Abstract
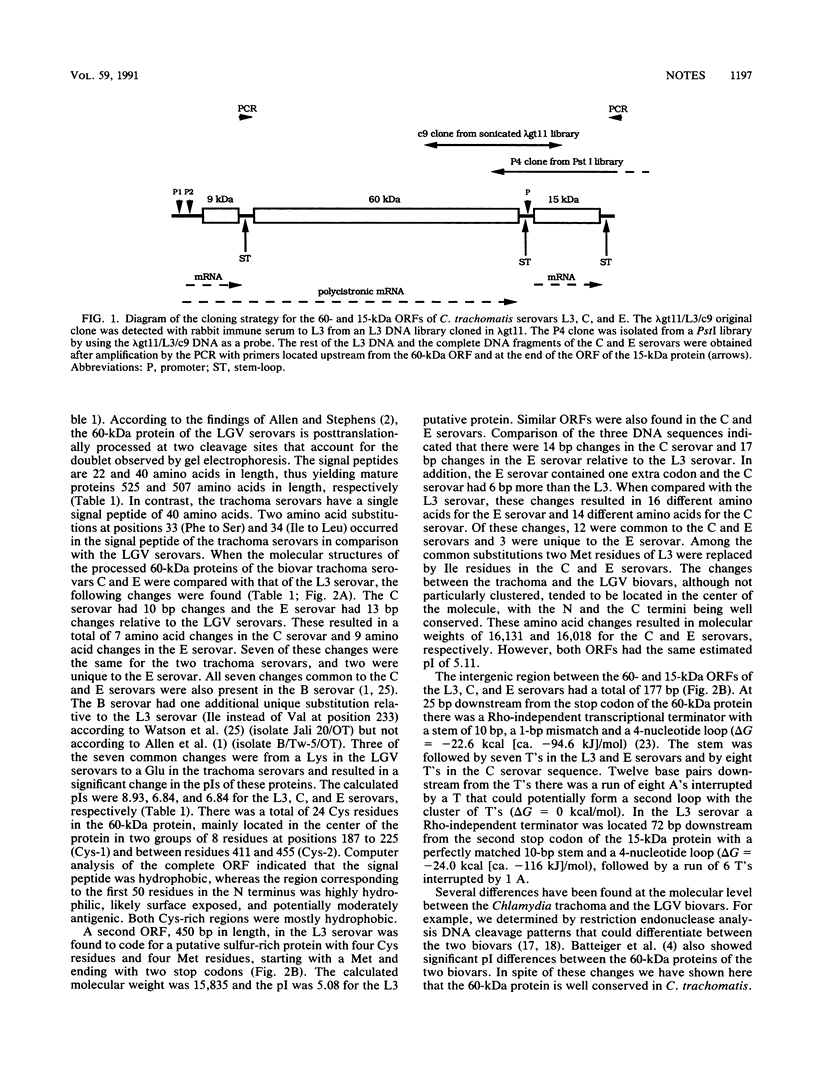
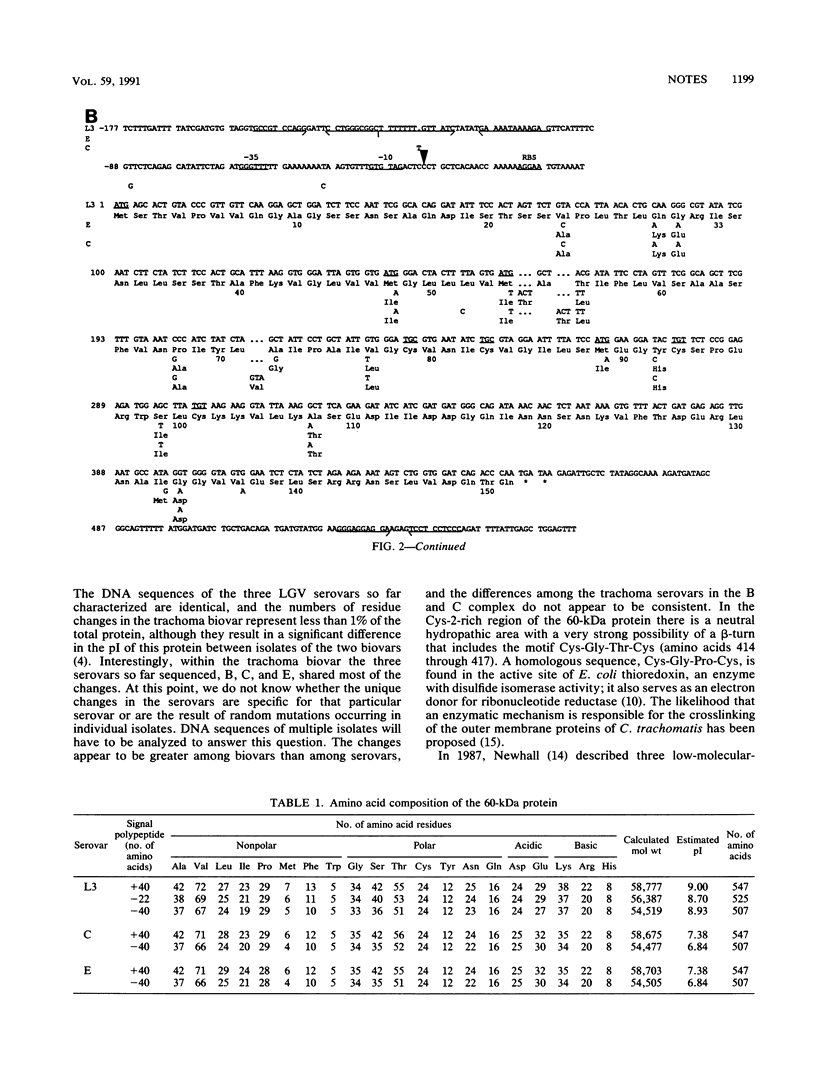
DNA from Chlamydia trachomatis serovars L3, C, and E corresponding to the open reading frames of the 60-kDa protein and of a putative 15-kDa protein was sequenced. The open reading frames coding for the 60-kDa protein had 1,641 bp in the three serovars. Compared with the L3 serovar, there were 9 and 11 amino acid changes in the C and E serovars, respectively. The open reading frames corresponding to the putative 15-kDa protein had 450, 456, and 453 bp for the L3, C, and E serovars, respectively. When compared with the L3 serovar, the C and E serovars had 14 and 16 amino acid differences, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Cerrone M. C., Beatty P. R., Stephens R. S. Cysteine-rich outer membrane proteins of Chlamydia trachomatis display compensatory sequence changes between biovariants. Mol Microbiol. 1990 Sep;4(9):1543–1550. doi: 10.1111/j.1365-2958.1990.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Allen J. E., Stephens R. S. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989 Jan;171(1):285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Newhall W. J., 5th, Jones R. B. Differences in outer membrane proteins of the lymphogranuloma venereum and trachoma biovars of Chlamydia trachomatis. Infect Immun. 1985 Nov;50(2):488–494. doi: 10.1128/iai.50.2.488-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E. J., Peterson E. M., de la Maza L. M. Cloning and characterization of a Chlamydia trachomatis L3 DNA fragment that codes for an antigenic region of the major outer membrane protein and specifically hybridizes to the C- and C-related-complex serovars. Infect Immun. 1989 Feb;57(2):487–494. doi: 10.1128/iai.57.2.487-494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke I. N., Ward M. E., Lambden P. R. Molecular cloning and sequence analysis of a developmentally regulated cysteine-rich outer membrane protein from Chlamydia trachomatis. Gene. 1988 Nov 30;71(2):307–314. doi: 10.1016/0378-1119(88)90047-9. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Roy K. L., Wenman W. M. Cloning, expression, and primary structure of a Chlamydia trachomatis binding protein. J Bacteriol. 1987 Nov;169(11):5152–5156. doi: 10.1128/jb.169.11.5152-5156.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Everson J. S., Ward M. E., Clarke I. N. Sulfur-rich proteins of Chlamydia trachomatis: developmentally regulated transcription of polycistronic mRNA from tandem promoters. Gene. 1990 Mar 1;87(1):105–112. doi: 10.1016/0378-1119(90)90500-q. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., 5th Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun. 1987 Jan;55(1):162–168. doi: 10.1128/iai.55.1.162-168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., Schachter J., de la Maza L. M. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid. 1990 Mar;23(2):144–148. doi: 10.1016/0147-619x(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Characterization of Chlamydia DNA by restriction endonuclease cleavage. Infect Immun. 1983 Aug;41(2):604–608. doi: 10.1128/iai.41.2.604-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Restriction endonuclease analysis of DNA from Chlamydia trachomatis biovars. J Clin Microbiol. 1988 Apr;26(4):625–629. doi: 10.1128/jcm.26.4.625-629.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wagar E. A., Schachter J., Bavoil P., Stephens R. S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990 Oct;162(4):922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- Watson M. W., Lambden P. R., Ward M. E., Clarke I. N. Chlamydia trachomatis 60 kDa cysteine rich outer membrane protein: sequence homology between trachoma and LGV biovars. FEMS Microbiol Lett. 1989 Dec;53(3):293–297. doi: 10.1016/0378-1097(89)90233-4. [DOI] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Watkins N. G., Stewart S., Caldwell H. D. The low-molecular-mass, cysteine-rich outer membrane protein of Chlamydia trachomatis possesses both biovar- and species-specific epitopes. Infect Immun. 1987 Nov;55(11):2570–2573. doi: 10.1128/iai.55.11.2570-2573.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


