificial cerebral spinal fluid (109 ± 8.4%, N = 7) (Supplementary Video 3) nor the β-adrenergic antagonist propranolol (50 μM; 129 ± 2.4%, N = 5) had significant effect on the response to foot stimulation (Fig. 6B,C), suggesting astrocyte Ca2+ increases are primarily mediated by an α-adrenergic pathway. Because NE has previously been shown to increase inhibitory activity in cortex (Motaghi et al. 2006), it is possible that astrocytes responded to gamma amino butyric acid (GABA) receptor stimulation as a result of α1-receptor mediated increases in GABA release. To address this possibility the GABAB antagonist CGP54626 was directly delivered to cortex as described above. CGP54626 had no significant effect on astrocyte Ca2+ responses (2–5 μM; 110 ± 22%, N = 4) (Fig. 6C) supporting the notion that NE is acting directly on astrocyte adrenergic receptors.
Figure 6.
The astrocytic Ca2+ response is mediated by α-adrenergic receptors. (A) Diagram illustrating imaging setup for local antagonist effects on footshock over nonhindlimb somatosensory cortex. Drug solution containing Alexafluor 594 is pressure ejected prior to footshock-mediated Ca2+ responses. (B) Cortical astrocyte Ca2+ responses to contralateral hindlimb stimulation during local drug application. Inset displays Alexafluor 594 from pipette at the same time point as the corresponding fluo-4 image for demonstrating drug delivery. (C) Effect of antagonist application as percentage of the surrounding area increase (P « 0.001, t-test). ACSF, artificial cerebral spinal fluid; CGP, CGP54626; F, fluorescence intensity; Phen, phentolamine; Prop, propranolol. Scale bar is 100 μm.
Discussion
This is the 1st study to demonstrate a direct link between the LC–NE modulatory network and astrocytes in vivo. The LC effect on astrocytic Ca2+ signaling across the cortex can only be established in in vivo experiments such as those presented here, as brain slices do not contain intact LC pathways. Astrocytes in ipsilateral and nonhindlimb areas responded to footshock with transient increases in Ca2+, effectively ruling out sensory pathway involvement. Astrocyte Ca2+ responses were competitively sensitive to α2-adrenergic agonist/antagonist combinations and local application of the nonspecific α-adrenergic antagonist phentolamine. Therefore, these studies demonstrate that astrocytes throughout the somatosensory cortex respond to robust foot stimulation via NE release from the LC. Although sensory pathways contributed to astrocytic Ca2+ responses in hindlimb somatosensory cortex, the LC provided the major drive throughout all cortical regions examined.
Both the anatomy and pharmacology of astrocytic Ca2+ responses to footshock stimulation point to the LC as the source of input. Electron microscopic studies have previously demonstrated both α- and β-adrenergic receptors in astrocytic membranes (Aoki 1992; Aoki et al. 1998) and the close apposition of astrocytic processes with LC-noradrenergic varicosities and terminals (Cohen et al. 1997; Aoki et al. 1998; Latsari et al. 2002) suggest that astrocytes are positioned as a target for LC–NE release. Astrocytes have also been shown to respond with Ca2+ transients in hippocampal brain slices to NE mediated primarily by α1-adrenergic receptors (Duffy and MacVicar 1995), consistent with our observations. Furthermore, astrocytes in culture express all adrenergic receptor subtypes that modulate many of their functions (Stone and Ariano 1989; Hertz et al. 2004). Although local delivery of propranolol did not reduce the footshock-mediated response in this study, the trend for an increased response suggests that α- and β-receptors may interact and alter astrocyte signaling properties. The fact that we were able to induce a β-adrenergic receptor dependent Ca2+ wave in cortical astrocytes demonstrates they have the ability to respond. The lack of a role in these studies may be due to masking effects of α-receptor–mediated Ca2+ transients. The best evidence for a lack of β-receptor involvement is the large local block mediated by phentolamine. Potentiation of the response with systemic delivery of an α2-adrenergic receptor antagonist suggests the response involves α1-adrenergic receptors. Again however, we cannot rule out a role for α2-receptors in response to NE as astrocytes are known to express them as well (Hertz et al. 2004). For purposes of this study we chose to use the nonspecific α- and β-adrenergic receptor antagonists to demonstrate NE-mediated effects on astrocytes directly.
Astrocyte responses to LC–NE release likely involve metabolic, homeostatic, and longer-lasting modulatory roles in synaptic function. As astrocytes are the primary source of brain glycogen and NE stimulates glycogenolysis, a possible function of LC activity is to mobilize energy substrates required to support stress evoked increases in synaptic transmission (Stone and Ariano 1989; Hertz et al. 2004). Furthermore, recent studies suggest that NE-mediated glycogenolysis may serve as a source of increased glutamate and glutamine in memory consolidation processes (Gibbs et al. 2006, 2007). Astrocyte transmitter and potassium homeostatic functions are also modulated by NE (Hertz et al. 2004). In addition, Ca2+-mediated release of D-serine (Panatier et al. 2006) and adenosine triphosphate (Cotrina et al. 1998; Gordon et al. 2005; Pascual et al. 2005; Serrano et al. 2006) from astrocytes have widespread effects and may serve to coordinate synaptic networks (Pascual et al. 2005) and affect memory formation (Panatier et al. 2006). The neuromodulators NE and acetylcholine have been shown to enhance the ability of a synapse to undergo long-term modification (Kirkwood et al. 1999; Dringenberg et al. 2006; Yamada et al. 2006). In this way, phasic LC activity that affects astrocytes across the somatosensory cortex may enable concurrently active sensory glutamatergic pathways to undergo NE/arousal-associated synaptic modification.
Astrocyte responses to LC stimulation or footshock are very broad responses that span the image field of view with no apparent wave propagation. Unlike responses to sensory stimulation (Wang et al. 2006), LC-mediated responses occur simultaneously. Occasionally the responses can be seen to initiate in cell processes that propagate rapidly to the soma. However, wave propagation from cell to cell is never observed consistent with the diffuse blanket-like nature of LC projections. As astrocytes are the primary source of D-serine in brain and it is known that D-serine regulates availability of N-methyl-D-aspartate (NMDA) receptors for activation (Panatier et al. 2006), astrocytes are unquestionably involved in regulating NMDA-dependent plasticity events in cortical synapses. Thus, LC effects on astrocytes may prime neural network modification for subsequent and long-term activity. Furthermore, given the importance of LC function in focus and memory and the fact that astrocytes serve to extend LC neuromodulatory functions to every synapse, it is becoming increasingly clear that astrocytes be considered a fundamental partner in LC–NE network neuromodulation.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Canadian Institutes of Health Research postdoctoral fellowship to L.K.B; National Institute of Health (NS30007) to M.N.; and National Institute of Neurological Disorders and Stroke (NS38073) to M.N.
Supplementary Material
Acknowledgments
We thank E. Vates, K.A. Kasischke, and N. Smith for comments on the manuscript and W. Libionka for discussion. Conflict of Interest: None declared.
References
- Aoki C. Beta-adrenergic receptors: astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J Neurosci. 1992;12:781–792. doi: 10.1523/JNEUROSCI.12-03-00781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Role of thalamocortical sensory suppression during arousal: focusing sensory inputs in neocortex. J Neurosci. 2002;22:9651–9655. doi: 10.1523/JNEUROSCI.22-22-09651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab. 1997;17:894–904. doi: 10.1097/00004647-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse. 2000;37:273–282. doi: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Hamze B, Wilson A, Speechley W, Kuo MC. Heterosynaptic facilitation of in vivo thalamocortical long-term potentiation in the adult rat visual cortex by acetylcholine. Cereb Cortex. 2006;17:839–848. doi: 10.1093/cercor/bhk038. [DOI] [PubMed] [Google Scholar]
- Dudley MW, Howard BD, Cho AK. The interaction of the beta-haloethyl benzylamines, xylamine, and DSP-4 with catecholaminergic neurons. Annu Rev Pharmacol Toxicol. 1990;30:387–403. doi: 10.1146/annurev.pa.30.040190.002131. [DOI] [PubMed] [Google Scholar]
- Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54:214–222. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Lloyd HG, Santa T, Hertz L. Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J Neurosci Res. 2007;85:3326–3333. doi: 10.1002/jnr.21307. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Rabin BC, Guo TZ, Maze M. Role of pertussis toxin-sensitive G-proteins in the analgesic and anesthetic actions of alpha 2-adrenergic agonists in the rat. Anesthesiology. 1995;83:816–822. doi: 10.1097/00000542-199510000-00022. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hertz L, Chen Y, Gibbs ME, Zang P, Peng L. Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders? Curr Drug Targets CNS Neurol Disord. 2004;3:239–267. doi: 10.2174/1568007043337535. [DOI] [PubMed] [Google Scholar]
- Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci. 2006;26:4426–4436. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Aston-Jones G. A novel long-latency response of locus coeruleus neurons to noxious stimuli: mediation by peripheral C-fibers. J Neurophysiol. 1994;71:1752–1761. doi: 10.1152/jn.1994.71.5.1752. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latsari M, Dori I, Antonopoulos J, Chiotelli M, Dinopoulos A. Noradrenergic innervation of the developing and mature visual and motor cortex of the rat brain: a light and electron microscopic immunocytochemical analysis. J Comp Neurol. 2002;445:145–158. doi: 10.1002/cne.10156. [DOI] [PubMed] [Google Scholar]
- Levitt P, Moore RY. Noradrenaline neuron innervation of the neocortex in the rat. Brain Res. 1978;139:219–231. doi: 10.1016/0006-8993(78)90925-3. [DOI] [PubMed] [Google Scholar]
- Motaghi S, Sheibani V, Farazifard R, Joneidi H. Electrical stimulation of locus coeruleus strengthens the surround inhibition in layer V barrel cortex in rat. Neurosci Lett. 2006;401:280–284. doi: 10.1016/j.neulet.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1:31–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- Origlia N, Kuczewski N, Aztiria E, Gautam D, Wess J, Domenici L. Muscarinic acetylcholine receptor knockout mice show distinct synaptic plasticity impairments in the visual cortex. J Physiol. 2006;577:829–840. doi: 10.1113/jphysiol.2006.117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Papadopoulos GC. Ultrastructural relationships between noradrenergic nerve fibers and non-neuronal elements in the rat cerebral cortex. Glia. 1996;17:133–146. doi: 10.1002/(SICI)1098-1136(199606)17:2<133::AID-GLIA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Ariano MA. Are glial cells targets of the central noradrenergic system? A review of the evidence. Brain Res Brain Res Rev. 1989;14:297–309. doi: 10.1016/0165-0173(89)90015-5. [DOI] [PubMed] [Google Scholar]
- Tsuruoka M, Arai YC, Nomura H, Matsutani K, Willis WD. Unilateral hindpaw inflammation induces bilateral activation of the locus coeruleus and the nucleus subcoeruleus in the rat. Brain Res Bull. 2003;61:117–123. doi: 10.1016/s0361-9230(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Yamada K, Inagaki T, Funahashi R, Yoshimura Y, Komatsu Y. High-frequency stimulation together with adrenoceptor activation facilitates the maintenance of long-term potentiation at visual cortical inhibitory synapses. Cereb Cortex. 2006;16:1239–1248. doi: 10.1093/cercor/bhj065. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.