Abstract
The primary activation of T-helper and T-cytotoxic cells following mucosal immunization with recombinant Streptococcus gordonii was studied in vivo by adoptive transfer of ovalbumin (OVA)-specific transgenic CD8+ (OT-I) and CD4+ (OT-II) T cells. A recombinant strain, expressing on the surface the vaccine antigen Ag85B-ESAT-6 from Mycobacterium tuberculosis fused to OVA T-helper and T-cytotoxic epitopes (peptides 323 to 339 and 257 to 264), was constructed and used to immunize C57BL/6 mice by the intranasal route. Recombinant, but not wild-type, bacteria induced OVA-specific CD4+ and CD8+ T-cell clonal expansion in cervical lymph nodes, lung, and spleen. OVA-specific CD4+ and CD8+ T-cell proliferation appeared first in cervical lymph nodes and later in the spleen, suggesting a possible migration of activated cells from the inductive site to the systemic district. A significant correlation between the percentages of CD4+ and CD8+ proliferating T cells was observed for each animal. The expression of CD69, CD44, and CD45RB on proliferating T lymphocytes changed as a function of the cell division number, confirming T-cell activation following the antigen encounter. These data indicate that intranasal immunization with recombinant S. gordonii is capable of inducing primary activation of naive antigen-specific CD4+ and CD8+ T cells, both locally and systemically.
Many pathogens invade the host at mucosal surfaces, which represent the first antimicrobial barrier through nonspecific and specific defense mechanisms. Mucosal vaccination is capable of targeting the inductive sites of the mucosa-associated lymphoid tissues, inducing local immune responses at the portal of entry of mucosal pathogens. Mucosal inductive sites, such as the nasopharynx-associated lymphoid tissue (NALT) and the gut-associated lymphoid tissue (GALT), are specialized in priming naive T and B cells that then move to other mucosal and glandular tissues and preferentially home back to the site where the antigen was initially encountered, carrying out their effector functions (18, 56).
The intranasal route of immunization is very efficient at inducing humoral and cellular immune responses in the respiratory mucosa and at distal mucosal sites (4, 24, 27, 54-56, 58). The NALT in rodents presents as the functional equivalent of the Waldeyer's ring in humans (26, 50) and is an important inductive tissue for the generation of mucosal immunity to inhaled antigens, capable of disseminating effector cells at distant mucosal sites (24, 54, 59). Different adjuvants and delivery systems have been proposed for the development of effective nasal vaccines (24). Our approach to intranasal immunization is based on the use of Streptococcus gordonii, a nonpathogenic gram-positive commensal bacterium component of the normal microbial flora of the human oral cavity (23), as the vaccine vector (36, 39, 41). A variety of antigens have been expressed on the surface of S. gordonii and were shown to be immunogenic by the systemic and the mucosal routes (oral, nasal, vaginal, and intragastric) in both mice and monkeys (11, 33-35, 37, 38, 40, 46). S. gordonii is efficiently internalized by both human and murine dendritic cells (DCs), inducing their maturation and activation (8, 9, 45). Using the model of the adoptive transfer of transgenic T lymphocytes, we recently demonstrated that intranasal immunization with recombinant S. gordonii induces an in vivo primary activation of antigen-specific CD4+ T cells in NALT, draining lymph nodes, and spleens of mice (32). Furthermore, its safety by the intranasal and oral routes has been demonstrated in a phase I clinical trial (25).
Intranasal immunization is particularly relevant against those pathogens that infect the respiratory tract, such us Mycobacterium tuberculosis, which usually enters the host by inhalation and infects through the mucosal surface of the lung. M. bovis bacille Calmette-Guérin (BCG) is the only vaccine currently available against tuberculosis (TB), and although it was originally developed as an oral vaccine, it is now administered by the intradermal route. BCG confers efficient protection in newborns but does not prevent the establishment of latent TB or reactivation of pulmonary disease in adults. A novel, effective vaccination strategy against adult pulmonary TB has therefore become an international research priority. Nasal TB vaccines have been shown to be capable of inducing both local and systemic responses and to confer protection from infection (1, 2, 7, 10, 15, 17, 20, 30, 48, 52, 57). The exact correlates of protection against TB are not yet fully understood; however, CD4+ and CD8+ T cells have been shown to play a crucial role in conferring protection from M. tuberculosis infection (2, 53). Most importantly, the localization of T cells in the airways at the time of M. tuberculosis infection has been shown to be of primary importance (5, 6, 21, 47, 48). One of the most promising antigens for vaccine generation against M. tuberculosis infection is represented by the Ag85B-ESAT-6 fusion protein, which includes a protein of the Ag85 mycolyl transferase complex and a member of the ESAT family (42). The Ag85B-ESAT-6 fusion protein has been shown to be immunogenic by both the parenteral and the mucosal routes and capable of conferring protection in many animal models, such as mice, guinea pigs, and nonhuman primates (10-13, 28, 42, 43). Furthermore, this TB vaccine antigen has recently entered human clinical trials (2).
The aim of the present work was to study the primary activation of both CD4+ and CD8+ T cells at mucosal and systemic sites following intranasal immunization with recombinant S. gordonii expressing on the cell surface the vaccine antigen Ag85B-ESAT-6 of M. tuberculosis fused to both T-helper and T-cytotoxic epitopes of ovalbumin (OVA). For this purpose, the adoptive transfer system that uses transgenic T lymphocytes with a T-cell receptor specific for the H-2b-restricted T-helper and T-cytotoxic epitopes of OVA (amino acids 323 to 339 [OVA323-339] and 257 to 264 [OVA257-264], respectively) (3, 19) was employed. Transgenic CD4+ and CD8+ T cells were adoptively cotransferred into the same recipient mice, and their in vivo primary activation was assessed in cervical lymph nodes, lungs, and spleens following intranasal immunization with our recombinant model strain of S. gordonii.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. gordonii GP1456 expressing the Ag85B-ESAT-6 antigen protein from M. tuberculosis, fused to OVA T-helper peptides 323 to 339 and T-cytotoxic epitopes 257 to 264, and the recipient control strain GP1295 (41) were used for immunization experiments. Bacteria were grown at 37°C with 5% CO2 in tryptic soy broth (TSB; Difco, Detroit, MI), except for the cytofluorimetric analysis experiments, for which they were cultured in TSB without dextrose (Difco). For solid medium, tryptic soy agar (Difco) was used and supplemented with 2% horse blood. Antibiotics were added at the following concentrations: erythromycin at 1 μg/ml and kanamycin at 500 μg/ml.
Construction of recombinant S. gordonii GP1456.
An S. gordonii strain surface displaying the Ag85B-ESAT-6 fusion protein was constructed by using the host-vector system previously described (41). The emm6-based gene fusion includes (i) the DNA coding for OVA323-339 (49), (ii) the DNA coding for OVA257-264 (16), and (iii) the sequence coding for the Ag85B-ESAT-6 fusion protein. Construction of the above-mentioned emm6-based gene fusion required two cloning steps. Oligonucleotides OVA 4-F (5′-GGT ACC GGA TCC ATT TCT CAA GCT GTT CAT GCC GCT CAT GCA GAA ATT AAT GAA GCT GGT CGT GAT ATC-3′ [restriction sites KpnI, BamHI, and EcoRV are underlined]) and OVA 4-R (5′-GAT ATC ACG ACC AGC TTC-3′ [the EcoRV restriction site is underlined]) were used as a template to synthesize OVA323-339 by using the Klenow enzyme (Roche Applied Science, Monza, Italy); the fragment was cloned into restriction sites KpnI and EcoRV of plasmid pSMB55 (41), generating plasmid pSMB342 (32). The OVA257-264 and Ag85B-ESAT-6 coding sequences were produced by PCR (annealing at 52°C for 30 s, extension at 72°C for 90 s, and denaturation at 95°C for 30 s for a total of 30 cycles) by using primers OVA 8-F (5′-GCG AAT TCT GCG AAC ATC CCA GTG ACG TTG-3′ [EcoRI restriction site is underlined]) and OVA 8-R (5′-ACG CGT CGA CCA TAG TAT TAT TAA TTT TGA AAA GTT GAT CGA GGG CAG ATC TTT CTC CCG GC-3′ [the SalI restriction site is underlined, and OVA257-264 is in italics]) and plasmid DNA pMCT (42) as the template. The resulting plasmid, named pSMB545, was used to transform competent cells of S. gordonii GP1295. An erythromycin/streptomycin-resistant transformant was selected that carried the expected emm6-based gene fusion and named GP1456. Procedures for cloning gene fusions in Escherichia coli, transformation of S. gordonii, scoring, and genetic analysis of streptococcal transformants were performed as previously described (44).
Analysis of recombinant bacteria.
The expression of the Ag85B-ESAT-6 fusion protein on the bacterial surface was verified by flow cytometry analysis. Bacteria were grown in TSB without dextrose to early stationary phase, washed, and resuspended in phosphate-buffered saline (PBS; pH 7.4) containing 1% bovine serum albumin and then incubated at 37°C for 30 min. Cells were incubated with anti-Ag85-ESAT-6 mouse serum (1:300; obtained from Staten Serum Institute, Denmark) for 1 h at 4°C. After two washes with PBS, fluorescein isothiocyanate (FITC)-conjugated anti-mouse (1:64; Sigma-Aldrich, Munich, Germany) was added to bacteria for 20 min at 37°C. Cells were washed twice in PBS and finally resuspended in 0.5 ml of PBS and analyzed by flow cytometry (FACScan, Becton Dickinson, San Diego, CA).
Mice.
Nine-week-old female OVA-specific CD8+ (OT-I) (19) and OVA-specific CD4+ (OT-II) (3) T-cell receptor-transgenic mice (H-2b) and C57BL/6 mice were purchased from Charles River (Lecco, Italy) and maintained in the animal facilities at the University of Siena. Transgenic mice were maintained under specific-pathogen-free conditions. All animal procedures were carried out in accordance with institutional guidelines.
In vitro interaction of OT-I and OT-II T cells and recombinant bacteria.
OT-I and OT-II cells were treated in vitro with recombinant or wild-type S. gordonii strains for assessing the expression of CD69 activation marker. Splenocytes were collected, homogenized to single-cell suspensions through nylon screens (Sefar Italia, Italy), and resuspended in RPMI medium (Gibco BRL, Life Technologies, Scotland) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), and 2 mM l-glutamine (Sigma-Aldrich). Cells were cultured at 1 × 106 cells/well in a 96-well round bottom plate (Sarstedt) and stimulated with recombinant strain GP1456 or wild-type strain GP1295 at doses of 0.1, 1, and 10 bacteria per cell, for 24 h. As control, cells were stimulated with soluble OVA (10 and 25 μg/well; Sigma-Aldrich) or left untreated. Antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin [Sigma-Aldrich]) were added 2 h after bacteria were added. Following incubation, cells were washed in ice-cold medium and incubated for 30 min with PerCP-conjugated anti-mouse CD8 (clone 53-6.7) or CD4 (clone RM 4-5) (BD Pharmingen) and FITC-conjugated anti-mouse CD69 (clone H1-2F3; eBioscences). Cells were then washed, resuspended in RPMI medium, and analyzed by flow cytometry.
Adoptive transfer of OT-I and OT-II T cells.
OT-I and OT-II transgenic mice were sacrificed, and lymphoid organs (spleen and cervical, brachial, axillary, mesenteric, and iliac lymph nodes) were removed by dissection. Tissues were mashed onto a nylon screen, and the cells obtained were pooled, washed twice in Hanks' balanced salt solution (HBSS; Sigma-Aldrich), and resuspended at 1 × 108 cells/ml. Transgenic OT-I CD8+ and OT-II CD4+ T cells were isolated by negative selection, using EasySep magnetic nanoparticles (StemCell Technologies, Vancouver, BC, Canada), according to the manufacturer's protocol. The purity of the CD4+ or the CD8+ T-cell population in the enriched fraction was >95%, as determined by flow cytometry analysis. CD4+ and CD8+ isolated T cells were pooled and stained with carboxy-fluorescein diacetate succinimidyl ester (CFSE; 7.5 μM [Invitrogen]) for 10 min at 37°C. An amount of 5 × 106 of CFSE-labeled T cells was injected into the tail vein of each recipient mouse.
Intranasal immunization.
Twenty-four hours after the adoptive cotransfer of CFSE-labeled OT-I CD8+ and OT-II CD4+ T cells, C57BL/6 mice were intranasally immunized with recombinant strain GP1456 or with the control strain GP1295. Mice were lightly anesthetized with an intraperitoneal injection of tiletamine and zolazepam hydrochloride (6 mg/kg of body weight; Zoletil 20 [Laboratoires Virbac, France]) and xylazine (3 mg/kg; Xilor 2% [Bio 98 Srl, Italy]) and then inoculated in a single nostril with 109 CFU of bacteria in a total volume of 15 μl of PBS. Groups of three or six mice were sacrificed on days 3, 5, and 7 following immunization.
Organ collection.
Cervical (superficial and posterior) lymph nodes, spleen, and lung were harvested from each mouse, immediately put in ice-cold Click's medium (Sigma-Aldrich), and mashed onto a nylon screen. For lung collection, blood was removed from the pulmonary vasculature by in situ perfusion with collagenase D (0.5 mg/ml) and DNase I (0.75 mg/ml) (Sigma-Aldrich) via the right ventricle. Lungs were removed, cut into small pieces, and digested in the presence of collagenase D and DNase I enzymes for 1 h at 37°C. Lungs were then mashed onto a nylon screen and mononuclear cell suspensions were obtained by Lympholyte M (Cedarlane Laboratories, Ontario, Canada) gradient centrifugation. Collected organs were washed twice in HBSS prior to cell staining for flow cytometry analysis.
Flow cytometry analysis.
Single-cell suspensions from lymph nodes, lungs, and spleens were incubated with Fc-blocking solution (0.5 mg CD16/CD32 monoclonal antibody [clone 2.4G2; BD Pharmingen], 5% mouse serum, 5% rat serum, 0.2% sodium azide [all from Sigma-Aldrich] in 100 ml of HBSS) for 30 min on ice. Cells were stained with PerCP-conjugated anti-mouse CD4 (clone RM 4-5) or CD8 (clone 53-6.7) (BD Pharmingen), phycoerythrin-conjugated anti-mouse CD69 (clone H1.2F3), CD44 (clone IM7), or CD45RB (clone C363.16A) (all from eBioscience) for 30 min at 4°C and analyzed by flow cytometry. Sample acquisition stopped when 2,000 CFSE-positive CD4+ or CFSE-positive CD8+ events with light scatter properties of lymphocytes were acquired. Data analysis was performed by using Cell Quest software (Becton Dickinson).
T-cell proliferation assay.
C57BL/G mice were intranasally immunized with GP1456 or GP1295, and splenocytes were collected 5 days after immunization and analyzed ex vivo for Ag85B-ESAT-6-specific T-cell proliferation. Splenocytes were seeded in triplicate in 96-well culture plates (Sarstedt) at a density of 2 × 105 and cultured in 200 μl of complete RPMI medium with or without Ag85B-ESAT-6 (20 μg/ml; obtained from Staten Serum Institute, Denmark). After 3 days, cells were pulsed with 1 μCi/well of [3H]thymidine (DuPont NEN, Boston, MA). Cells were harvested after 18 h on paper discs (Cell Harvester; Skatron Instruments, Norway), and radioactivity was measured in a liquid-scintillation counter. The stimulation index, calculated as the ratio of the mean counts per minute obtained in the presence of the antigen to the mean counts per minute obtained in untreated cells, was used to express the results.
IFN-γ enzyme-linked immunospot assay.
For the gamma interferon (IFN-γ) enzyme-linked immunospot assay, 96-well nitrocellulose plates (Milititer HA; Millipore, Bedford, MA) were coated with 10 μg/ml of the purified rat IFN-γ monoclonal antibody (BD Pharmingen, CA). Free binding sites were blocked with complete RPMI medium, followed by washing with PBS-0.05% Tween 20. Splenocytes, collected from mice 5 days after intranasal immunization with GP1456 or GP1295, were seeded in triplicate starting from 3 × 106 cells/well and diluted by threefold up to 3 × 105 cells/well. Cells were stimulated with 20 μg/ml of the Ag85B-ESAT-6 vaccine antigen for 18 h at 37°C. Plates were washed and then incubated with biotinylated anti-mouse IFN-γ monoclonal antibody and then streptavidin-horseradish peroxidase (all from BD Pharmingen, CA). The enzyme reaction was developed by adding 1 mg/ml of the substrate 3,3′-diaminobenzidine (Sigma-Aldrich). Spots were counted by using a stereomicroscope (Leica).
Statistical analysis.
Statistical significance was assessed by Student's t test. The Spearman rank correlation coefficient (ρ) was used for assessing the correlation among the percentages of proliferating CD4+ and CD8+ T cells in each animal. A P value of ≤0.05 was considered significant.
RESULTS
Construction of the vaccine strain.
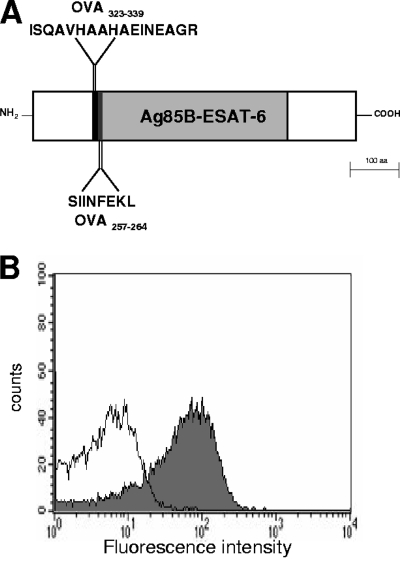
A recombinant strain of S. gordonii displaying on the cell surface a fusion protein containing the Ag85B-ESAT-6 vaccine antigen from M. tuberculosis fused to the OVA323-339 and OVA257-264 peptides was constructed and named GP1456 (Fig. 1A).
FIG. 1.
Fusion protein expressed on the surface of recombinant S. gordonii GP1456. (A) Schematic structure shows the fusion protein displayed on the surface of S. gordonii GP1456. The recombinant protein consists of OVA323-339 (ISQAVHAAHAEINEAGR, black area), OVA257-264 (SIINFEKL, black area), and the Ag85B-ESAT-6 fusion protein (388 amino acids, gray area). (B) Flow cytometry analysis of S. gordonii GP1456 (gray histogram) and control strain (open histogram). Bacterial cells were reacted with Ag85B-ESAT-6-specific mouse serum and then with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G.
The expression of the Ag85B-ESAT-6 antigen on the surface of S. gordonii GP1456 was assessed by flow cytometry on whole streptococcal cells by using anti-Ag85B-ESAT-6 mouse serum (Fig. 1B). Eighty percent of bacterial cells were positive for the expression of Ag85B-ESAT-6, and the mean fluorescence intensity (MFI) value was 68 versus 6 for the control strain (Fig. 1B). This recombinant strain of S. gordonii is therefore a vaccine vector for a TB antigen that also expresses the model peptides OVA323-339 and OVA257-264 for studying the in vivo primary activation of both CD4+ and CD8+ T cells in the same animal, using the adoptive transfer model.
In vitro activation of OT-I and OT-II T cells.
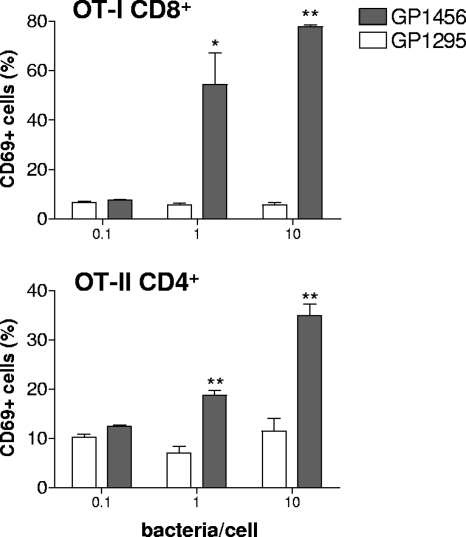
The activation of transgenic OT-I CD8+ and OT-II CD4+ T lymphocytes following interaction with recombinant S. gordonii GP1456 was first assessed in vitro. Splenocytes from OT-I and OT-II transgenic mice were stimulated for 24 h with recombinant or wild-type S. gordonii strains at doses of 0.1, 1, and 10 bacteria/cell and then analyzed for the expression of the early activation marker CD69 (51). The treatment with recombinant strain GP1456 induced a significant upregulation of the activation marker CD69 on both OT-I CD8+ and OT-II CD4+ cells in a dose-dependent manner (Fig. 2). The dose of 10 bacteria/cell induced the highest percentage of CD69-positive OT-I (78%) and OT-II (35%) T cells, while the treatment with wild-type bacteria or with soluble OVA was not effective (Fig. 2 and data not shown).
FIG. 2.
Expression of CD69 on OT-I CD8+ and OT-II CD4+ T cells after in vitro interaction with recombinant S. gordonii. CD69 expression was assessed in OT-I and OT-II cells incubated for 24 h with control strain GP1295 (open histogram bars) or recombinant vaccine strain GP1456 (filled histogram bars) at different doses (0.1, 1, and 10 bacteria per cell). The percentage of CD69-positive OT-I CD8+ or OT-II CD4+ T cells is reported as the mean ± the standard error of the means of two individual experiments, performed in triplicate. *, P ≤ 0.05; **, P ≤ 0.01 versus GP1295.
In vivo antigen-specific OT-I CD8+ and OT-II CD4+ T-cell proliferation and activation.
To study the primary activation of cytotoxic and helper T cells in vivo, transgenic OT-I CD8+ and OT-II CD4+ T cells were labeled with CFSE and then cotransferred into C57BL/6 syngeneic recipient mice. CFSE is a vital dye that can label cells very stably by covalently coupling to intracellular molecules, and it can be used to monitor lymphocyte proliferation due to the progressive halving of the dye fluorescence following cell division (31). The distribution of transgenic T cells among different lymphoid organs following intravenous injection was evaluated in untreated mice; the percentage of transgenic T cells was about 0.5% of resident lymphocytes in lymph nodes and about 0.25% in the spleen, while it was almost undetectable in the lung (data not shown).
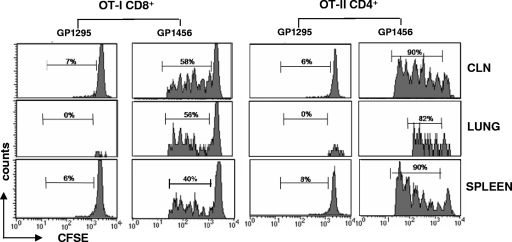
OVA-specific proliferation of both OT-II CD4+ and OT-I CD8+ T cells was observed for cervical lymph nodes, lung, and spleen of mice immunized intranasally with recombinant S. gordonii as shown by the progressive dilution of CFSE (Fig. 3). In all organs, OT-I and OT-II T cells had divided between one and six times. On the contrary, a high level of the CFSE fluorescence was maintained in mice inoculated with the control strain GP1295, indicating that no cell division had occurred (Fig. 3). Furthermore, in control mice, we can observe the absence of transgenic T cells in the lung, suggesting an antigen-specific T-cell recruitment following intranasal immunization.
FIG. 3.
Clonal expansion of OT-I CD8+ and OT-II CD4+ T cells following intranasal immunization with S. gordonii. CFSE-labeled OT-I CD8+ and OT-II CD4+ T cells were adoptively cotransferred into C57BL/6 mice, and then the mice were intranasally immunized with 109 CFU of the recombinant vaccine strain GP1456 or control strain GP1295. OVA-specific proliferation of OT-I CD8+ and OT-II CD4+ T cells was assessed in cervical lymph nodes (CLN), lung, and spleen collected 5 days after immunization and analyzed as CFSE dilution (x axis) on the gated CFSE-positive CD8+ or CFSE-positive CD4+ populations with light scatter properties of lymphocytes. The percentage of proliferating cells is reported above peaks.
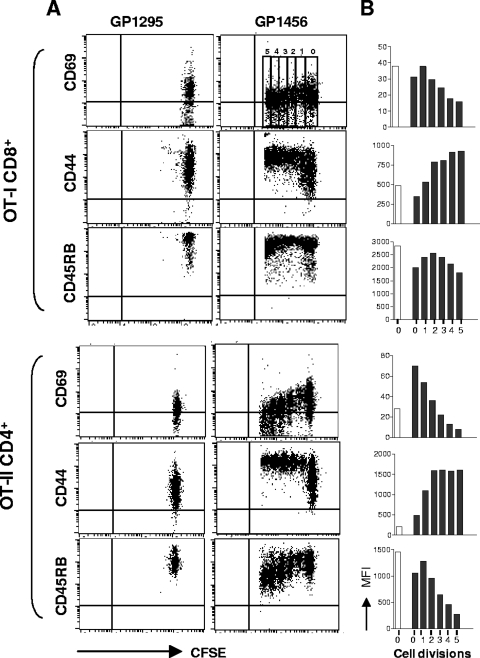
The phenotype of both OT-I CD8+ and OT-II CD4+ proliferating T cells was assessed in cervical lymph nodes 3 days following immunization by analyzing the expression of activation markers as a function of cell division number. To this aim, the expression of markers CD69, CD44, and CD45RB was analyzed in each generation (from zero to five) and identified by CFSE intensity (Fig. 4A), and the MFI values of the surface markers in each cell division are reported in Fig. 4B. The early activation marker CD69 was rapidly expressed and then downregulated with advancing cycles of cell division on both OT-I CD8+ and OT-II CD4+ T cells (Fig. 4). The expression of the activation marker CD44 increased throughout the early cycles of cell division and remained high on both OT-I CD8+ and OT-II CD4+ T cells (Fig. 4). The expression of CD45RB, a marker of naive cells (29), was down-modulated especially in OT-II CD4+ T cells (Fig. 4). The phenotypic analysis of transgenic proliferating CD8+ and CD4+ T cells confirm that cells are activated by the antigen encounter following intranasal immunization.
FIG. 4.
Phenotypic analysis of proliferating OT-I CD8+ and OT-II CD4+ T cells. The expression of CD69, CD44, and CD45RB was analyzed as a function of cell division on OT-I CD8+ and OT-II CD4+ T cells from cervical lymph nodes 3 days after immunization with recombinant vaccine strain GP1456 or control strain GP1295. (A) Dot plot analysis of CFSE dilution (x axis) versus CD69, CD44, or CD45RB expression (y axis) on OT-I CD8+ (top panels) and OT-II CD4+ (bottom panels) T cells following treatment with control (GP1295, left panels) or vaccine strain (GP1456, right panels). (B) MFI of CD69, CD44, and CD45RB expression per each cell generation of OT-I CD8+ (top panels) and OT-II CD4+ (bottom panels) T cells from mice immunized with control (GP1295, open histogram bars) or vaccine strain (GP1456, filled histogram bars). Cell divisions (from 0 to 5) are identified by CFSE intensity dilution, as shown in the upper right portion of panel A. Phenotypic analyses were performed with cells pooled from six animals. Results are representative of two separate experiments.
Time course analysis of antigen-specific OT-I CD8+ and OT-II CD4+ T-cell proliferation.
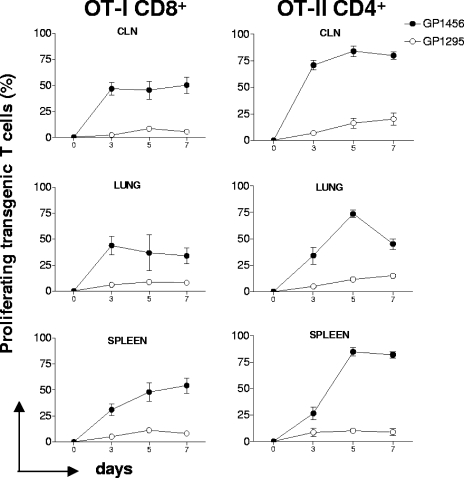
The OVA-specific primary activation of OT-I CD8+ and OT-II CD4+ T cells was analyzed 3, 5, and 7 days following intranasal immunization with recombinant GP1456 or the control strain in mice into which OT-I and OT-II transgenic T cells were cotransferred. The time course analysis of the OT-I CD8+ T-cell activation showed a high level of proliferation in cervical lymph nodes and lung from the third day, with approximately 48% proliferating cells, while in the spleen, numbers of proliferating T cells were lower at day 3 and increased at day 5, with more than 50% of T cells proliferating (Fig. 5). The time course analysis of the OT-II CD4+ T-cell activation showed a high level of proliferation in cervical lymph nodes since the third day, with approximately 75% of cells proliferating. The CD4+ OT-II proliferation in lung and spleen reached the peak at day 5, with more that 75% of cells proliferating (Fig. 5). Mice immunized with the wild-type strain did not respond at any time point in any lymphoid organ. These data demonstrate that the clonal expansion of OT-I and OT-II T cells is antigen specific and not due to other vector antigens. The delayed appearance of OT-I and OT-II proliferating cells in the spleen suggests a possible migration of activated antigen-specific CD4+ or CD8+ T cells from the inductive mucosal site to the systemic district.
FIG. 5.
Time course analysis of the clonal expansion of OT-I CD8+ and OT-II CD4+ T cells following intranasal immunization with recombinant S. gordonii. The clonal expansion of adoptively transferred OT-I CD8+ and OT-II CD4+ T cells following intranasal immunization with recombinant vaccine strain GP1456 or control strain GP1295 was analyzed on days 3, 5, and 7 postimmunization in cervical lymph nodes (CLN), lung, and spleen. Transgenic OT-I and OT-II T cells were identified on the gated CD8+ CFSE-positive and CD4+ CFSE-positive populations, respectively, with light scatter properties of lymphocytes. The percentages of proliferating cells are shown on the y axis. Bars represent the means ± standard errors of the means of values from nine mice assessed in three separate experiments.
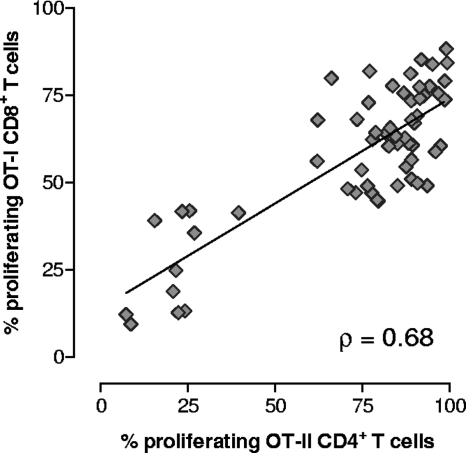
Since transgenic OT-I and OT-II T cells were simultaneously injected into the same recipient mice, the activation of CD8+ and CD4+ T lymphocytes following immunization with recombinant S. gordonii could be monitored and compared in the same animal. A positive correlation between the percentages of OT-I CD8+ and OT-II CD4+ proliferating T cells was observed with each animal (Spearman rank correlation coefficient [ρ] = 0.68; P ≤ 0.001) (Fig. 6).
FIG. 6.
Correlation between OT-I CD8+ and OT-II CD4+ T-cell proliferation in each animal. Transgenic OT-I CD8+ and OT-II CD4+ T cells were adoptively cotransferred into C57BL/6 mice, and then the mice were immunized with recombinant S. gordonii. The percentages of proliferating OT-I CD8+ (y axis) and OT-II CD4+ (x axis) T cells detected in each animal at various time points are shown. The Spearman rank correlation coefficient (ρ) and regression line are shown.
T-cell priming specific for Ag85B-ESAT-6.
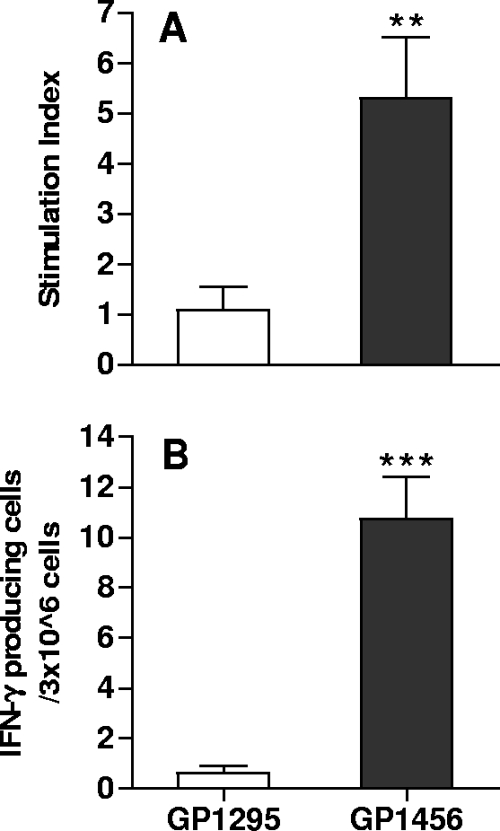
T-cell priming specific for M. tuberculosis Ag85B-ESAT-6 antigen was studied in mice immunized intranasally with recombinant vaccine strain GP1456. The TB-specific proliferative response and IFN-γ production were assessed in splenocytes 5 days after the inoculum. Ag85B-ESAT-6-specific T cells were primed by nasal immunization with the recombinant vaccine strain, as shown by the significant T-cell proliferative response (P = 0.01) (Fig. 7A) and IFN-γ production (P = 0.00006) (Fig. 7B). These data indicate that a single nasal immunization with recombinant S. gordonii expressing Ag85B-ESAT-6 is effective in inducing a TB-specific primary T-cell activation and suggest that the OVA-specific response observed in vivo using the adoptive transfer model can be considered representative of the vaccine-specific response.
FIG. 7.
Ag85B-ESAT-6-specific T-cell proliferative response and IFN-γ production. Mice were immunized by the nasal route with a single dose of GP1456 or control strain GP1295, and the Ag85B-ESAT-6-specific T-cell response was assessed in splenocytes 5 days after the inoculum. (A) Ag85B-ESAT-6-specific proliferation of splenocytes was assessed by tritiated thymidine incorporation. (B) IFN-γ production of splenocytes was restimulated with 20 μg/ml of Ag85B-ESAT-6. Bars represent the means ± standard errors of the means of values from 10 mice assessed in two separate experiments. **, P ≤ 0.01; ***, P ≤ 0.0001 versus control.
DISCUSSION
The local and systemic in vivo primary activation of T-helper and T-cytotoxic cells following intranasal immunization with recombinant S. gordonii was demonstrated by using the model of adoptive cotransfer of antigen-specific transgenic CD8+ and CD4+ T cells. Mucosal inductive sites, such as the NALT, are specialized for priming naive T and B cells that then move to other mucosal effector sites but also home back to the site where the antigen was initially encountered for the generation of antigen-specific immune responses, which function as the first line of defense at mucosal surfaces (18, 56).
S. gordonii has been previously used as a vaccine vector for mucosal immunization, and it was shown to be immunogenic in both mice and monkeys (11, 32-35, 37, 38, 40, 46). Its safety by the intranasal and oral routes has been demonstrated in a phase I clinical trial (25).
In the present study, CD8+ and CD4+ T-cell activation was studied following intranasal immunization with recombinant S. gordonii expressing OVA CD4+ and CD8+ T-cell epitopes fused to an M. tuberculosis vaccine antigen. Antigen-specific proliferation of OT-I CD8+ and OT-II CD4+ T cells was observed for cervical lymph nodes, lung, and spleen. In all organs, transgenic T cells had divided between one and six times. A positive correlation between the percentages of OT-I CD8+ and OT-II CD4+ proliferating T cells in each animal was also observed. The T-cell proliferation observed for the OVA model antigen can be considered representative of T-cell responses that are also induced with the M. tuberculosis vaccine antigen, as confirmed by the Ag85B-ESAT-6-specific proliferative response and the IFN-γ production observed following a single intranasal immunization with the vaccine strain. Furthermore, Ag85B-ESAT-6-specific humoral and cellular responses are induced following repeated intranasal immunizations with this recombinant S. gordonii strain, as indicated by a serum antibody response and splenocyte IFN-γ release (A. Ciabattini, unpublished data).
Even if the exact correlates of protection against TB are not yet fully understood, however, experimental evidence supports the idea that protection is dominated by CD4+ and CD8+ T cells (22). Furthermore, the localization of T cells in the airways has been shown to play a key role in immune protection from TB (5, 6, 21, 47, 48). Therefore, the CD4+ and CD8+ T-cell proliferation observed in the lung following intranasal immunization with recombinant S. gordonii is of primary importance.
Antigen-specific OT-I CD8+ T cells proliferated first in cervical lymph nodes and lung from the third day, with approximately 48% proliferating cells, and only later in the spleen (day 7). OT-II CD4+ T cells were also activated in cervical lymph nodes from the third day, with approximately 75% proliferating cells, while in lung and spleen, the peak of CD4+ T cell activation was reached on day 5. These data are in agreement with previous results obtained using the model of DO11.10 transgenic mice, in which we demonstrated that intranasal immunization with recombinant S. gordonii induces CD4+ T-cell primary activation first in NALT and subsequently in the spleen (32). The delayed appearance of proliferating cells in the spleen compared to their appearance in draining lymph nodes, observed for both CD4+ and CD8+ T cells, suggests a possible migration of activated antigen-specific CD4+ or CD8+ T cells from the immunization site to the systemic district.
The present work shows, for the first time, that the S. gordonii vaccine vector, an extracellular commensal bacterium, is effective in eliciting a CD8+ T-cell response in vivo. This may occur via cross-presentation of heterologous antigens on major histocompatibility complex class I (MHC-I) after phagocytosis. We have previously shown in vitro that DCs efficiently process and present the recombinant antigen delivered by S. gordonii not only with MHC-II but also with MHC-I molecules (8, 9, 45). Furthermore, it was shown that this exogenous pathway of MHC-I presentation was TAP (transporter associated with antigen processing) dependent, indicating that there is a transport from phagolysosome to cytosol inside DCs (45). We can therefore hypothesize that also in vivo, the DCs present in cervical lymph nodes might be stimulated by recombinant S. gordonii for antigen presentation on both MHC-I and -II, thereby inducing the CD8+ and CD4+ T-cell activation observed.
Proliferating CD4+ and CD8+ T cells in cervical lymph nodes were also characterized for the expression of surface markers, such as CD69, CD44, and CD45RB, in each cell generation. As expected, the early activation marker CD69 (51) was rapidly expressed and then downregulated with advancing cycles of cell division for both OT-II CD4+ and OT-I CD8+ T cells, while the expression of the activation marker CD44 (14) increased throughout the early cycles of cell division and remained high for both OT-II CD4+ and OT-I CD8+ T cells. CD45RB, a marker expressed by naive cells (29), was down-modulated as cell division proceeded, especially in OT-II CD4+ T cells. The phenotypic analysis of OVA-specific CD4+ and CD8+ T cells demonstrates that proliferating T cells are also activated by the antigen encounter following intranasal immunization with recombinant S. gordonii.
These data indicate that intranasal immunization with recombinant S. gordonii is capable of inducing primary activation of naive antigen-specific CD4+ and CD8+ T cells, both locally and systemically, and further support the use of S. gordonii as the mucosal vaccine vector.
Acknowledgments
We thank Rita Privitera for help in construction of recombinant bacteria and Anna Maria Cuppone and Edoardo Ficai for help with the experimental procedures.
This study has been carried out with the financial support from the Commission of the European Communities, Sixth Framework Programme, contract LSHP-CT-2003-503240, Mucosal Vaccines for Poverty-Related Diseases (MUVAPRED), and contract 037611, European Vaccines and Microbicides Enterprise (EUROPRISE), and from Azione Concertata Italiana per lo Sviluppo di un Vaccino HIV/AIDS (ICAV) of the Istituto Superiore di Sanità, contract no. 45G.27.
Editor: A. Camilli
Footnotes
Published ahead of print on 6 October 2008.
REFERENCES
- 1.Andersen, C. S., J. Dietrich, E. M. Agger, N. Y. Lycke, K. Lovgren Bengtsson, and P. Andersen. 2007. The combined CTA1-DD/ISCOMs vector is an effective intranasal adjuvant for boosting prior Mycobacterium bovis BCG immunity to Mycobacterium tuberculosis. Infect. Immun. 75408-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P. 2007. Tuberculosis vaccines: an update. Nat. Rev. Microbiol. 5484-487. [DOI] [PubMed] [Google Scholar]
- 3.Barnden, M. J., J. Allison, W. R. Heath, and F. R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 7634-40. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist, C., E. L. Johansson, T. Lagergard, J. Holmgreen, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 652676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccamo, N., G. Sireci, S. Meraviglia, F. Dieli, J. Ivanyi, and A. Salerno. 2006. Gammadelta T cells condition dendritic cells in vivo for priming pulmonary CD8 T cell responses against Mycobacterium tuberculosis. Eur. J. Immunol. 362681-2690. [DOI] [PubMed] [Google Scholar]
- 6.Chackerian, A. A., T. V. Perera, and S. M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 692666-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L., J. Wang, A. Zganiacz, and Z. Xing. 2003. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect. Immun. 72238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi-Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant Gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 1633029-3036. [PubMed] [Google Scholar]
- 9.Corinti, S., D. Medaglini, C. Prezzi, G. Pozzi, and G. Girolomoni. 2000. Human dendritic cells are superior to B cells at presenting a major histocompatibility complex class-II restricted heterologous antigen expressed on recombinant Streptococcus gordonii. Infect. Immun. 681879-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich, J., C. Andersen, R. Rappuoli, T. M. Doherty, C. G. Jensen, and P. Andersen. 2006. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J. Immunol. 1776353-6360. [DOI] [PubMed] [Google Scholar]
- 11.Di Fabio, S., D. Medaglini, C. M. Rush, G. Corrias, G. L. Panzini, M. Pace, P. Verani, G. Pozzi, and F. Titti. 1998. Vaginal immunization of Cynomolgus monkeys with Streptococcus gordonii expressing HIV-1 and HPV 16 antigens. Vaccine 16485-492. [DOI] [PubMed] [Google Scholar]
- 12.Doherty, T. M., A. W. Olsen, J. Weinschenfeldt, K. Huygen, S. D'Souza, T. K. Kondratieva, V. V. Yeremeev, A. S. Apt, B. Raupach, and L. Grode. 2004. Comparative analysis of different vaccine constructs expressing defined antigens from Mycobacterium tuberculosis. J. Infect. Dis. 1902146-2153. [DOI] [PubMed] [Google Scholar]
- 13.Doherty, T. M., A. Weinrich Olsen, L. van Pinxteren, and P. Andersen. 2002. Oral vaccination with subunit vaccines protects animals against aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 703111-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutton, R., L. Bradley, and S. Swain. 1998. T cell memory. Annu. Rev. Immunol. 16201-223. [DOI] [PubMed] [Google Scholar]
- 15.Falero-Diaz, G., S. Challacombe, D. Banerjee, G. Douce, A. Boyd, and J. Ivanyi. 2000. Intranasal vaccination of mice against infection with Mycobacterium tuberculosis. Vaccine 183223-3229. [DOI] [PubMed] [Google Scholar]
- 16.Fremont, D. H., E. A. Stura, M. Marsumura, P. A. Peterson, and I. A. Wilson. 1995. Crystal structure of an H-2Kb-ovalbumin peptide complex reveals the interplay of primary and secondary anchor positions in the major histocompatibility complex binding groove. Proc. Natl. Acad. Sci. USA 922479-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri, P. K., S. B. Sable, I. Verma, and G. K. Khuller. 2005. Comparative evaluation of intranasal and subcutaneous route of immunization for development of mucosal vaccine against experimental tuberculosis. FEMS Immunol. Med. Microbiol. 4587-93. [DOI] [PubMed] [Google Scholar]
- 18.Haneberg, B., D. Kendall, H. M. Amerongen, F. M. Apter, J.-P. Kraehenbuhl, and M. R. Neutra. 1994. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect. Immun. 6215-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogquist, K. A., S. C. Jameson, W. R. Heath, L. J. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell 7617-27. [DOI] [PubMed] [Google Scholar]
- 20.Kallenius, G., A. Pawlowski, P. Brandtzaeg, and S. Svenson. 2007. Should a new tuberculosis vaccine be administered intranasally? Tuberculosis 87257-266. [DOI] [PubMed] [Google Scholar]
- 21.Kamath, A., J. Woodworth, X. Xiong, C. Taylor, Y. Weng, and S. M. Behar. 2004. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J. Exp. Med. 61479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann, S. H. 2006. Envisioning future strategies for vaccination against tuberculosis. Nat. Rev. Immunol. 6699-704. [DOI] [PubMed] [Google Scholar]
- 23.Kilian, M., L. Mikkelsen, and J. Henrichsen. 1989. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrews and Horder 1906). Int. J. Syst. Bacteriol. 39471-484. [Google Scholar]
- 24.Kiyono, H., and S. Fukuyama. 2004. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. 4699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotloff, K. L., S. S. Wassermann, K. F. Jones, S. Livio, D. E. Hruby, C. A. Franke, and V. A. Fischetti. 2005. Clinical and microbiological responses of volunteers to combined intranasal and oral inoculation with a Streptococcus gordonii carrier strain intended for future use as a group A Streptococcus vaccine. Infect. Immun. 732360-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuper, C. F., P. J. Koornstra, D. M. H. Hameleers, J. Biewenga, B. J. Spit, A. M. Duijevestijn, P. J. C. van Breda Vriesman, and T. Sminia. 1992. The role of nasopharyngeal lymphoid tissue. Immunol. Today 13219-223. [DOI] [PubMed] [Google Scholar]
- 27.Kurono, Y., M. Yamamoto, K. Fujihashi, S. Kodama, M. Suzuki, G. Mogi, J. R. McGhee, and H. Kiyono. 1999. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J. Infect. Dis. 180122-132. [DOI] [PubMed] [Google Scholar]
- 28.Langermans, J. A., T. M. Doherty, R. A. Vervenne, T. Van Der Laan, K. Lyashchenko, R. Greenwald, E. M. Agger, C. Aagaard, H. Weiler, D. van Soolingen, W. Dalemans, A. W. Thomas, and P. Andersen. 2005. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 232740-2750. [DOI] [PubMed] [Google Scholar]
- 29.Lee, W. T., X. Yin, and E. S. Vitetta. 1990. Functional and ontogenetic analysis of murine CD45Rhi and CD45Rlo CD4+ T cells. J. Immunol. 1443288-3295. [PubMed] [Google Scholar]
- 30.Lyadova, I. V., H. M. Vordermeier, E. B. Eruslanov, S. V. Khaidukov, A. S. Apt, and R. G. Hewinson. 2001. Intranasal BCG vaccination protects BALB/c mice against virulent Mycobacterium bovis and accelerates production of IFN-gamma in their lungs. Clin. Exp. Immunol. 126274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171131-137. [DOI] [PubMed] [Google Scholar]
- 32.Medaglini, D., A. Ciabattini, A. M. Cuppone, C. Costa, S. Ricci, M. Costalonga, and G. Pozzi. 2006. In vivo activation of naive CD4+ T cells in nasal mucosa-associated lymphoid tissue following intranasal immunization with recombinant Streptococcus gordonii. Infect. Immun. 742760-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medaglini, D., A. Ciabattini, M. R. Spinosa, T. Maggi, H. Marcotte, M. R. Oggioni, and G. Pozzi. 2001. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 191931-1939. [DOI] [PubMed] [Google Scholar]
- 34.Medaglini, D., M. R. Oggioni, and G. Pozzi. 1998. Vaginal immunization with recombinant Gram positive bacteria. Am. J. Reprod. Immunol. 39199-208. [DOI] [PubMed] [Google Scholar]
- 35.Medaglini, D., G. Pozzi, T. P. King, and V. A. Fischetti. 1995. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc. Natl. Acad. Sci. USA 926868-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medaglini, D., S. Ricci, T. Maggi, C. M. Rush, R. Manganelli, M. R. Oggioni, and G. Pozzi. 1997. Recombinant Gram-positive bacteria as vehicles of vaccine antigens. Biotechnol. Annu. Rev. 3297-312. [Google Scholar]
- 37.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 151330-1337. [DOI] [PubMed] [Google Scholar]
- 38.Oggioni, M. R., R. Manganelli, M. Contorni, M. Tommasino, and G. Pozzi. 1995. Immunization of mice by oral colonization with live recombinant commensal streptococci. Vaccine 13775-779. [DOI] [PubMed] [Google Scholar]
- 39.Oggioni, M. R., D. Medaglini, T. Maggi, and G. Pozzi. 1999. Engineering the Gram-positive cell surface for construction of bacterial vaccine vectors. Methods 19163-173. [DOI] [PubMed] [Google Scholar]
- 40.Oggioni, M. R., D. Medaglini, L. Romano, F. Peruzzi, T. Maggi, L. Lozzi, L. Bracci, M. Zazzi, F. Manca, P. E. Valensin, and G. Pozzi. 1999. Antigenicity and immunogenicity of the V3 domain of HIV-1 gp120 expressed on the surface of Streptococcus gordonii. AIDS Res. Hum. Retroviruses 15451-459. [DOI] [PubMed] [Google Scholar]
- 41.Oggioni, M. R., and G. Pozzi. 1996. A host-vector system for heterologous gene expression in Streptococcus gordonii. Gene 16985-90. [DOI] [PubMed] [Google Scholar]
- 42.Olsen, A. W., L. A. H. van Pinxteren, L. M. Okkels, P. B. Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on fusion protein of antigen 85B and ESAT-6. Infect. Immun. 692773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen, A. W., A. Williams, L. M. Okkels, G. Hatch, and P. Andersen. 2004. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect. Immun. 726148-6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pozzi, G., R. A. Musmanno, P. M. J. Lievens, M. R. Oggioni, P. Plevani, and R. Manganelli. 1990. Methods and parameters for genetic transformation of Streptococcus sanguis Challis. Res. Microbiol. 141659-670. [DOI] [PubMed] [Google Scholar]
- 45.Rescigno, M., S. Citterio, C. Thery, M. Rittig, D. Medaglini, G. Pozzi, S. Amigorena, and P. Ricciardi-Castagnoli. 1998. Bacterial-induced neo-biosynthesis, stabilization and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl. Acad. Sci. USA 955229-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricci, S., D. Medaglini, C. M. Rush, A. Marcello, R. Manganelli, G. Palù, and G. Pozzi. 2000. Immunogenicity of the B monomer of the Escherichia coli heat-labile toxin expressed on the surface of Streptococcus gordonii. Infect. Immun. 68760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santosuosso, M., S. McCormik, E. Roediger, X. Zhang, and A. Zganiacz. 2007. Mucosal luminal manipulation of T cell geography switches on protective efficacy by otherwise ineffective parenteral genetic immunization. J. Immunol. 1782387-2395. [DOI] [PubMed] [Google Scholar]
- 48.Santosuosso, M., X. Zhang, S. McCormik, J. Wang, M. Hitt, and Z. Xing. 2005. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 1747986-7994. [DOI] [PubMed] [Google Scholar]
- 49.Shimonkevitz, R., S. Colon, J. W. Kappler, P. Marrack, and H. M. Grey. 1984. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J. Immunol. 1332067-2074. [PubMed] [Google Scholar]
- 50.Spit, B. J., E. G. Hendriksen, J. P. Bruijntjes, and C. F. Kuper. 1989. Nasal lymphoid tissue in the rat. Cell Tissue Res. 255193-198. [DOI] [PubMed] [Google Scholar]
- 51.Testi, R., J. H. Phillips, and L. L. Lanier. 1989. T cell activation via Leu-23 (CD69). J. Immunol. 1431123-1128. [PubMed] [Google Scholar]
- 52.Wang, J., L. Thorson, R. W. Stokes, M. Santosuosso, K. Huygen, A. Zganiacz, M. Hitt, and Z. Xing. 2004. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 1736357-6365. [DOI] [PubMed] [Google Scholar]
- 53.Woodworth, J., and S. M. Behar. 2006. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit. Rev. Immunol. 26317-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, H. Y., H. H. Nguyen, and M. W. Russel. 1997. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand. J. Immunol. 46506-513. [DOI] [PubMed] [Google Scholar]
- 55.Wu, H. Y., and M. W. Russel. 1993. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect. Immun. 61314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, H. Y., and M. W. Russell. 1997. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol. Res. 16187-201. [DOI] [PubMed] [Google Scholar]
- 57.Xing, Z., and B. D. Lichty. 2006. Use of recombinant virus-vectored tuberculosis vaccines for respiratory mucosal immunization. Tuberculosis 86211-217. [DOI] [PubMed] [Google Scholar]
- 58.Zuercher, A. W. 2003. Upper respiratory tract immunity. Viral Immunol. 16279-289. [DOI] [PubMed] [Google Scholar]
- 59.Zuercher, A. W., S. E. Coffin, M. C. Thurnheer, P. Fundova, and J. J. Cebra. 2002. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J. Immunol. 1681796-1803. [DOI] [PubMed] [Google Scholar]