Abstract
Alcohol use has become far too prevalent in our society. Alcohol kills 6.5 times more youth than all other illicit drugs combined. In combination with traumatic and hemorrhagic injuries, alcohol results in a much higher mortality rate. Alcohol, alone and in high dosages, also causes great damage to the body, often leading to death as well. Thus, it is of utmost importance that research is conducted to help explain the pathological mechanism of high fatalities and injuries associated with alcohol use. In order to simulate this complex situation in vitro, a rat hepatoma cell line (H-II-4-E) was exposed to various concentrations of ethanol as well as the condition of hypoxia. Hypoxia mimics the primary level of tissue damage caused by hemorrhage after impact in a car accident. In this way, we tested the hypothesis that the presence of ethanol in combination with hypoxia causes greater cellular damage compared to conditions of ethanol or hypoxia alone. Ethanol, alone and in high concentrations, was found to greatly affect cell function as shown by decreased cellular ATP levels, increased LDH release, and a downregulated expression of CYP2E1 gene. By adding the condition of hypoxia to low concentrations of ethanol, cellular damage increased dramatically as well. Decreased gene expression and protein levels of CYP2E1 correlated with increased hepatocyte injury and thus, this enzyme may significantly contribute to the severity of cellular damage. These results provide useful information for future research on the effects of ethanol in combination with hemorrhage on cells in vitro, simulating the condition of driving while intoxicated and binge drinking.
Keywords: Ethanol, hypoxia, adenosine triphosphate, cytochrome P450 2E1, lactate dehydrogenase
Introduction
In today's industrialized society, car accidents occur frequently on busy highways, slippery roads, and even in safe neighborhoods. It has been reported that an estimated 1 in 50 people gets into a car accident every year. In fact, according to the National Highway Traffic Safety Administration (NHTSA), in 2005 alone, more than six million people in the United States were registered as having had some sort of motor vehicle crash. Over 30% of those accidents resulted in either death or severe injury (http://www-nrd.nhtsa.dot.gov/pdf/nrd-30/NCSA/TSFAnn/TSF2005.pdf). Due to either harsh impact or sharp pieces of debris, severe injuries are often sustained during vehicle accidents. One of the most common and often the most deadly injuries is severe bleeding or hemorrhage, either externally or internally [1]. Trauma victims who experience hemorrhage often undergo a period of shock due to blood loss, causing the cardiovascular system to begin to deteriorate as blood flow is reduced. The reduction in the delivery of oxygen and other nutrients to vital organs due to the decreased blood flow causes damage to the body and, if not corrected, will result in death.
The NHTSA has also found that 39% of the total fatal crashes that occurred in 2005 were alcohol-related. The frightening reality is that alcohol-related motor vehicle crashes kill someone every 31 minutes. Even more frightening is that, excluding accidents and homicides, alcohol alone kills someone every 25 minutes (http://www.cdc.gov/nchs/fastats/alcohol.htm). Although only a few of these deaths are caused by alcohol poisoning, over 65% of the 11 million underage drinkers in the United States are binge drinkers, according to a 2005 National Survey on Drug Use and Health. Binge drinking, which is defined in the United States as having five or more drinks on occasion if one is male, or four or more drinks on occasion if one is female, puts the drinker at a huge risk for alcohol poisoning and possibly death (http://www.msnbc.msn.com/id/17491440/). Young adults who are in their late teens to early twenties also have the highest risk of getting into fatal motor vehicle accidents involving alcohol. Alcohol-related death is not only extremely prevalent within our society, but also affects the bright, young students who are the future of our nation. Thus, it is of utmost importance that research be performed to help explain the pathological mechanisms why so much damage occurs during an alcohol-related vehicle crash and during acute alcohol consumptions such as binge drinking.
In the present report, we used an in vitro cell culture system to reproduce the situation similar to that of bodily injury after driving while intoxicated or binge drinking. Rat hepatoma cells (H-4-II-E cell line) were incubated with different concentrations of ethanol to simulate acute alcohol intake. These cells were either cultured in a normal incubator under room air conditions (21% O2) or under the conditions of hypoxia (1% O2). Hypoxia simulates severe bleeding and organ injury in the cell culture since, during hemorrhage, the reduced blood flow due to a depleted blood supply, deprives the entire body, including vital organs, of life-supporting oxygen. Thus, in vitro hypoxia mimics the primary damage caused by severe hemorrhage. The objective of this study was therefore to determine alterations in the overall function of the hepatocytes by measuring the extent of cellular damage that occurs when cells are exposed to both ethanol and hypoxia or ethanol alone. We hypothesized that the presence of ethanol during hypoxia causes greater damage to the cells compared to the conditions of ethanol alone or hypoxia alone. The extent of cellular damage was assessed by measuring levels of adenosine triphosphate (ATP), lactate dehydrogenase (LDH), and cytochrome P450 2E1 (CYP2E1) following incubation with ethanol alone or in the presence of hypoxia.
Materials and Methods
H-4-II-E Cell Culture
H-4-II-E cells from ATCC (American Tissue Culture Collection; Manassas, VA), originated from Rattus norvegicus hepatoma, were cultured in Eagle's Minimum Essential Medium (ATCC), also called MEM-FBS, containing 10% fetal bovine serum and 100 U/mL penicillin and streptomycin. Cells were cultured at a density of 5 × 104 cells/mL overnight, treated with different concentrations of ethanol (0, 100, 500, 1000 mM), and then cultured either in a normal cell culture incubator (21% O2) or under conditions of hypoxia (1% O2) at 37°C. These concentrations of ethanol have been widely used by other investigators forin vitro studies [2,3]. Hypoxia, which is a subnormal concentration of oxygen, was produced in a sealed chamber containing 1% O2, 5% CO2, and 94% N2, using a tank containing this air mixture. After 24-h incubation, the cell supernatant was collected for LDH measurement. Some of the cells were also lysed for use in ATP assays, and some were also set aside for real-time RT-PCR measurement and Western blot analysis of cytochrome P450 2E1 (CYP2E1), as described below.
ATP Detection Assay
ATP levels were determined using a luminescence ATP detection assay system, ATPlite (PerkinElmer; Boston, MA). ATP is the currency of life [4]. In fact, stored ATP powers all the physiological mechanisms that require energy for operation in the cell. Reduced ATP levels in the liver are a major factor that contributes to liver cell death [5]. The assay was performed as recommended by the manufacturer. Cells were cultured in 100 μL/well 96-well plates. 50 μL of mammalian cell lysis solution was added, and the plate was placed on a shaker for 5 min. Then, 50 μL of the substrate buffer solution (luciferace/lucerin) was added, and the plate was shook again. The plate was placed afterwards in the dark for 10 min and then measured on a luminescence plate reader. The protein concentration in the cell suspension was determined by a DC protein assay kit (Bio-Rad; Hercules, CA) and used to calculate the ratio of ATP in light units per mg of protein.
LDH Measurement
LDH is an intracellular enzyme that participates in the energy conversion of lactate to pyruvate and is relatively rich in the liver [6]. As cells die in the human body, their LDH is released and finds its way into the blood. The LDH in the cell culture supernatant was measured according to the protocol provided by the manufacturer (Pointe Scientific; Canton, MI) in order to assess the extent of damage of the cultured cell. A greater concentration of LDH present in the supernatant would indicate greater amounts of dead and dying cells.
Real-time RT-PCR Assay for CYP2E1 Gene Expression
CYP2E1 is a protein whose expression is induced by the presence of ethanol, among other causes [7]. This enzyme helps metabolize ethanol, as well as other endogenous and exogenous substances. In order to examine and measure the expression of CYP2E1 gene after treatment with ethanol, total RNA was first isolated from the H-4-II-E cells by using TRIzol Reagent (Invitrogen). 2 μg of the isolated RNA was reverse transcribed. The resulting cDNA was diluted, and PCR was performed for CYP2E1 and the house-keeping gene, GAPDH. The forward and reverse primers for the rat CYP2E1 gene were TCCCCAAGTCTTTCACCAAGTT and GAGCCAAGGTGCAGTGTGAAC (Accession #NM_031543), respectively. GAPDH was used as a reference gene (Accession #M17701). Its forward primer was ATGACTCTACCCACGGCAAG, and its reverse primer was CTGGAAGATGGTGATGGGTT. The real-time PCR was performed using the 7300 Real-Time PCR System (Applied Biosystems). The reaction was carried in a 24 μL final reaction volume containing 0.08 μmol forward and reverse primers, 2 μL cDNA, 9.2 μL H2O and 12 μL SYBR Green PCR Mast Mix (Applied Biosystems). The thermal profile for the real-time PCR was 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The gene expression was then expressed in fold changes, according to the formula for real-time PCR data analysis as given in the real-time PCR kit. Also, a melting curve analysis was performed in order to confirm the specificity of the PCR product in this experiment.
CYP2E1 Protein Expression Assessment
Total protein (10 μg) from H-4-II-E cells was loaded on 4-12% Bis-Tris gels (Invitrogen; Carlsbad, CA) and electrophoretically fractionated in MES-SDS running buffer (Invitrogen). The protein on the gel was then transferred to a 0.45-μm nitrocellulose membrane, and the non-specific binding sites were blocked with 5% nonfat milk in 10 mM Tris-HCl with 0.1% Tween 20, pH 7.5 (TBST). The membrane was incubated with 1:1000 dilution of rabbit anti-rat CYP2E1 polyclonal antibody (Santa Cruz Biotechnology, CA) overnight at 4°C followed by incubation in 1:10,000 HRP-linked anti-rabbit IgG for 1 h at room temperature. The same membrane was stripped and re-blotted with anti-β-actin monoclonal antibody (1:20,000; Sigma; St. Louis, MO) as the loading control. The changes of Western blots were detected with chemiluminescence (ECL) Western blot detection reagent (Amersham; Piscataway, NJ) and exposed on X-ray films. A Bio-Rad GS-800 Calibrated Densitometer analysis system (BioRad; Hercules, CA) was used to quantitate the density of the bands in the Western blots.
Statistical Analysis
All data was expressed as mean ± SE (n=6-8) and compared with the one-way analysis of variance (ANOVA) and the Tukey's test. Differences in values were considered significant if P<0.05.
Results
Effects of Ethanol on H-II-4-E Cells
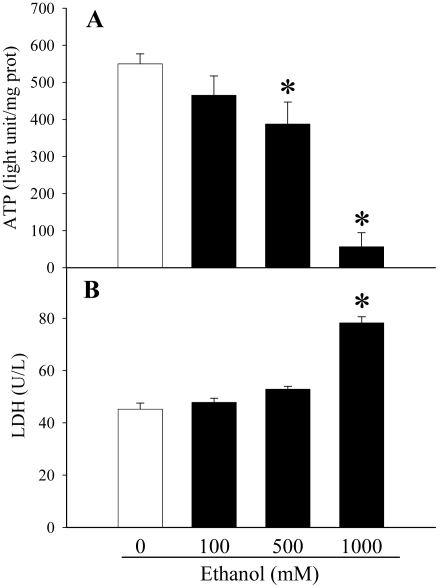
We first determined the effects of different concentrations of ethanol (0, 100, 500, 1000 mM) on the hepatoma cells to assess the damage caused to the cells by ethanol alone using ATP detection assays, LDH assays, and real-time RT-PCR for CYP2E1. The results of the ATP detection assays demonstrated that the concentration of ATP decreased as the concentrations of ethanol increased. With the addition of 500 mM ethanol to the cells, ATP levels had already decreased significantly compared to those of the control (0 mM ethanol) (decreased by 29.6%, P<0.05, Figure 1A). An especially dramatic decrease was observed at 1000 mM ethanol (decreased by 89.9%, P<0.05, Figure 1A).
Figure 1.
Alterations in cellular ATP and supernatant LDH levels in ethanol-treated H-4-II-E cells. A. Cellular levels of ATP were assayed from H-4-II-E cells treated with 0, 100, 500, or 1000 mM ethanol for 24 h. B. Supernatant levels of LDH were measured from H-4-II-E cells treated with 0, 100, 500, or 1000 mM ethanol for 24 h. Data is presented as mean ± SE (n=6-8) and compared by one-way ANOVA and Tukey's test: *P<0.05 versus 0 mM ethanol control group.
The data collected from LDH assays followed a similar but opposite pattern. The LDH concentration outside of the cells increased as the concentration of ethanol exposed to the cell increased. LDH, which was measured by testing the cell supernatant, is a marker for cellular damage. Thus, it is not surprising that the data showed a significant increase of the concentration of LDH when comparing 1000 mM ethanol to the control (increased by 72.9%, P<0.05, Figure 1B).
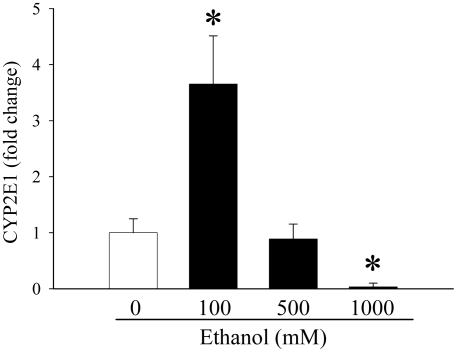
As shown in Figure 2, CYP2E1 gene expression in the H-II-4-E cells was found to have increased as ethanol concentration was raised to 100 mM, jumping by 265% (P<0.05). However, when ethanol concentration was quintupled to 500 mM, CYP2E1 gene expression actually decreased dramatically, dropping 75.7% as compared to 100 mM ethanol. Thus, it seems that if the cells are exposed to a higher concentration of ethanol, such as 500 mM, CYP2E1 gene expression is actually inhibited to levels similar to that of the control. CYP2E1 gene expression at an ethanol concentration of 1000 mM followed this pattern and thus, was suppressed even further (decreased by 96.6% compared to 0 mM ethanol), indicating an even greater inhibition of CYP2E1 production within the cell (Figure 2). The data prove that CYP2E1, an enzyme which is able to metabolize ethanol, cannot be effectively expressed. This may be one of the primary reasons why cellular damage increases so dramatically as the concentrations of ethanol increase to 1000 mM.
Figure 2.
Alterations in CYP2E1 gene expression in ethanol-treated H-4-II-E cells. The expression of CYP2E1 was determined in H-4-II-E cells treated with 0, 100, 500, or 1000 mM ethanol for 24 h. Data (fold change) is presented as mean ± SE (n=6-8) and compared by one-way ANOVA and Tukey's test: *P<0.05 versus 0 mM ethanol control group.
Effects of Ethanol in Combination with Hypoxia on H-II-4-E Cells
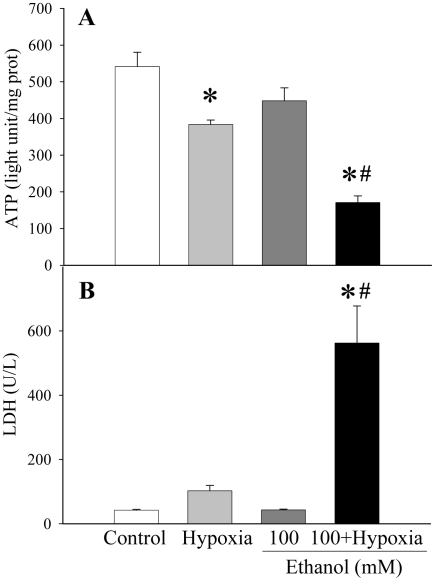
Based on the above observations, the presence of high concentration of ethanol alone has already caused damage to the hepatocyte. By introducing hypoxia, another condition that causes cellular injury, the damage should, thus, increase. Based on the data collected from ATP assays, as shown in Figure 3A, hypoxia alone had a modest, but statistically significant effect on mitochondrial function within a cell (reduction of cellular ATP levels by 29.1%). Similarly, comparing the concentration of ATP in 100 mM ethanol-treated cells to that of the control, the decrease of ATP was 17.2% (no statistical significance). However, when comparing 100 mM ethanol in combination with hypoxia (1% O2) to the control, the decrease in ATP was 68.4% greater (P<0.05 vs control, hypoxia alone, or ethanol alone) (Figure 3A). Thus, hypoxia, in combination with a low-concentration ethanol, causes much greater cellular damage than ethanol or hypoxia does alone.
Figure 3.
Alterations in cellular ATP and supernatant LDH levels in H-4-II-E cells. A. Cellular levels of ATP were assayed from H-4-II-E cells treated with hypoxia (1% O2) alone, 100 mM ethanol alone, or combination of both for 24 h. B. Supernatant levels of LDH were measured from H-4-II-E cells with hypoxia (1% O2) alone, 100 mM ethanol alone, or combination of both for 24 h. Data is presented as mean ± SE (n=6-8) and compared by one-way ANOVA and Tukey's test: *P<0.05 versus control group; #P<0.05 versus hypoxia alone or 100 mM ethanol only group.
The data from the LDH assays reinforces the idea that hypoxia combined with ethanol causes greater damage to cells than ethanol or hypoxia alone (Figure 3B). When the ethanol concentration was raised from 0 to 100 mM, the LDH concentration in the cell supernatant barely changed from less than 50 U/L. Although hypoxia increased LDH levels by 144%, the increase is not statistically significant (Figure 3B). In contrast, as soon as the 100 mM ethanol-treated cells were placed under the condition of hypoxia, the LDH concentration sky-rocketed by more than 12.4 folds (P<0.05, Figure 3B).
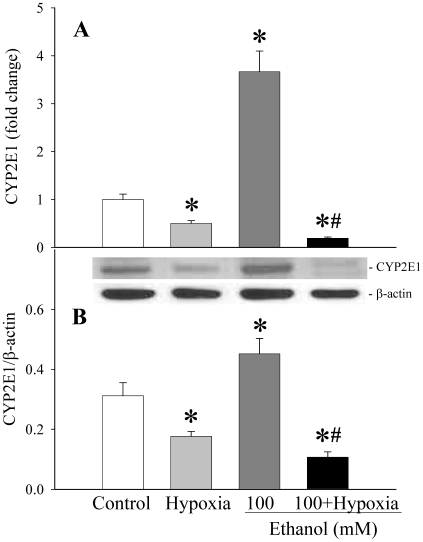
Even at a low concentration of ethanol in combination with hypoxia, the expression of CYP2E1 gene was inhibited. Figure 4A shows that although the expression of CYP2E1 increased dramatically in 100 mM ethanol (P<0.05), the same concentration of ethanol in combination with hypoxia inhibited the expression of CYP2E1 by 94.8% compared to 100 mM ethanol alone (decreased by 81% compared to control, P<0.05). Similarly, CYP2E1 protein levels increased by 44.9% at 100 mM ethanol (P<0.05), and ethanol plus hypoxia decreased CYP2E1 protein levels in the cell by 76.3% compared to ethanol alone (P<0.05, Figure 4B). In contrast, hypoxia alone only decreased CYP2E1 gene and protein expression by 50% and 43.6% (P<0.05), respectively (Figures 4A–4B).
Figure 4.
Alterations in CYP2E1 gene and protein expression levels in H-4-II-E cells. The CYP2E1 gene (A) and protein expression levels (B) were determined in H-4-II-E cells treated with hypoxia (1% O2) alone, 100 mM ethanol alone, or in combination for 24 h. Data is presented as mean ± SE (n=6-8) and compared by one-way ANOVA and Tukey's test: *P<0.05 versus control group; #P<0.05 versus 100 mM ethanol only group.
Discussion
Drunk driving has been a major problem in our society, and especially affects young adults in their late teens to early twenties [8]. Binge drinking, which is defined as having five or more drinks per occasion for males and four or more drinks per occasion for females [9], is also extremely relevant and again, affects youth of our modern world. Thus, in order to reduce the percentage of alcohol-related deaths, more research must be conducted and a better method of health care must be implemented. Not including drunk driving scenarios, excessive alcohol consumption is the third leading cause of controlled death in the United States with 12,000 deaths each year, and also contributes to alcoholic liver disease [10]. The pathogenesis of chronic and acute alcohol consumption is complex and has diverse consequences in different tissues and cell types. Alcohol-induced liver injury is marked by pathological changes in the liver ranging from steatosis, steatohepatitis to cirrhosis and even hepatocellular carcinoma [11]. Proinflammatory cytokines such as TNF-α and IL-1β, appear to play an important role in chronic alcohol-induced liver injury [11–15]. Another factor is reactive oxygen species (ROS), which can negatively affect hepatocyte function [16]. It is widely accepted that ROS not only causes direct hepatocyte injury but also contributes to increased inflammatory responses which, in turn, cause further liver injury. Alcohol-induced production of ROS can cause mitochondrial damage, leading to mitochondrial dysfunction and causing lipid peroxidation and protein modification [17]. Moreover, hepatic metabolism of alcohol can cause oxygen deficiencies or hypoxia, which in turn impedes the liver cells’ ability to produce ATP and thus contributes to cell death [18]. While chronic alcohol consumption causes liver injury through multiple factors such as oxidative stress, hypoxia, and cytokines, acute alcohol injury, especially in combination with trauma and hemorrhage, appears to have different pathophysiological responses.
In this study, the simulation of acute alcohol injury was accomplished by exposing hepatoma cells to various concentrations of ethanol for 24 h, as if to mimic acute alcohol consumption. As such, this project does not determine the effects of long-term (chronic) alcohol use or alcoholism. The hepatoma cells were also exposed to the condition of hypoxia (1% O2). This low level of oxygen simulates the aspect of a dunk driving accident in which the passenger or driver experiences internal or external hemorrhage caused by the impact. Cells exposed to high concentrations of ethanol simulated binge drinking. The goal of this project was to determine whether or not the combination of ethanol and hypoxia would cause greater damage than the condition of ethanol alone and to discover the possible reasons for alcohol-induced cellular damage both in the presence or absence of hypoxia. Although both cases (drunk driving accidents and binge drinking) can result in death, greater damage is expected in the case of exposing cells to both ethanol and hypoxia.
The results of the ATP detection assays revealed that mitochondrial function decreased more significantly in cells exposed to both ethanol and hypoxia compared to cells exposed only to ethanol. Thus, cells under the influence of ethanol and hypoxia were less able to properly function since ATP powers all the physiological mechanisms that require energy for operation in the cell. In fact, since reduced ATP levels in the liver is one major factor that contributes to liver cell death [18], it is highly probable that cells exposed to concentrations of 500 mM and especially 1000 mM of ethanol were dying. Therefore, binge drinking does have a significant effect on cellular ATP production. A similar low concentration of ATP in the cells was found when cells exposed to 100 mM and hypoxia were tested. Thus, the data shows that low concentrations of ethanol in vitro can cause as much damage as high concentrations of ethanol when combined with the condition of hypoxia.
The results of the LDH assay proved that when ethanol concentrations increase, the LDH concentration outside of the cell (in the cell supernatant) increases as well. LDH is an intracellular enzyme and therefore, is not naturally found in the cell supernatant. When cells die or when the cell membrane is damaged, LDH is released into the environment. Therefore, when cells are exposed to large concentrations of ethanol such as 1000 mM, they are greatly damaged and are perhaps already dying or dead, as was shown in the ATP data above. On the other hand, when 100 mM ethanol is combined with hypoxia, the LDH concentration in the cell supernatant increases dramatically, far above the concentration of LDH in the supernatant of cells treated with 1000 mM ethanol alone. Thus, although cells are affected by high concentrations of ethanol, far more damage and cell death seems to occur when the condition of hypoxia is added even when the ethanol concentration is considered to be low.
The CYP2E1 gene expression in the cells revealed a different, but equally interesting finding. CYP2E1 is induced by ethanol, and its most important function is to metabolize toxic substances such as ethanol. Thus, with the acute presence of low levels of ethanol, the expression of CYP2E1 gene should have a detoxicating effect on the cells. However, the data collected from real-time RT-PCR revealed that at higher concentrations of ethanol such as 500 mM, CYP2E1 gene expression is decreased to a level similar to that of the control. At 1000 mM ethanol, the expression of CYP2E1 gene was much lower than that of the control. It, therefore, seems that high concentrations of ethanol inhibited the production of the beneficial enzyme, CYP2E1. This decrease in the production of CYP2E1 would allow the ethanol to cause greater damage to the cells which are thus, unable to metabolize the toxic substance. The inhibition of CYP2E1 at least in part explains the cellular and bodily damage that is caused by binge drinking. The data also shows that low concentrations of ethanol in combination with hypoxia inhibit the expression of CYP2E1 at mRNA and protein levels as well. Thus, damage is being done to the cells since CYP2E1 would be unable to metabolize ethanol.
It should be pointed out that CYP2E1 is responsible for producing ROS in chronic alcohol consumptions and ROS is related to liver injury under such conditions [19–21]. Thus, overexpression of CYP2E1 for a prolonged period of time may cause liver damage. In the present study, since the induced CYP2E1 is also responsible for metabolizing ethanol [20, 22], this enzyme was measured to represent the cell's ability to metabolize cellular ethanol. The findings that high concentrations of ethanol or low concentrations of ethanol in combination with hypoxia markedly downregulated CYP2E1 expression suggest that hepatoma cells lost their capacity to metabolize the cellular ethanol.
In summary, the presence of ethanol during hypoxia causes greater damage to the cells than similar concentrations of ethanol alone. High concentrations of alcohol cause a comparable degree of damage as low concentrations of alcohol in combination with hypoxia. The induced enzyme, CYP2E1, appears to have a significant effect on the cell's ability to protect itself from the damage caused by acute ethanol consumption. When CYP2E1 gene expression is inhibited, greater damage is inflicted on the cell by high concentrations of ethanol or low concentrations of ethanol in combination with hypoxia. This experiment provides much useful information for future research on the effects of ethanol in combination with hemorrhage on in vitro cells in the simulation of driving while intoxicated and binge drinking. In our future studies, we will further investigate the effects of ethanol and hypoxia on hepatic functions using primary cultures of hepatocytes as well as alcohol-intoxicated hemorrhaged rats.
Acknowledgments
The authors greatly appreciate the constructive suggestions and comments received from Mrs. Irena Tsarevsky. We also would like to sincerely thank Wayne Chaung, PhD, for his kind help with the experiment.
References
- 1.Sommers MS, Dyehouse JM, Howe SR, Fleming M, Fargo JD, Schafer JC. Effectiveness of brief interventions after alcohol-related vehicular injury: A randomized controlled trial. J Trauma. 2006;61:523–531. doi: 10.1097/01.ta.0000221756.67126.91. [DOI] [PubMed] [Google Scholar]
- 2.Mitra SK, Varma SR, Godavarthi A, Nandakumar KS. Liv.52 regulates ethanol induced PPARgamma and TNF alpha expression in HepG2 cells. Mol Cell Biochem. 2008;315:9–15. doi: 10.1007/s11010-008-9782-9. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramaniyan V, Shukla R, Murugaiyan G, Bhonde RR, Nalini N. Mouse recombinant leptin protects human hepatoma HepG2 against apoptosis, TNF-alpha response and oxidative stress induced by the hepatotoxinethanol. Biochim Biophys Acta. 2007;1770:1136–1144. doi: 10.1016/j.bbagen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida M, Kinosita K. Mechanically driven ATP synthesis by F1-ATPase. Nature. 2004;427:465–468. doi: 10.1038/nature02212. [DOI] [PubMed] [Google Scholar]
- 5.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad LO, Mirandola SR, Maciel EN, Castilho RF. Lactate dehydrogenase activity is inhibited by methylmalonate in vitro. Neurochem Res. 2006;31:541–548. doi: 10.1007/s11064-006-9054-6. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Windle M. Alcohol use among adolescents and young adults. Alcohol Res Health. 2003;27:79–85. [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffer M, Jeglic EL, Stanley B. The Relationship between Suicidal Behavior, Ideation, and Binge Drinking among College Students. Arch Suicide Res. 2008;12:124–132. doi: 10.1080/13811110701857111. [DOI] [PubMed] [Google Scholar]
- 10.Mantena SK, King AL, Andringa KK, Landar A, rley-Usmar V, Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver disease. World J Gastroenterol. 2007;13:4967–4973. doi: 10.3748/wjg.v13.i37.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H, Takei Y, Hirose M, Shimizu H, Miyazaki A, Brenner DA, Sato N, Thurman RG. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15:D20–D25. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 12.Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 13.Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis. 2004;24:257–272. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- 14.Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–1326. doi: 10.1002/hep.510280521. [DOI] [PubMed] [Google Scholar]
- 15.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 16.Zima T, Kalousova M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:110S–115S. doi: 10.1097/01.alc.0000189288.30358.4b. [DOI] [PubMed] [Google Scholar]
- 17.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham CC, Van Horn CG. Energy availability and alcohol-related liver pathology. Alcohol Res Health. 2003;27:291–299. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuge J, Cederbaum AI. Increased toxicity by transforming growth factor-beta 1 in liver cells overexpressing CYP2E1. Free Radic Biol Med. 2006;41:1100–1112. doi: 10.1016/j.freeradbiomed.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuge J, Cederbaum AI. Depletion of Sadenosyl-l-methionine with cycloleucine potentiates cytochrome P450 2E1 toxicity in primary rat hepatocytes. Arch Biochem Biophys. 2007;466:177–185. doi: 10.1016/j.abb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koop DR. Alcohol metabolism's damaging effects on the cell: a focus on reactive oxygen generation by the enzyme cytochrome P450 2E1. Alcohol Res Health. 2006;29:274–280. [PMC free article] [PubMed] [Google Scholar]