Abstract
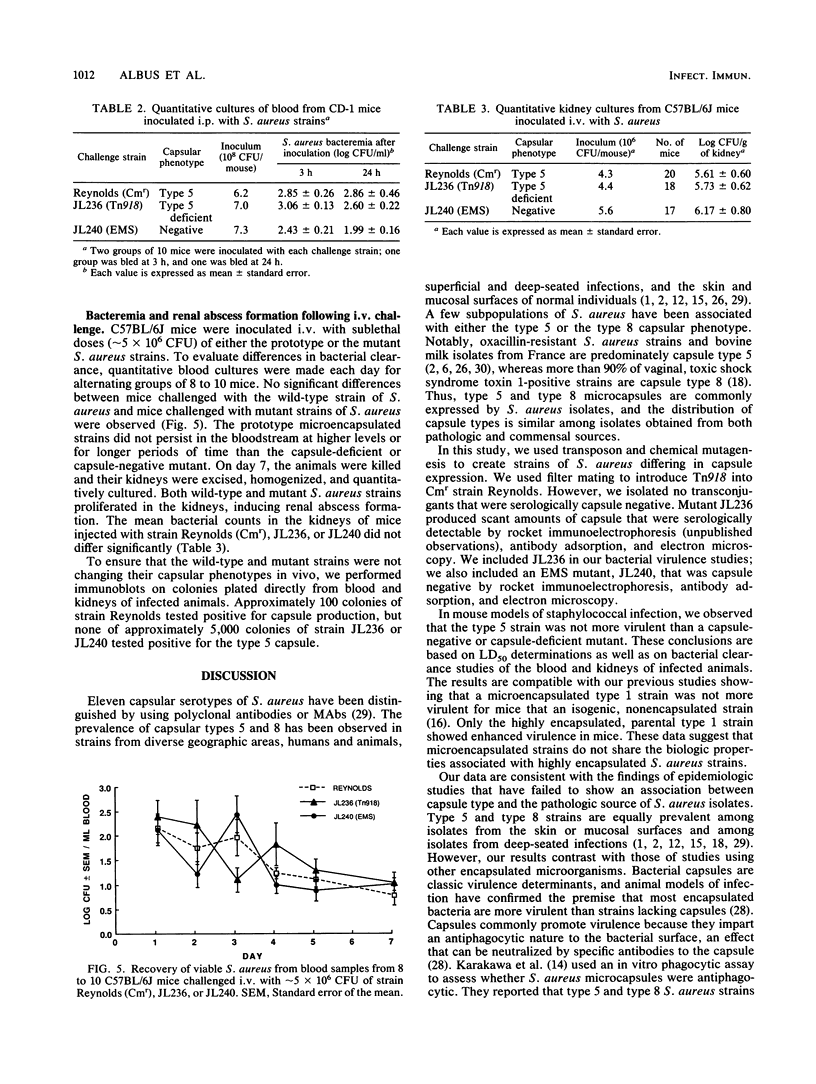
Most clinical isolates of Staphylococcus aureus produce microcapsules (uronic acid-containing extracellular polysaccharides) that are detectable by serologic methods but are not visible by negative staining. Among the 11 reported serotypes, capsule types 5 and 8 comprise approximately 75% of all isolates. Transposon mutagenesis was performed on S. aureus to create mutants altered in capsule expression. Tn918 was introduced into the capsule type 5 strain Reynolds by filter mating, and a capsule-deficient transconjugate, JL236, was isolated. The wild-type strain was transformed with JL236 chromosomal DNA to confirm that transfer of the appropriate-size chromosomal fragment containing Tn918 generated a capsule-deficient transformant. Strain Reynolds was mutagenized with ethyl methanesulfonate to obtain a capsule-negative mutant (strain JL240). Capsular phenotypes were determined by colony immunoblots, antibody adsorption experiments, and transmission electron microscopy. The virulences of the parental and mutant strains in mice were compared. The 50% lethal doses for strains Reynolds, JL236, and JL240 were similar (10(8.59), 10(8.98), and 10(8.93) CFU, respectively). Animals injected intraperitoneally with either wild-type or mutant strains had comparable levels of bacteremia at 3 and 24 h after challenge. Quantitative cultures of blood and kidneys from animals challenged intravenously with sublethal doses of the S. aureus strains also showed no differences in bacterial clearance or renal abscess formation. These studies indicate that the type 5 S. aureus microcapsule does not promote bacterial virulence in the animal models tested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus A., Fournier J. M., Wolz C., Boutonnier A., Ranke M., Høiby N., Hochkeppel H., Döring G. Staphylococcus aureus capsular types and antibody response to lung infection in patients with cystic fibrosis. J Clin Microbiol. 1988 Dec;26(12):2505–2509. doi: 10.1128/jcm.26.12.2505-2509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeit R. D., Karakawa W. W., Vann W. F., Robbins J. B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984 Apr;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Arbeit R. D., Nelles M. J. Capsular polysaccharide antigenemia in rats with experimental endocarditis due to Staphylococcus aureus. J Infect Dis. 1987 Feb;155(2):242–246. doi: 10.1093/infdis/155.2.242. [DOI] [PubMed] [Google Scholar]
- Augustin J., Götz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., An F. Y., White B. A., Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918). J Bacteriol. 1985 Jun;162(3):1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J. M., Bouvet A., Boutonnier A., Audurier A., Goldstein F., Pierre J., Bure A., Lebrun L., Hochkeppel H. K. Predominance of capsular polysaccharide type 5 among oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1987 Oct;25(10):1932–1933. doi: 10.1128/jcm.25.10.1932-1933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J. M., Hannon K., Moreau M., Karakawa W. W., Vann W. F. Isolation of type 5 capsular polysaccharide from Staphylococcus aureus. Ann Inst Pasteur Microbiol. 1987 Sep-Oct;138(5):561–567. doi: 10.1016/0769-2609(87)90041-x. [DOI] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert I. Dissociation in an Encapsulated Staphylococcus. J Bacteriol. 1931 Mar;21(3):157–160. doi: 10.1128/jb.21.3.157-160.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- HALLANDER H. O. PRODUCTION OF LARGE QUANTITIES OF ENTEROTOXIN B AND OTHER STAPHYLOCOCCAL TOXINS ON SOLID MEDIA. Acta Pathol Microbiol Scand. 1965;63:299–305. doi: 10.1111/apm.1965.63.2.299. [DOI] [PubMed] [Google Scholar]
- Hochkeppel H. K., Braun D. G., Vischer W., Imm A., Sutter S., Staeubli U., Guggenheim R., Kaplan E. L., Boutonnier A., Fournier J. M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987 Mar;25(3):526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Sutton A., Schneerson R., Karpas A., Vann W. F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988 May;56(5):1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Betley M. J., Hopkins C. A., Perez N. E., Pier G. B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987 Nov;156(5):741–750. doi: 10.1093/infdis/156.5.741. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Liu M. J., Parsonnet J., Arbeit R. D. Expression of type 8 capsular polysaccharide and production of toxic shock syndrome toxin 1 are associated among vaginal isolates of Staphylococcus aureus. J Clin Microbiol. 1990 Dec;28(12):2612–2615. doi: 10.1128/jcm.28.12.2612-2615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Mathieu L. G., Lecomte J., deRepentigny J. Adherence of gram-positive and gram-negative bacterial strains to human lung fibroblasts in vitro. Exp Biol. 1986;45(4):323–334. [PubMed] [Google Scholar]
- Mathieu L. G., Dubreuil D., Gadbois T., de Repentigny J. In vitro adhesion of Staphylococcus strains to rabbit tissues. Rev Can Biol Exp. 1982 Mar;41(1):3–12. [PubMed] [Google Scholar]
- Mills J. T., Dodel A. W., Kass E. H. Regulation of staphylococcal toxic shock syndrome toxin-1 and total exoprotein production by magnesium ion. Infect Immun. 1986 Sep;53(3):663–670. doi: 10.1128/iai.53.3.663-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A., Karakawa W. W., Vann W. F., Arbeit R. D. Reactivity of type-specific monoclonal antibodies with Staphylococcus aureus clinical isolates and purified capsular polysaccharide. Infect Immun. 1985 Jul;49(1):14–18. doi: 10.1128/iai.49.1.14-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Sabath L. D., Quie P. G. Effect of protein A on staphylococcal opsonization. Infect Immun. 1977 Mar;15(3):760–764. doi: 10.1128/iai.15.3.760-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phonimdaeng P., O'Reilly M., O'Toole P. W., Foster T. J. Molecular cloning and expression of the coagulase gene of Staphylococcus aureus 8325-4. J Gen Microbiol. 1988 Jan;134(1):75–83. doi: 10.1099/00221287-134-1-75. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z., Karakawa W. W., Vann W. F., Schneerson R., Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985 Nov;22(5):828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutra L., Mendolia C., Rainard P., Poutrel B. Encapsulation of Staphylococcus aureus isolates from mastitic milk: relationship between capsular polysaccharide types 5 and 8 and colony morphology in serum-soft agar, clumping factor, teichoic acid, and protein A. J Clin Microbiol. 1990 Mar;28(3):447–451. doi: 10.1128/jcm.28.3.447-451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]