Introduction
Cocaine dependence is a devastating disorder with no pharmacological treatments available. Genetic studies estimate that 65–78% of the vulnerability risk for cocaine dependence is heritable (Kendler et al 2000; Kendler and Prescott 1998); however, identification of genetic risk factors remains difficult due to the complex mode of inheritance, clinical and genetic heterogeneity and likely multiple genes involved, each only contributing a small effect to the overall risk. Dopaminergic brain systems have been implicated to play a major role in drug reward (Dackis and O'Brien 2005; Dackis and O'Brien 2001; Hyman et al 2006), thus making genes involved in these circuits plausible candidates for susceptibility to substance use disorders (Lachman 2006). The enzyme catechol-O-methyltransferase (COMT) is involved in the degradation of catecholamines, including dopamine (Axelrod and Tomchick 1958). The functional COMT polymorphism Val158Met affects enzyme activity, with the Val-allele resulting in higher enzyme activity relative to the Met-allele (Aksoy et al 1993; Boudikova et al 1990; Lachman et al 1996; Lotta et al 1995; Spielman and Weinshilboum 1981). Several studies suggest that the Val158Met polymorphism is involved in psychiatric phenotypes, including schizophrenia and bipolar disorder (Craddock et al 2006; Tunbridge et al 2006), and might further contribute to the co-morbid substance abuse/dependence spectrum across psychiatric disorders. Although cocaine blocks the dopamine transporter (DAT) primarily, leading to enhanced postsynaptic effects of dopamine signaling, COMT remains an important regulatory element in dopamine homeostasis. Emerging evidence suggests that COMT variation influences prefrontal cortex (PFC) dopamine regulation and might modulate aspects of cognition, emotions and behavior (Egan et al 2001; Tunbridge et al 2006). PFC dysfunction might be an important component in cocaine dependence that contributes to loss of control and denial (Dackis and O'Brien 2005). Individual differences in COMT activity might therefore influence vulnerability to cocaine dependence and other substance use disorders. In this study we tested the hypothesis that the functional Val158Met variation of the COMT gene increases susceptibility to cocaine dependence in individuals of African descent.
Materials and Methods
DNA samples from cocaine dependent individuals of African descent were collected during clinical studies of cocaine dependence at the University of Pennsylvania Treatment Research Center. Subjects were at least 18 years of age and had a clinical diagnosis of cocaine dependence as defined by DSM-IV. Control samples from persons of African descent were collected at the University of Pennsylvania, Thomas Jefferson University (Dahl et al 2006) and through the National Institute of Mental Health Genetics Initiative (www.nimhgenetics.org). Control individuals were screened for history of substance abuse disorders or other psychiatric illness. Subjects with a history of substance dependence or a history of major psychiatric illness (schizophrenia and unipolar or bipolar illnesses) as defined by DSM-IV criteria were excluded from this study (Berrettini and Persico 1996). All protocols were approved by the Institutional Review Boards at Thomas Jefferson University and the University of Pennsylvania and all subjects provided written informed consent before DNA sample collection.
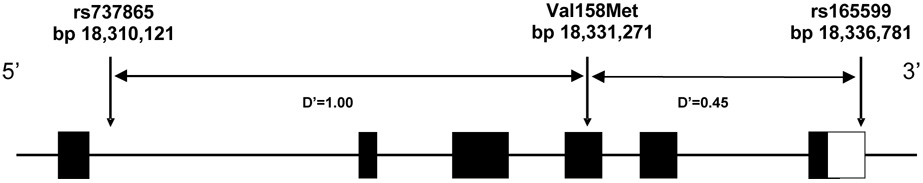
Genotyping of three SNPs across the COMT gene was performed using the Applied Biosystems Inc. (ABI) “Assays-on-demand” (ABI, Foster City, CA, USA) SNP genotyping assay as per manufacturers protocol [SNP1: rs737865; SNP2: rs165688 (Val158Met - rs4680); SNP3: rs165599]. SNPs were selected based on linkage disequilibrium (LD) structure of the gene, SNP allele frequency, available HapMap data (Figure 1) and previous studies (Berrettini et al 2007; Shifman et al 2002). Genotyping quality control was assured by genotyping 10% duplicates for cases and controls. Concordance rate of genotypes was > 99.5%.
Figure 1.
Diagram of the COMT gene and the three SNPs examined, with linkage disequilibrium D′ values. Values in bp refer to chromosome 22, taken from the March 2006 assembly of the human genome sequence at www.genome.ucsc.edu. Exons are indicated by shaded boxes. The unshaded box represents the 3′UTR in exon 6. D′ values represent African-American sample results.
Statistical Analyses
Genotypes and allele frequencies were compared between groups using Chi square contingency analysis. A two-tailed type I error rate of 5% was chosen for the analysis. Linkage disequilibrium (LD) and haplotype frequencies were estimated using the COCAPHASE program (Dudbridge 2003). The COCAPHASE program uses standard unconditional logistic regression analysis. Correction for multiple testing was performed using permutation correction by the COCAPHASE program. This approach corrects for multiple testing but takes into account the correlation between markers. It is thus less conservative than a Bonferroni correction, which is appropriate for independent tests such as unlinked markers. For the single-marker analyses, 10,000 permutations were carried out to estimate the significance of the best results, correcting for the three loci tested. Haplotype analysis was performed using a 2 and 3 marker window. Rare haplotypes were excluded from analysis since the EM algorithm does not accurately estimate haplotype frequencies <1% (Fallin and Schork 2000). The most significant p value was corrected by permutation analysis as described above.
Our sample size had reasonable power to detect a disease association at a P value less than or equal to 0.05, assuming an odds ratio of 1.5 and a minor allele frequency (MAF) of 30% (99% for a log additive mode of inheritance, 92% for a dominant and 56% for a recessive mode of inheritance). Power analysis was performed using the Quanto program (Gauderman 2002).
Results
None of the genotype counts deviated significantly from those expected from Hardy-Weinberg equilibrium for cases or controls. Genotype and allele frequencies differed significantly for the Val158Met polymorphism between cocaine dependent individuals (f(Met)=35%) and normal controls (f(Met)=27%) (p=0.004; corrected p=0.014). SNP1 and SNP3 did not show a statistical difference between cases and controls (Table 1). Haplotype analysis showed significant associations for two marker analysis and a trend for the three marker combination (Table 2); however, after correction for multiple testing only the rs737865 - rs165688 haplotype remained statistically significant. The patient group carried the major allele of rs737865 and the Met158 allele more often (33%) then compared to controls (26%) (p=0.005; corrected p=0.02; OR: 1.44). Haplotype analysis results appear to be driven by the Val158Met SNP, supporting the hypothesis that the Val158Met polymorphism is a causative functional SNP.
Table 1.
Variation in the COMT gene
| SNP | Sample | n | Genotype frequency | P*a | Allele frequency | P*b | ||
|---|---|---|---|---|---|---|---|---|
| rs737865 | A/A | A/G | G/G | f(A) | ||||
| Cocaine | 330 | 0.715 | 0.255 | 0.030 | 0.312 | 0.842 | 0.766 | |
| Controls | 253 | 0.688 | 0.296 | 0.016 | 0.836 | |||
| Val158Metc | Val/Val | Val/Met | Met/Met | f(Met) | ||||
| Cocaine | 324 | 0.417 | 0.469 | 0.114 | 0.011 | 0.349 | 0.004d | |
| Controls | 255 | 0.541 | 0.376 | 0.082 | 0.271 | |||
| rs165599 | A/A | A/G | G/G | f(x) | ||||
| Cocaine | 324 | 0.454 | 0.441 | 0.105 | 0.258 | 0.674 | 0.196 | |
| Controls | 255 | 0.486 | 0.447 | 0.067 | 0.710 | |||
type-I error rates for comparison of genotype and allele frequencies between BPD patients and controls.
Val158Met=rs4680=rs165688 (Val=G)
Global significance after permutation correction for multiple testing: p=0.014 Standard Error (SE): 0.001175
Table 2.
Analysis of haplotypes in the COMT gene
| Haplotype | Case | freq | Control | freq | OR | chisq | p |
|---|---|---|---|---|---|---|---|
| rs737865 - rs165688* | |||||||
| A-Val | 325 | 0.520 | 289 | 0.580 | 1 | 3.962 | 0.046 |
| A-Met | 211 | 0.338 | 130 | 0.261 | 1.443 | 7.84 | 0.005 |
| G-Val | 88 | 0.141 | 79 | 0.158 | 0.990 | 0.675 | 0.411 |
| rs165688* - rs165599 | |||||||
| Val-A | 341.5 | 0.542 | 308.2 | 0.604 | 1 | 4.547 | 0.032 |
| Val-G | 73.51 | 0.116 | 63.77 | 0.125 | 1.04 | 0.277 | 0.598 |
| Met-A | 86.51 | 0.137 | 53.77 | 0.105 | 1.452 | 2.829 | 0.092 |
| Met-G | 128.5 | 0.204 | 84.23 | 0.165 | 1.377 | 3.282 | 0.070 |
| rs737865 - rs165599 | |||||||
| A-A | 358.8 | 0.555 | 295.2 | 0.583 | 1 | 0.879 | 0.348 |
| A-G | 184.2 | 0.285 | 127.8 | 0.252 | 1.186 | 1.535 | 0.215 |
| G-A | 76.22 | 0.118 | 62.81 | 0.124 | 0.998 | 0.175 | 0.675 |
| G-G | 26.78 | 0.041 | 20.19 | 0.039 | 1.091 | 0.107 | 0.742 |
| rs737865 - rs165688* - rs165599 | |||||||
| A-Val-A | 259.6 | 0.437 | 230.7 | 0.470 | 1 | 1.274 | 0.259 |
| A-Val-G | 59.42 | 0.10 | 57.27 | 0.116 | 0.922 | 0.929 | 0.335 |
| A-Met-A | 78.42 | 0.132 | 47.27 | 0.096 | 1.475 | 3.673 | 0.055 |
| A-Met-G | 120.6 | 0.203 | 81.73 | 0.166 | 1.311 | 2.566 | 0.109 |
| G-Val-A | 76 | 0.127 | 73 | 0.149 | 0.925 | 0.998 | 0.317 |
Best p-value 0.00511
Global significance after permutation correction: p=0.0263 SE: 0.0016
rs165688 = Val158Met (Val=G) = rs4680
Discussion
In the present study, we show an association between the Val158Met polymorphism of the COMT gene and cocaine dependence in individuals of African descent. Furthermore, we identify a risk haplotype contributing to the susceptibility for cocaine dependence. To our knowledge, this is the first report of an association of the COMT Val158Met polymorphism in cocaine dependence in individuals of African descent; however, given the high comorbidity of polysubstance abuse in individuals using cocaine, our result might reflect an association of a broader phenotype of substance use disorders. In fact, several previous studies have implicated the Val158Met polymorphism in a variety of substance abuse disorders (Table 3) (Beuten et al 2005; Enoch et al 2006; Horowitz et al 2000; Hosak et al 2006; Li et al 2004; Samochowiec et al 2006; Sery et al 2006; Tiihonen et al 1999; Vandenbergh et al 2000; Vandenbergh et al 1997; Wang et al 2001); however, others could not replicate results (Cevoli et al 2006; Guo et al 2007; Hallikainen et al 2000; Kauhanen et al 2000; Kweon et al 2005) and here is no clear consensus on whether the Val-allele or the Met-allele increases risk (Table 3). This locus heterogeneity might indicate that the COMT Val158Met polymorphism is only one important variation in a cascade of regulatory systems, and depending upon other genes or environmental factors, a higher or lower enzyme activity might predispose to substance use disorders. The COMT Val158Met polymorphism, rather then a single allele, might thus have an effect on pathways commonly shared between all substance use disorders. Such pathways include the reward system and cognitive functions influencing substance use behavior. Interestingly, recent neuroimaging data suggest that the frontal cortex is involved in the reward process (Goldstein and Volkow 2002; Volkow et al 2002). Dysregulation of COMT, which is the major enzyme involved in the degradation of dopamine in the frontal cortex (Karoum et al 1994), might thus have direct effects on cognitive processes involved in substance dependence and indirect downstream effects on the reward system.
Table 3.
COMT Val158Met in substance use disorders
| Author | Year | Ethnicity | Substance use disorder | Sample size | Val 158 frequency | Met 158 frequency | p value allele freq |
|---|---|---|---|---|---|---|---|
| Vandenbergh et al | 1997 | Caucasian | Polysubstance abuse (lifetime use) |
Cases = 185 Controls = 124 |
|||
| Vandenbergh et al | 2000 | ||||||
| Kauhanen et al | 2000 | ||||||
| Li et al | 2004 | Han Chinese |
Methamphetamine | Cases = 416 Controls = 435 |
0.74 0.68 |
0.26 0.32 |
0.02 |
| Beuten et al | 2005 | ||||||
| Enoch et al | 2006 | ||||||
| Horowitz et al | 2000 | ||||||
| Hosak et al | 2006 | ||||||
| Samochowiec et al | 2006 | ||||||
| Sery et al | 2006 | ||||||
| Tiihonen et al | 1999 | ||||||
| Wang et al | 2001 | ||||||
| Cevoli et al | 2006 | ||||||
| Guo et al | 2007 | ||||||
| Kweon et al | 2005 | ||||||
| Hallikainen et al | 2000 | ||||||
Our results suggest an increased Met allele (low activity allele) frequency in cocaine users (35%) compared to normal controls (27%) (p=0.004; corrected p=0.014) in individuals of African descent. Our finding is in line with the results of Hosak et al (2006) who showed higher novelty seeking scores in methamphetamine individuals with a Met- allele (Hosak et al 2006) and the observation that Met/Met carrier have decreased efficiency of PFC information processing in response amphetamine (Mattay et al 2003). Individuals with the low-activity COMT allele may have longer lasting and more effective dopamine release in the brain, thus increasing the duration and intensity of reward derived from cocaine use which ultimately might result in cocaine dependence.
Although our study provides evidence for an association between the Val158Met polymorphism and cocaine dependence, it could be possible that other variations which are in LD with the Val158Met SNP might contribute to the observed association or that a haplotype confers risk rather then a single SNP. Consistent with this is that several studies found haplotypes to be associated with disease rather then a single SNP (Berrettini et al 2007; Shifman et al 2002). Recent reports show that COMT haplotypes code for differences in enzyme activity (Diatchenko et al 2005) and haplotype-specific mRNA secondary structure has functional effects on COMT protein synthesis and enzyme activity (Nackley et al 2006). Our haplotype analysis indicates a risk haplotype for rs737865 and Val158Met (p=0.005; corrected p=0.02; OR: 1.44). Interestingly both markers (rs737865 and Val158Met) are in strong LD and incorporate the SNPs in the functional haplotypes described by Nackley et al. (2006). Additional studies are necessary to elucidate potential functional haplotypic variations.
Even though we report a positive association between the COMT gene and cocaine dependence, it is possible that our finding might be a false positive result due to population stratification. Case-control association studies of subjects with self-reported ancestries are not immune to population stratification (Freedman et al 2004), even though all cases and controls in this study were of African descent. In fact, the Met allele is less frequent in individuals of African descent (Ameyaw et al 2000; DeMille et al 2002; McLeod et al 1994), and haplotypes in COMT show marked differences across populations (Palmatier et al 1999). One strategy to minimize the issue of population stratification would be the use of a family-based association design that matches the genotype of an affected offspring with those parental alleles not inherited by the offspring (Spielman and Ewens 1996). Ultimately, our results require confirmation in an independent population of patients and controls.
In summary, we show that the Val158Met polymorphism in the COMT gene is associated with cocaine dependence. In addition we have identified a risk haplotype for cocaine dependence. Our results require confirmation in other populations and additional studies are required to elucidate the role of COMT in the pathophysiology of substance use disorders.
Acknowledgement
This work was supported by the Center for Neurobiology and Behavior, Department of Psychiatry, University of Pennsylvania. Financial support is gratefully acknowledged from National Institutes of Health grants MH59553, MH63876 (to W.H.B.), NIDA grant P60-051186 (to C.P.O.) and R25 MH060490 and T32 MH014654-29A1 (F.W.L.), the VISN4 Mental Illness Research and Clinical Center grant from the Veterans Affairs Administration, grants from the National Alliance for Research on Schizophrenia and Depression (to W.H.B.), the 2006 NARSAD Marshall-Reynolds Foundation Investigator Award (to F.W.L.), a grant from the Tzedakah Foundation (to W.H.B.), the Daland Fellowship Award by the American Philosophical Society (to F.W.L.) and the APIRE/AstraZeneca Young Minds in Psychiatry Award (to F.W.L.). We thank Candice Schwebel for technical assistance. Most importantly, we thank the subjects who have participated in and contributed to these studies.
References
- Aksoy S, Klener J, Weinshilboum RM. Catechol O-methyltransferase pharmacogenetics: photoaffinity labelling and western blot analysis of human liver samples. Pharmacogenetics. 1993;3:116–122. doi: 10.1097/00008571-199304000-00008. [DOI] [PubMed] [Google Scholar]
- Ameyaw MM, Syvanen AC, Ulmanen I, Ofori-Adjei D, McLeod HL. Pharmacogenetics of catechol-O-methyltransferase: frequency of low activity allele in a Ghanaian population. Hum Mutat. 2000;16:445–446. doi: 10.1002/1098-1004(200011)16:5<445::AID-HUMU13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- Berrettini WH, Persico AM. Dopamine D2 receptor gene polymorphisms and vulnerability to substance abuse in African Americans. Biol Psychiatry. 1996;40:144–147. doi: 10.1016/0006-3223(96)00036-4. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Wileyto EP, Epstein L, et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biol Psychiatry. 2007;61:111–118. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Beuten J, Payne TJ, Ma JZ, Li MD. Significant Association of Catechol-O-Methyltransferase (COMT) Haplotypes with Nicotine Dependence in Male and Female Smokers of Two Ethnic Populations. 2005;31:675–684. doi: 10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- Cevoli S, Mochi M, Scapoli C, et al. A genetic association study of dopamine metabolism-related genes and chronic headache with drug abuse. Eur J Neurol. 2006;13:1009–1013. doi: 10.1111/j.1468-1331.2006.01415.x. [DOI] [PubMed] [Google Scholar]
- Craddock N, Owen MJ, O'Donovan MC. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol Psychiatry. 2006;11:446–458. doi: 10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- Dackis C, O'Brien C. Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci. 2005;8:1431–1436. doi: 10.1038/nn1105-1431. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Dahl JP, Cubells JF, Ray R, et al. Analysis of variations in the tryptophan hydroxylase-2 (TPH2) gene in cocaine dependence. Addict Biol. 2006;11:76–83. doi: 10.1111/j.1369-1600.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- DeMille MM, Kidd JR, Ruggeri V, et al. Population variation in linkage disequilibrium across the COMT gene considering promoter region and coding region variation. Hum Genet. 2002;111:521–537. doi: 10.1007/s00439-002-0809-0. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–121. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M-A, Waheed JF, Harris CR, Albaugh B, Goldman D. Sex Differences in the Influence of COMT Val158Met on Alcoholism and Smoking in Plains American Indians. Alcoholism: Clinical and Experimental Research. 2006;30:399–406. doi: 10.1111/j.1530-0277.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- Fallin D, Schork NJ. Accuracy of haplotype frequency estimation for biallelic loci, via the expectation-maximization algorithm for unphased diploid genotype data. Am J Hum Genet. 2000;67:947–959. doi: 10.1086/303069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ML, Reich D, Penney KL, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ. Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol. 2002;155:478–484. doi: 10.1093/aje/155.5.478. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Chen DF, Zhou DF, et al. Association of functional catechol O-methyl transferase (COMT) Val108Met polymorphism with smoking severity and age of smoking initiation in Chinese male smokers. Psychopharmacology. 2007;190:449–456. doi: 10.1007/s00213-006-0628-4. [DOI] [PubMed] [Google Scholar]
- Hallikainen T, Lachman H, Saito T, et al. Lack of association between the functional variant of the catechol-o-methyltransferase (COMT) gene and early-onset alcoholism associated with severe antisocial behavior. Am J Med Genet. 2000;96:348–352. doi: 10.1002/1096-8628(20000612)96:3<348::aid-ajmg22>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Horowitz R, Kotler M, Shufman E, et al. Confirmation of an excess of the high enzyme activity COMT val allele in heroin addicts in a family-based haplotype relative risk study. Am J Med Genet. 2000;96:599–603. [PubMed] [Google Scholar]
- Hosak L, Libiger J, Cizek J, Beranek M, Cermakova E. The COMT Val158Met polymorphism is associated with novelty seeking in Czech methamphetamine abusers: preliminary results. Neuro Endocrinol Lett. 2006;27:799–802. [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. NEURAL MECHANISMS OF ADDICTION: The Role of Reward-Related Learning and Memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine Is the Major Metabolite of Released Dopamine in the Rat Frontal Cortex: Reassessment of the Effects of Antipsychotics on the Dynamics of Dopamine Release and Metabolism in the Frontal Cortex, Nucleus Accumbens, and Striatum by a Simple Two Pool Model. Journal of Neurochemistry. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kauhanen J, Hallikainen T, Tuomainen TP, et al. Association between the functional polymorphism of catechol-O-methyltransferase gene and alcohol consumption among social drinkers. Alcohol Clin Exp Res. 2000;24:135–139. [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cocaine use, abuse and dependence in a population-based sample of female twins. Br J Psychiatry. 1998;173:345–350. doi: 10.1192/bjp.173.4.345. [DOI] [PubMed] [Google Scholar]
- Kweon YS, Lee HK, Lee CT, Pae CU. Association study of catechol-O-methyltransferase gene polymorphism in Korean male alcoholics. Psychiatr Genet. 2005;15:151–154. doi: 10.1097/00041444-200506000-00014. [DOI] [PubMed] [Google Scholar]
- Lachman HM. An overview of the genetics of substance use disorders. Curr Psychiatry Rep. 2006;8:133–143. doi: 10.1007/s11920-006-0013-3. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Li T, Chen CK, Hu X, et al. Association analysis of the DRD4 and COMT genes in methamphetamine abuse. Am J Med Genet B Neuropsychiatr Genet. 2004;129:120–124. doi: 10.1002/ajmg.b.30024. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod HL, Fang L, Luo X, Scott EP, Evans WE. Ethnic differences in erythrocyte catechol-O-methyltransferase activity in black and white Americans. J Pharmacol Exp Ther. 1994;270:26–29. [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, et al. Human Catechol-O-Methyltransferase Haplotypes Modulate Protein Expression by Altering mRNA Secondary Structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Kucharska-Mazur J, Grzywacz A, et al. Family-based and case-control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neuroscience Letters. 2006;410:1–5. doi: 10.1016/j.neulet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Sery O, Didden W, Mikes V, Pitelova R, Znojil V, Zvolsky P. The association between high-activity COMT allele and alcoholism. Neuro Endocrinol Lett. 2006;27:231–235. [PubMed] [Google Scholar]
- Shifman S, Bronstein M, Sternfeld M, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman RS, Ewens WJ. The TDT and other family-based tests for linkage disequilibrium and association. Am J Hum Genet. 1996;59:983–989. [PMC free article] [PubMed] [Google Scholar]
- Spielman RS, Weinshilboum RM. Genetics of red cell COMT activity: analysis of thermal stability and family data. Am J Med Genet. 1981;10:279–290. doi: 10.1002/ajmg.1320100311. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Hallikainen T, Lachman H, et al. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Mol Psychiatry. 1999;4:286–289. doi: 10.1038/sj.mp.4000509. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Rodriguez LA, Hivert E, et al. Long forms of the dopamine receptor (DRD4) gene VNTR are more prevalent in substance abusers: no interaction with functional alleles of the catechol-o-methyltransferase (COMT) gene. Am J Med Genet. 2000;96:678–683. doi: 10.1002/1096-8628(20001009)96:5<678::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Rodriguez LA, Miller IT, Uhl GR, Lachman HM. High-activity catechol-O-methyltransferase allele is more prevalent in polysubstance abusers. Am J Med Genet. 1997;74:439–442. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Goldstein RZ. Role of Dopamine, the Frontal Cortex and Memory Circuits in Drug Addiction: Insight from Imaging Studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Wang T, Franke P, Neidt H, et al. Association study of the low-activity allele of catechol-O-methyltransferase and alcoholism using a family-based approach. Mol Psychiatry. 2001;6:109–111. doi: 10.1038/sj.mp.4000803. [DOI] [PubMed] [Google Scholar]