Abstract
Background
Chronic granulomatous disease (CGD) is a rare genetic disorder, predisposing affected individuals to recurrent infectious complications and reduced survival. Liver involvement in CGD includes vascular abnormalities, which may lead to non-cirrhotic portal hypertension.
Methods
To evaluate the impact of non-cirrhotic portal hypertension on survival in CGD, all records from 194 patients followed at the NIH with CGD were reviewed. Cox proportional hazards regression was used to determine factors associated with mortality.
Results
Twenty-four patients died, all of infectious complications. By Cox regression, factors associated with mortality were: (1) decreases in platelet count (>9,000/µl per year; HR 4.7, p=0.007), (2) alkaline phosphatase elevations (>0.25 per year; HR 4.5, p=0.01) and (3) history of liver abscess (HR 3.1, p=0.03). By regression analysis, decreasing platelet count was associated with increasing portal vein diameter, splenomegaly, elevated serum IgG and increasing number of ALT elevations; greater number of alkaline phosphatase elevations and abscess were both associated with increasing age and number of infections. Prospective evaluation revealed elevated hepatic-venous pressure gradients in two patients with progressive thrombocytopenia, suggestive of portal hypertension.
Conclusions
These data suggest mortality in patients with CGD is associated with the development of non-cirrhotic portal hypertension, likely due to injury to the microvasculature of the liver from repeated systemic and hepatic infections. The slope of decline in platelet count may be a useful measure of progression of portal hypertension over time. Furthermore the data illustrate the potential independent effect of portal hypertension on clinical outcome outside the setting of cirrhosis.
Keywords: chronic granulomatous disease (CGD), non-cirrhotic portal hypertension, hepatic venous pressure gradient (HVPG), mortality, thrombocytopenia, platelet slope, nodular regenerative hyperplasia (NRH), venopathy
Introduction
Chronic granulomatous disease (CGD) is a rare inherited disorder characterized by impaired ability of phagocytic cells in killing certain bacteria and fungi1. Affected individuals are predisposed to recurrent infectious complications leading to significantly reduced long-term survival1–3. Of the four known genotypes of CGD, the most common mutation is the X-linked gp91phox, which is found in 70% of individuals and portends a worse prognosis. Aggressive surgical and medical management, including prophylactic antimicrobials and interferon-gamma, have resulted in a dramatic reduction in mortality, with many patients now surviving well into adulthood4–6.
As patients are living longer, non-infectious consequences of CGD are emerging. Liver disease was recently reported to be a common occurrence in patients with CGD7. In addition to liver enzyme abnormalities, many patients develop hepatic and splenic enlargement. Vascular abnormalities including portal and central venopathy as well as established nodular regenerative hyperplasia (NRH) are commonly found and are known causes of portal hypertension.
In patients with cirrhosis, the development of portal hypertension portends a poor prognosis. In addition to overt complications of increased portal pressure, patients with cirrhosis and portal hypertension are less able to tolerate systemic insults, particularly infection8–10. Furthermore, infection itself may predispose portal hypertensive patients to develop potentially fatal secondary complications such as variceal hemorrhage and hepatorenal syndrome11, 12. Septic episodes may further exacerbate existing portal hypertension as a consequence of the hyperdynamic circulatory response13.
Little is known about the consequences of portal hypertension in the absence of cirrhosis. The recognition that patients with CGD appear to develop non-cirrhotic portal hypertension raises the question of how to assess this complication longitudinally and whether it impacts on disease outcome.
Materials and Methods
A large cohort of patients with CGD has been followed at the Clinical Center of the National Institutes of Health as part of a natural history protocol. All patients had the diagnosis of CGD confirmed by either nitroblue tetrazolium reduction or dihydrorhodamine oxidation14. The specific gene defect was determined by immunoblotting and/or sequencing.
Clinical Data
All patient charts were reviewed and laboratory and clinical data were recorded. Autopsy reports were reviewed when available.
Radiological studies were reviewed with particular attention to liver and spleen size (in relation to body size) and portal hypertensive changes. Portal vein diameter was measured on the most recent contrast-enahanced CT scan available. Measurements were made between the confluence of the splenic and superior mesenteric veins and the bifurcation of the portal vein. If possible, measurements were made on multiple CT images and the final value was taken as the average of at least three measurements. One hepatopathologist reviewed all liver biopsy and wedge resection specimens. All specimens were examined at least 2 cm distant from liver abscesses to avoid peri-abscess changes.
Because data were collected longitudinally, liver enzyme elevations were categorized based on the predominant pattern over time using age-appropriate reference ranges7. Alkaline phosphatase (ALP) elevations were confirmed of liver origin by measuring gamma-glutamyl transpeptidase (GGT). Elevations of ALT or ALP were considered distinct only if a normal value was noted between elevations. Baseline laboratory values were averaged over the first 3 months of follow-up. Due to the association with portal hypertension, immunoglobulin G (IgG) levels were averaged over the final 6 months of follow-up.
Improvements in the treatment of CGD have occurred as antifungal treatment has evolved. Amphotericin was introduced in 1975 and used extensively until it was replaced with voriconazole (1995) and subsequently with posaconazole (2003)6. Interferon gamma 15 and trimethoprim-sulfamethoxazole16 prophylaxis were started in 1990 and fluoroquinolone use became widespread in 1995. To evaluate the potential confounding effect of treatment modifications, patients were divided into treatment cohorts of pre-1975, 1975–1990, 1990–1995 and 1995–2007.
Portal Pressure Measurement
Hepatic-portal venous pressure gradient (HVPG) was determined using standard venous catheterization techniques17. All measurements were repeated at least 3 times. Portal hypertension was defined as HVPG greater than 5 mmHg.
Platelet Slopes
To evaluate the change in platelet count over time, the slope of platelet change per year of follow-up was calculated. To reduce the effect of frequent repeated measures and transient fluctuations, average platelet counts for each week were used for slope determination. Platelet slopes were calculated for patients with at least 4 months of follow-up and platelet measurements in 3 separate weeks.
Statistical Analyses
Baseline categorical variables were compared using X2 or Fisher’s exact test and continuous variables using the student’s t-test or one-way ANOVA. Statistical analyses were performed using SAS version 9.1 and Stata version 9.2.
Factors associated with mortality were evaluated using Cox proportional hazards regression. Time to event was calculated using date of first laboratory data at the NIH and date of final follow-up or death. Variables collected longitudinally were converted to time-dependent covariates for Cox regression. Covariates with p values ≤ 0.05 by univariate analysis were entered into multivariable models and factors of clinical importance were also evaluated to exclude important confounding. Final survival models were developed using multivariable Cox regression to estimate hazard ratios (HR) with associated 95% confidence intervals (95% CI). Proportional hazards assumptions were evaluated using Schoenfeld’s residuals and plots of hazard functions versus time. Using Cox regression models, estimates of survival functions for each covariate and the model as a whole were plotted18 19.
After development of a model for mortality, univariate and multivariable logistic regression were used to determine odds ratios (OR) for factors associated with each determinant of mortality.
Results
A total of 194 patients with CGD were evaluated. Thirty-one patients were excluded because of inadequate clinical follow-up (<4 months), including 2 patients who died. Characteristics of the remaining 163 patients are shown in Table 1.
Table 1.
Characteristics of patients who died and those alive at the end of follow-up
| Factor | Patients Alive at End of Follow-Up | Patients who Died | p-value |
|---|---|---|---|
| Mean ± SD/Number (%) | n=141 | n=22 | |
| Male (%) | 108 (77) | 19 (86) | 0.304 |
| Race (%) | |||
| Caucasian | 119 (84) | 19 (86) | 0.729 |
| African American | 18 (13) | 3 (14) | |
| Asian | 4 (3) | 0 (0) | |
| Age (median, range) [years] | 19.3 | 21.6 | 0.468 |
| [3.1–48.5] | [5.6–63.9] | ||
| Years of Follow-up | 10.9 ± 6.7 | 8.0 ± 5.4 | 0.054 |
| (mean ± sd), [range] | [0.40–28.9] | [0.34–18.3] | |
| Body Mass Index (kg/m2) | 21.2 ± 4.9 | 18.9 ± 4.4 | 0.048 |
| CGD Genotype | |||
| gp91phox | 86 (61) | 15 (68) | 0.754 |
| p47phox | 46 (33) | 5 (23) | |
| p67phox | 1 (1) | 1 (5) | |
| p22phox | 4 (3) | 0 | |
| Unknown | 4 (3) | 1 (5) | |
| Hepatomegaly (%) | 42 (30) | 12 (55) | 0.022 |
| Splenomegaly (%) | 72 (51) | 15 (68) | 0.134 |
| Hepatic Calcifications (%) | 29 (21) | 9 (41) | 0.036 |
| Ascites (%) | 11 (8) | 8 (36) | <0.0001 |
| Portal Vein Diameter [mm]# | 12.7 ± 2.6 | 13.7 ± 1.5 | 0.32 |
| Portal Vein > 13mm (%) | 28/65 (43) | 6/7 (86) | 0.032 |
| Baseline Laboratory Values* | |||
| Hemoglobin (g/dl) | 11.9 ± 1.5 | 12.2 ± 1.7 | 0.401 |
| White Blood Cell (k/µl) | 9.2 ± 9.8 | 7.9 ± 2.6 | 0.557 |
| Platelet (k/µl) | 326 ± 109 | 286 ± 102 | 0.095 |
| Prothrombin Time (seconds) | 12.9 ± 0.78 | 13.2 ± 0.8 | 0.149 |
| ALT (U/L) | 27.4 ± 22.2 | 24.9 ± 15.7 | 0.610 |
| AST (U/L) | 32.7 ± 19.6 | 26.2 ± 9.1 | 0.127 |
| ALP (U/L) | 197 ± 102 | 199 ± 79 | 0.906 |
| GGT (U/L) † | 44.2 ± 79 | 199 ± 346 | <0.0001 |
| Bilirubin, Total (mg/dl) | 0.38 ± 0.28 | 0.43 ± 0.30 | 0.492 |
| Bilirubin, Direct (mg/dl) | 0.09 ± 0.17 | 0.12 ± 0.19 | 0.565 |
| Albumin (g/dl) | 4.1 ± 0.45 | 4.0 ± 0.45 | 0.153 |
| Creatinine (mg/dl) | 0.69 ± 0.30 | 0.87 ± 0.31 | 0.013 |
| Blood Urea Nitrogen (mg/dl) | 13.2 ± 12.4 | 16.3 ± 8.4 | 0.017 |
| IgG (mg/dl)‡ | 1160 ± 472 | 1315 ± 496 | 0.173 |
| Platelet slope (k/µl/year) | −6.70 ± 15.8 | −20.5 ± 32.2 | 0.003 |
| Platelet Slope < −9 k/µl/year (%) | 40/132 (30) | 15/19 (79) | <0.0001 |
| ALP elevations/year | 0.22 ± 0.03 | 0.50 ± 0.36 | 0.0007 |
| ALP elevations > 0.25/year (%) | 44 (31) | 15 (68) | 0.001 |
| Number of Liver abscesses | 0.45 ± 0.78 | 0.86 ± 0.71 | 0.022 |
| History of Liver Abscess (%) | 47 (33) | 15 (68) | 0.002 |
| ALT elevations/year | 0.23 ± 0.31 | 0.34 ± 0.34 | 0.132 |
| Number of Hospital Admissions | 11.9 ± 15 | 16.6 ± 17 | 0.179 |
| CGD Treatment Cohort | |||
| 1 (pre-1975) | 0 | 0 | <0.0001 |
| 2 (1975–1990) | 2 (1) | 2 (9) | |
| 3 (1990–1995) | 3 (2) | 6 (27) | |
| 4 (1995–2006) | 136 (97) | 14 (64) | |
| Infections | |||
| Bacterial | 3.2 ± 4.6 | 3.8 ± 3.9 | 0.544 |
| Fungal | 1.4 ± 2.3 | 2.8 ± 2.5 | 0.009 |
| Total | 4.6 ± 6.0 | 6.6 ± 5.9 | 0.143 |
| Liver Enzyme Elevation Pattern(7) | |||
| Hepatocellular (%) | 30 (21) | 3 (14) | 0.237 |
| Cholestatic (%) | 35 (25) | 5 (23) | |
| Mixed (%) | 25 (18) | 8 (36) | |
| Normal/Near Normal (%) | 51 (36) | 6 (27) | |
| Liver Biopsy Findings | |||
| Central Venopathy (%) | 14/21 (67) | 6/9 (67) | 1.000 |
| Portal Venopathy (%) | 18/21 (86) | 6/9 (67) | 0.232 |
| Any Venopathy (%) | 19/21 (91) | 7/9 (78) | 0.348 |
| Regenerative Changes (%) | 10/19 (53) | 6/9 (67) | 0.646 |
| NRH (%) | 2/19 (11) | 6/15 (40) | 0.044 |
Portal vein measurements made from most recent contrast-enhanced CT scans were available for 72 patients.
Baseline laboratory values equal to mean of first 3 months of follow-up
Mean GGT reflects average value for 103 patients with available data
IgG values represent average value for final 6 months of follow-up
Mortality
Of the 24 (12%) patients who died, the two with inadequate follow-up were not included in the analysis. All 22 deaths resulted from infectious complications. Median age of death was 21.6 years (range 5.6–63.9 years). Disseminated fungal infections and/or fungal pneumonia accounted for 12 deaths (55%) and Aspergillus sp. were the most commonly identified organisms. Bacterial pneumonia, liver abscess, septic shock and spontaneous bacterial peritonitis were reported as the cause of death in 5, 3, 2 and 1 patient respectively. Autopsies were available in 17 of the 24 (71%) patients and findings for all 24 patients are summarized in Table 2.
Table 2.
Summary of Findings in CGD Patients who Died
| Pt | Cause of Death | Age /Yrs FU | Sex Race | CGD genotype | Liver*$ | Spleen$ | Portal HTN | Other | Liver Abscess | Plt Slope (1000/µl/yr) | ALP Elevations/ yr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Disseminated | 20.2 | M | gp91phox | 2300 g | 800g | Yes | −18.2 | 1.17 | ||
| Aspergillus | 3.5 | C | HM | 21×12cm | |||||||
| PV | SM | ||||||||||
| 2 | Sepsis | 28.2 | M | gp91phox | HM | 556g | Splenectomy | Yes | 20.6 | 1.25 | |
| 5.6 | C | PV + CV | 20×10cm | (rise posts-plenectomy) | |||||||
| NRH | SM | ||||||||||
| 3 | Disseminated | 34.8 | M | gp91phox | 1180g | 300g | Massive | HIV | Yes | −10.7 | 0.57 |
| Aspergillus | 8.8 | C | HM | 14×11cm | ascites | Normal bone | |||||
| AIDS | NRH | SM | marrow | ||||||||
| 4 | Pneumonia | 19.8 | M | p47phox | 3050g | 400g | Yes | −125.8 | 0.48 | ||
| Septic Shock | 2.2 | AA | HM | SM | |||||||
| Liver Abscess | |||||||||||
| 5 | Pneumonia | 37.5 | M | p47phox | PV + CV | SM | Coronary | Yes | −14.5 | 0.31 | |
| Liver Abscess | 16.4 | C | NRH | Artery Disease | |||||||
| 6 | Disseminated | 22.1 | M | p47phox | Yes | −16.2 | 0.20 | ||||
| Aspergillus | 15.0 | C | |||||||||
| 7 | Pneumonia | 18.2 | M | gp91phox | 1630g | 745g | Ascites | Bone Marrow | No | −15.2 | 0.25 |
| Post-operative | 16.4 | C | HM | 17×16cm | Transplant | ||||||
| DILI | SM | Splenectomy | |||||||||
| GH | |||||||||||
| NRH | |||||||||||
| 8 | Aspergillus | 36.1 | F | p47phox | 2060g | 410g | Ascites | End-Stage | Yes | −12.5 | 0.38 |
| Pneumonia | 18.3 | AA | CV | Multi- | Renal Disease | ||||||
Liver findings - HM – hepatomegaly, DILI – drug-induced liver injury diagnosed on liver biopsy, PV – portal venopathy, CV – Central venopathy, NRH – nodular regernative hyperplasia, GH – granulomatous hepatitis, CN – centrilobular necrosis
Normal adult liver 1500 gm, normal adult spleen 150 gm
No autopsy performed
Limited autopsy performed
Not included in survival analysis because of inadequate follow-up (less than 4 months) or insufficient data to calculate platelet slope
Factors Associated with Mortality
Cox proportional hazards regression was used to assess the association with mortality of factors in table 1 (except liver biopsy findings). To account for changes during follow-up, platelet slope, number of ALT and ALP elevations and CGD treatment cohort were modeled as time-dependent covariates.
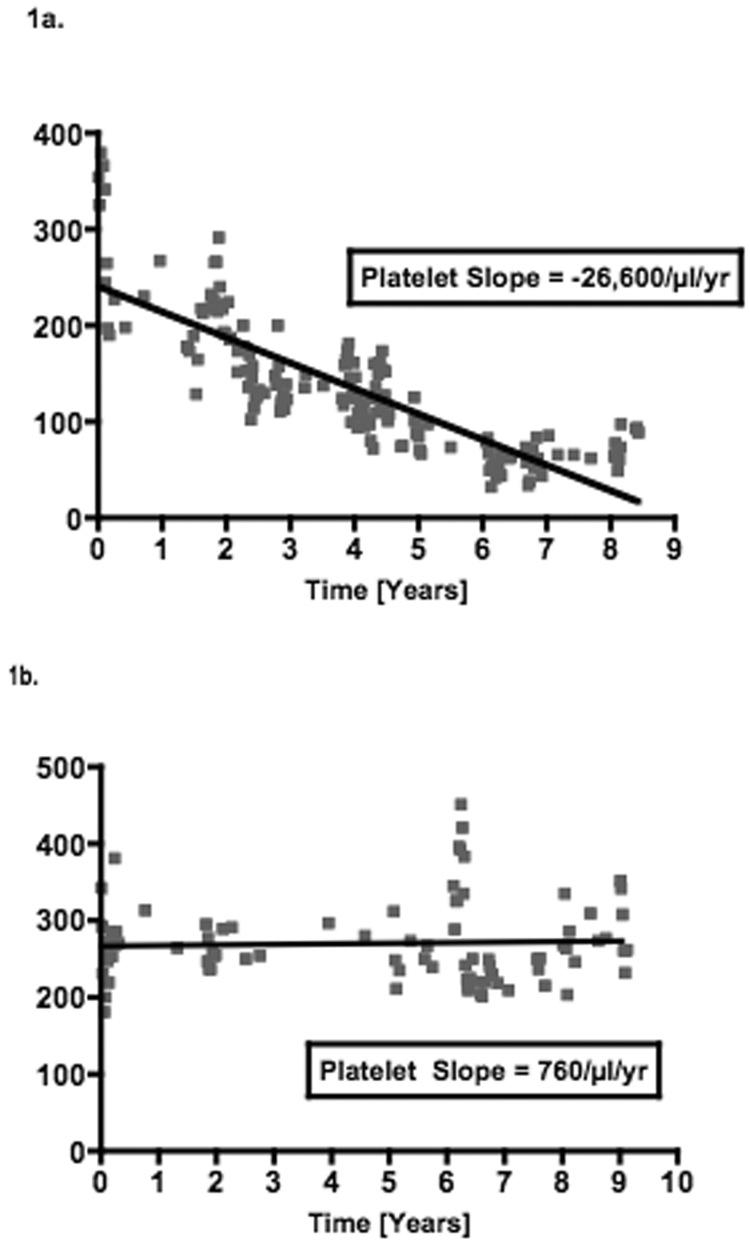
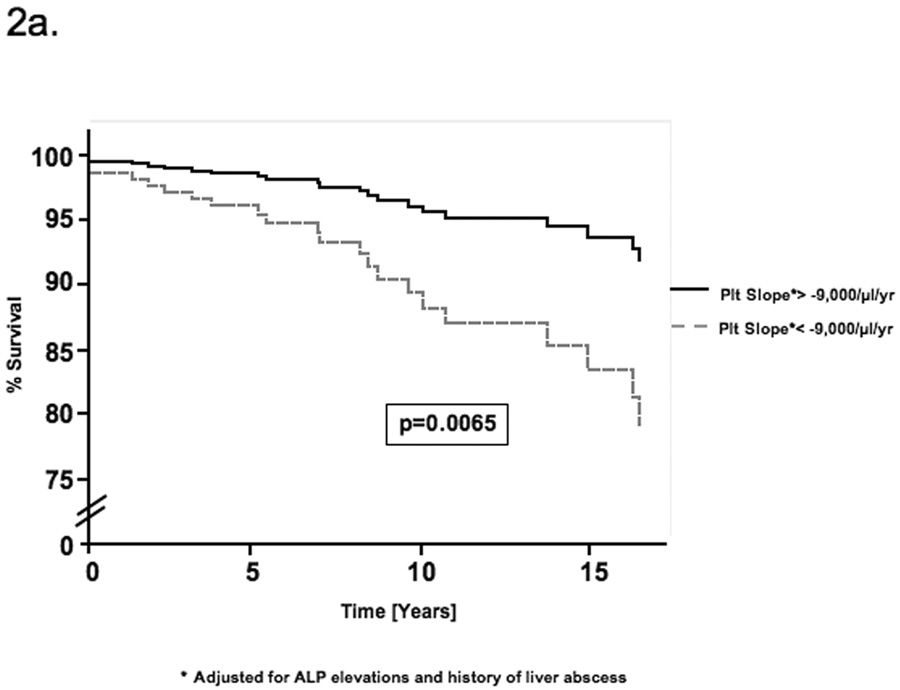
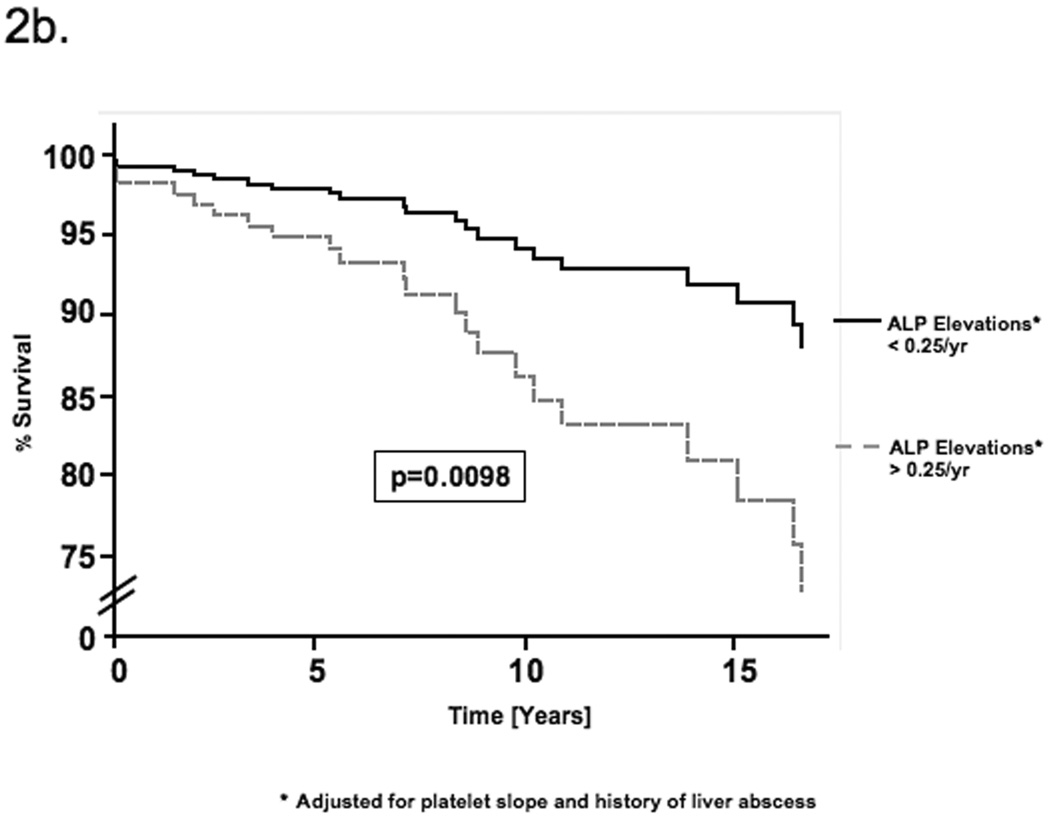
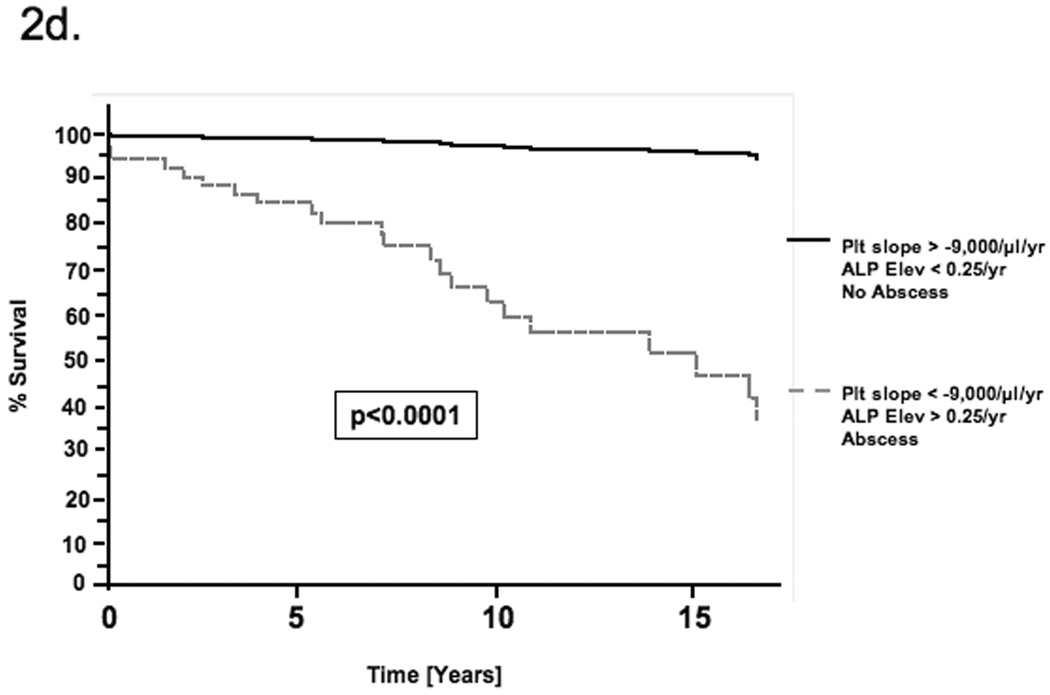
By univariate Cox regression, negative platelet slope, increasing number of ALP elevations and a history of liver abscess were associated with mortality (Table 3). These factors were first evaluated as continuous variables but were then dichotomized using receiver operator characteristic (ROC) curves to reduce the effect of outlier values and improve clinical utility. All three factors remained significant by multivariable Cox regression (Table 3). Patients with a platelet decline of greater than 9,000/µl per year had a 4.7-fold [95% CI 1.54–14.4, p=0.0065] increased risk of mortality. Similarly, greater than 0.25 ALP elevations per year (i.e. 1 elevation per 4 years) and a history of liver abscess increased the risk of mortality 4.5-fold [95% CI 1.43–13.8, p=0.0098] and 3.2-fold [95% CI 1.11–8.81, p=0.031] respectively. No significant confounding or effect-modification was noted when other covariates were evaluated in the multivariable model. Schoenfeld’s residuals showed that covariates and the full model satisfied proportional hazards assumptions (p=0.367). Figure 1 illustrates the platelet changes in (a) a representative patient who died with evidence of portal hypertension and (b) a patient alive at the end of follow-up without portal hypertension. Based on the Cox model, estimates of the survival functions for each of the significant covariates and for the full model are shown in figures 2a–d.
Table 3.
Univariate and Multivariable Cox Proportional Hazards Regression for Factors Associated with Mortality in CGD.
| Factor | HR | p-value | Adjusted HR* | p-value |
|---|---|---|---|---|
| [95% CI] | [95% CI] | |||
| Platelet Slope† | 4.54 | 0.0078 | 4.72 | 0.0065 |
| (below vs above −9,000/µl/yr) | [1.49–13.8] | [1.54–14.4] | ||
| ALP elevations† | 4.80 | 0.0057 | 4.45 | 0.0098 |
| (above vs below 0.25 per year) | [1.58–14.6] | [1.43–13.8] | ||
| History of Liver Abscess | 4.26 | 0.0056 | 3.13 | 0.031 |
| (yes vs no) | [1.53–11.9] | [1.11–8.81] | ||
Adjusted for other variables shown
Evaluated as time-dependent covariate
Figure 1.
Platelet slopes from representative patients who a) died and b) survived to end of follow-up.
Figure 2.
Estimate of the survival function for each of the determinants of mortality based on the Cox proportional hazards model. The curves compare the survival estimates for patients with and without a) declining platelet slope b) ALP elevations and c) a history of liver abscess. Each curve is adjusted for all factors in the multivariable Cox model. d) This figure compares the survival function for patients with all three determinants of mortality with that for patients with none of these factors.
Factors Associated with Determinants of Mortality
To evaluate factors associated with each of the three covariates in the model of mortality, logistic regression was performed. Because a platelet decline of greater than 9,000/µl per year was strongly associated with CGD genotype gp91phox (OR vs. p47phox 2.70 95% CI 1.14–6.39, p=0.023), logistic regression was performed after stratification by genotype. Using backward or forward stepwise multivariable logistic regression, progressive thrombocytopenia was associated with splenomegaly (OR 3.13 95% CI 1.10–8.86, p=0.032), serum IgG (OR 1.11 95% CI 1.00–1.24, p=0.047) and increasing number of ALT elevations per year (OR 5.32 95% CI 1.08–26.3, p=0.040). For patients with CGD genotype p47phox only BMI remained significant by multivariable analysis (Table 4).
Table 4.
Factors associated with determinants of mortality in CGD by univariate and multivariable logistic regression
| Determinant of Mortality | Factor | OR [95% CI] | p-value | Adjusted OR† [95% CI] | p-value |
|---|---|---|---|---|---|
| Progressive | gp91phox | ||||
| Thrombocytopenia‡ | Splenomegaly | 3.37 | 0.012 | 3.13 | 0.032 |
| [1.31–8.67] | [1.10–8.86] | ||||
| IgG* | 1.12 | 0.013 | 1.11 | 0.047 | |
| [1.03–1.22] | [1.00–1.24] | ||||
| ALT elevations/yr | 6.74 | 0.021 | 5.32 | 0.040 | |
| [1.33–34.0] | [1.08–26.3] | ||||
| Total Infections | 1.08 | 0.044 | |||
| [1.00–1.16] | |||||
| Hepatomegaly | 2.47 | 0.038 | |||
| [1.05–5.78] | |||||
| Ascites | 5.88 | 0.031 | |||
| [1.18–29.4] | |||||
| Race | 4.18 | 0.042 | |||
| AA vs Caucasian | [1.05–16.6] | ||||
| p47phox | |||||
| BMI | 0.83 | 0.028 | 0.83 | 0.028 | |
| [0.70–0.98] | [0.70–0.98] | ||||
| ALT elevations/yr | 23.6 | 0.023 | |||
| [1.54–372] | |||||
| ALP Elevations | Age | 1.07 | <0.0001 | 1.09 | <0.0001 |
| [1.04–1.12] | [1.04–1.13] | ||||
| Total Infections | 1.14 | <0.0001 | 1.16 | <0.0001 | |
| [1.07–1.22] | [1.08–1.24] | ||||
| History of Liver | 3.62 | <0.0001 | |||
| Abscess | [1.83–7.16] | ||||
| Splenomegaly | 2.69 | 0.005 | |||
| [1.36–5.34] | |||||
| Hepatomegaly | 2.01 | 0.044 | |||
| [1.02–3.97] | |||||
| Ascites | 3.13 | 0.022 | |||
| [1.17–8.30] | |||||
| Albumin | 0.43 | 0.021 | |||
| [0.20–0.88] | |||||
| BUN | 1.08 | 0.023 | |||
| [1.01–1.15] | |||||
| History of Liver | Age | 1.08 | <0.0001 | 1.08 | <0.0001 |
| Abscess | [1.03–1.12] | [1.04–1.12] | |||
| Total Infections | 1.07 | 0.012 | 1.08 | 0.008 | |
| [1.02–1.13] | 1.02–1.14 | ||||
| Hospital Admissions | 1.02 | 0.035 | |||
| [1.00–1.04] | |||||
| ALP Elevations/yr | 3.38 | 0.020 | |||
| [1.21–9.42] | |||||
| Hemoglobin | 1.20 | 0.080 | |||
| [0.98–1.48] | |||||
Adjusted model based on forward and backward stepwise regression
Analysis performed after stratification by CGD genotype.
Each unit of IgG represents units of 100 mg/dl. Values based on average of final months of follow-up.
The relationship between portal vein diameter and platelet slope was also evaluated. This was not included in multivariable regression models because portal vein measurements and corresponding platelet slopes were available for only 68 patients. Platelet slope decreased by 84 platelets per year for every 1 mm increase in portal vein diameter (p=0.026). Portal vein diameter above 13 mm has been documented as a marker of portal hypertension20. Patients with portal vein diameter ≥ 13 mm had a mean platelet slope of −7,218 platelets per year compared to −2,623 platelets per year among patients with smaller portal veins (p=0.034). Furthermore, all but 1 patient with portal vein diameter ≥ 13 mm had a negative platelet slope (32 of 33, 97%) compared to 24 of 35 (69%) with portal vein diameters <13 mm (p=0.002) (Table 5).
Table 5.
Relationship between platelet slope and portal vein diameter
| PV Diameter < 13mm | PV Diameter ≥ 13mm | P value | |
|---|---|---|---|
| Platelet Slope (mean ± sd) | −2,623 ± 9,838 | −7,218 ± 5,752 | 0.034 |
| Negative Platelet Slope | 24/35 (69%) | 32/33 (97%) | 0.002 |
By stepwise logistic regression, greater than 0.25 ALP elevations per year and a history of liver abscess were both associated with increasing age and number of infections (Table 4).
Histologic findings were not included in regression models because only 36 individuals had a liver biopsy or evaluation of the liver at autopsy. Of the 15 patients who died and had a liver biopsy or autopsy, 6 (40%) had NRH. In contrast, only 2 of 19 (10.5%) biopsies from patients alive at the end of follow-up showed established NRH (p=0.044). Early regenerative changes were noted in of 19 biopsies from living patients and 6 of 9 autopsy specimens (p=0.65). Portal venopathy was common, present in 18 of 21 living patients and 6 of 9 who died (p=0.35). Central venopathy was also common in both groups (14 of 21 living patients vs. 6 of 9 who died, p=0.23).
Prospective Evaluation
With recognition of the association between mortality and progressive thrombocytopenia, two surviving patients with declining platelet counts were evaluated prospectively. Hematologic evaluation including bone marrow biopsy revealed no evidence of bone marrow suppression but rather hypercellularity and normal numbers of megakaryocytes. Hemoglobin and white blood cell counts were normal and stable. Both patients had splenomegaly (19.5 cm and 15 cm) with enlarged portal veins (16mm and 15mm). Patient 1 had retroperitoneal collaterals, a recanalized umbilical vein and multiple hepatic calcifications. In Patient 1, the platelet count declined from 384,000/µl to 73,000/µl between 1988 and 2006, yielding a platelet slope of −20,300/µl per year. In Patient 2, platelets declined from 334,000/µl to 151,000/µl between 1984 and 2005 yielding a platelet slope of −4,100/µl per year. Notably despite a significant decline, the platelet count of Patient 2 was still in the low normal range (normal >150,000/µl).
To determine if portal hypertension was the cause of the progressive thrombocytopenia, both patients underwent HVPG measurement. HVPG was 19 mmHg in Patient 1 and 9 mmHg in Patient 2, indicating significant sinusoidal portal hypertension in both. Histology was reviewed from 4 specimens for Patient 1 (3 liver abscess wedge resections and 1 needle biopsy) and revealed portal and lobular infiltrates, but no fibrosis. Marked intimal thickening and near obliteration of portal veins was noted with many portal tracts lacking veins. Veno-occlusive changes were seen in central veins. Regenerative changes, but not established NRH, were present. Findings were consistent across specimens. In Patient 2, two separate biopsies showed venopathy of central and portal veins as well as early regenerative changes. On upper endoscopy, Patient 1 had gastric antral vascular ectasia but neither had esophageal or gastric varices. Associated with the negative platelet slope, the patients had 1.2 and 0.71 ALT elevations per year respectively (75th percentile=0.7 for cohort) and serum IgG was 1540 mg/dl in Patient 1 and 2560 g/dl in Patient 2 (75th percentile=1440 for cohort).
Discussion
Major advances in the management of patients with CGD have led to marked improvements in survival; however premature mortality due to repeated infectious complications is still the reality for patients with this disease. In an attempt to understand why after surviving repeated infections patients die from a given infectious episode, we examined predictors of mortality in a large cohort of well-characterized patients with CGD. Although all deaths were due to infection, regression analysis identified progressive thrombocytopenia, repeated ALP elevations and a history of liver abscess as independent predictors of mortality.
Beyond the predisposition to liver abscess, little is known about the impact of CGD on the liver. A recent description of this cohort showed that other liver abnormalities are common in CGD7. Patients had frequent liver enzyme elevations but more importantly, of those that had a liver biopsy, 80% were found to have damage or obliteration of central and/or portal veins. Associated with this venopathy, NRH was seen in 9 liver biopsies including 6 of 12 autopsy specimens7. NRH is a recognized cause of non-cirrhotic portal hypertension thought to result from a progressive vasculopathy with obliteration of small portal vein branches21–23. NRH and venopathy increase resistance to sinusoidal blood flow leading to portal hypertensive changes, however, unlike in cirrhosis, there is no intrinsic impairment of hepatic synthetic function and minimal or no fibrosis.
The results of the mortality analysis suggest that the development of non-cirrhotic portal hypertension may herald a poor prognosis in CGD. Patients with CGD have many reasons to develop thrombocytopenia, including bone marrow suppression from infections and/or medications. However, bone marrow biopsy in a number of patients revealed no evidence of marrow dysfunction and full hematological evaluation including platelet antibodies was unrevealing. Progressive thrombocytopenia was associated with the most severe form of CGD (gp91phox), splenomegaly, elevation of IgG and repeated ALT elevations as well as increased portal vein diameter, a reliable marker of portal hypertension20. In this setting, ALT elevations may be markers of recurrent hepatic insults from infection and/or drug hepatotoxicity while IgG elevation and splenomegaly are likely consequences of portal hypertension24, 25. The association with splenomegaly and the absence of platelet antibodies make autoimmune thrombocytopenia unlikely26. Immunoglobulin levels are thought to increase in cirrhosis due to reduced antigenic clearance by the liver27. Although this partially results from reduced Kupffer cell mass, structural abnormalities that lead to shunting are also likely important and are very similar to the findings seen with the venopathy and NRH found in many patients with CGD21. Recurrent infections may also lead to splenomegaly, however without portal hypertension, splenic sequestration of platelets is less common28. As a result, progressive thrombocytopenia, but not splenomegaly alone, was found to be a predictor of mortality in this cohort of patients with CGD.
Autopsy data, prospective evaluation of sinusoidal portal pressure and measurements of portal vein diameter support the connection between progressive thrombocytopenia and portal hypertension. Fifty percent of patients who died had a history of ascites compared to 7.8% who were alive at the end of follow-up (p<0.0001). Although factors other than portal hypertension may cause ascites, 10 of the 11 patients who died with a history of ascites had splenomegaly, one had hepatic encephalopathy and none had other known causes of ascites. Two patients with declining platelet counts were evaluated and had raised HVPG measurements. NRH and other causes of intrahepatic pre-sinusoidal portal hypertension have been reported to increase the HVPG29–32. Although prospective evaluation of portal pressure was only available in two patients, portal vein diameter, a validated surrogate for portal hypertension, showed a strong correlation with platelet slope in the 68 patients for whom data was available20. These findings suggest that declining platelet count is a sensitive and early maker for the development portal hypertension, likely as a consequence of venopathy and NRH in the setting of CGD.
ALP elevations were associated with hepatic and systemic infections, but also with signs of portal hypertension (splenomegaly and ascites), further suggesting that repeated infections likely play a role in the development of portal hypertension over time. Elevations of ALP are commonly observed with NRH21, reinforcing the connection between infection, NRH, portal hypertension and mortality.
Together these data suggest that a subset of patients with CGD develop progressive non-cirrhotic portal hypertension likely as a consequence of damage to the hepatic microvasculature caused by repeated liver abscesses and possibly contributed to by drug-induced liver injury21, 32, 33. Repeated systemic infections may also increase portal pressure through modulation of vasoactive substances, as has been described in cirrhosis34, 35 and recently in an animal model of non-cirrhotic portal hypertension36. Once portal hypertension develops, patients with CGD generally do not die of direct sequelae of increased portal pressure, but rather of sepsis. Portal hypertension itself may increase the risk of subsequent infection through increased bacterial translocation, particularly in the setting of systemic immunosuppression37, 38. This has also been described in patients with other causes of non-cirrhotic portal hypertension, namely congenital hepatic fibrosis (CHF) and schistosomiasis39–41. As in the setting of cirrhosis, this interaction potentially sets up a vicious cycle with infections worsening portal hypertension and portal hypertension increasing the risk of infection. This relationship is borne out in this study population; patients with portal hypertension, as defined by platelet slope, had more episodes of infection than those without (6.8 vs. 4.0, p=0.032). Similarly, patients undergoing renal transplantation for autosomal recessive polycystic kidney disease (ARPKD), which is caused by the same genetic defect as CHF, were found to have an increased rate of infection and sepsis was the most common cause of mortality in this group39. Which is cause and which is effect is difficult to discern. Once infection occurs, the hyperdynamic circulatory state associated with portal hypertension puts patients at greater risk of developing complications including ascites, spontaneous bacterial peritonitis and renal failure11, 12.
Although CGD is a rare disease, the findings from this retrospective and prospective study highlight the importance of portal hypertension as a contributor to mortality outside the setting of cirrhosis, a factor that is likely under-recognized. A progressive decline in platelet count may provide a useful non-invasive means to evaluate progression of portal hypertension over time. Whether non-cirrhotic portal hypertension contributes to morbidity and mortality in other chronic conditions and how its effects can be modulated will require further investigation.
Acknowledgement
This research was supported by the Intramural Research Programs of the NIDDK, NIAID, CC, and NCI, NIH. None of the authors has any financial interest or conflict of interest related to this research.
Abbreviations
- CGD
Chronic granulomatous disease
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- ULN
upper limit of normal
- NRH
nodular regenerative hyperplasia
- HVPG
Hepatic-venous pressure gradient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jurkowska M, Bernatowska E, Bal J. Genetic and biochemical background of chronic granulomatous disease. Arch Immunol Ther Exp (Warsz) 2004;52:113–120. [PubMed] [Google Scholar]
- 2.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 3.Johnston RB., Jr Clinical aspects of chronic granulomatous disease. Curr Opin Hematol. 2001;8:17–22. doi: 10.1097/00062752-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lublin M, Bartlett DL, Danforth DN, Kauffman H, Gallin JI, Malech HL, Shawker T, Choyke P, Kleiner DE, Schwartzentruber DJ, Chang R, DeCarlo ES, Holland SM. Hepatic abscess in patients with chronic granulomatous disease. Ann Surg. 2002;235:383–391. doi: 10.1097/00000658-200203000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marciano BE, Wesley R, De Carlo ES, Anderson VL, Barnhart LA, Darnell D, Malech HL, Gallin JI, Holland SM. Long-term interferon-gamma therapy for patients with chronic granulomatous disease. Clin Infect Dis. 2004;39:692–699. doi: 10.1086/422993. [DOI] [PubMed] [Google Scholar]
- 6.Gallin JI, Alling DW, Malech HL, Wesley R, Koziol D, Marciano B, Eisenstein EM, Turner ML, DeCarlo ES, Starling JM, Holland SM. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003;348:2416–2422. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 7.Hussain N, Feld JJ, Kleiner DE, Hoofnagle JH, Garcia-Eulate R, Ahlawat S, Koziel DE, Anderson V, Hilligoss D, Choyke P, Gallin JI, Liang TJ, Malech HL, Holland SM, Heller T. Hepatic abnormalities in patients with chronic granulomatous disease. Hepatology. 2007;45:675–683. doi: 10.1002/hep.21524. [DOI] [PubMed] [Google Scholar]
- 8.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 9.Thulstrup AM, Sorensen HT, Schonheyder HC, Moller JK, Tage-Jensen U. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis. 2000;31:1357–1361. doi: 10.1086/317494. [DOI] [PubMed] [Google Scholar]
- 10.Deschenes M, Villeneuve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol. 1999;94:2193–2197. doi: 10.1111/j.1572-0241.1999.01293.x. [DOI] [PubMed] [Google Scholar]
- 11.Bernard B, Cadranel JF, Valla D, Escolano S, Jarlier V, Opolon P. Prognostic significance of bacterial infection in bleeding cirrhotic patients: a prospective study. Gastroenterology. 1995;108:1828–1834. doi: 10.1016/0016-5085(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 12.Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, Rimola A, Gassull MA, Arroyo V, Rodes J. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–1501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- 13.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 14.Vowells SJ, Sekhsaria S, Malech HL, Shalit M, Fleisher TA. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 15.The International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic ranulomatous disease. N Engl J Med. 1991;324:509–516. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 16.Margolis DM, Melnick DA, Alling DW, Gallin JI. Trimethoprim-sulfamethoxazole prophylaxis in the management of chronic granulomatous disease. J Infect Dis. 1990;162:723–726. doi: 10.1093/infdis/162.3.723. [DOI] [PubMed] [Google Scholar]
- 17.Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280–282. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 18.Grambsch P, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 19.Cleves MA. Multiple curves plotted with stcurve command. Stata Technical Bulletin. 2000;54:2–4. [Google Scholar]
- 20.Schepis F, Camma C, Niceforo D, Magnano A, Pallio S, Cinquegrani M, D'Amico G, Pasta L, Craxi A, Saitta A, Raimondo G. Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology. 2001;33:333–338. doi: 10.1053/jhep.2001.21410. [DOI] [PubMed] [Google Scholar]
- 21.Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44:7–14. doi: 10.1002/hep.21258. [DOI] [PubMed] [Google Scholar]
- 22.Kondo F, Koshima Y, Ebara M. Nodular lesions associated with abnormal liver circulation. Intervirology. 2004;47:277–287. doi: 10.1159/000078479. [DOI] [PubMed] [Google Scholar]
- 23.Shimamatsu K, Wanless IR. Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology. 1997;26:343–350. doi: 10.1002/hep.510260214. [DOI] [PubMed] [Google Scholar]
- 24.Czaja AJ, Wolf AM, Baggenstoss AH. Clinical assessment of cirrhosis in severe chronic active liver disease: specificity and sensitivity of physical and laboratory findings. Mayo Clin Proc. 1980;55:360–364. [PubMed] [Google Scholar]
- 25.Watt K, Uhanova J, Gong Y, Kaita K, Doucette K, Pettigrew N, Minuk GY. Serum immunoglobulins predict the extent of hepatic fibrosis in patients with chronic hepatitis C virus infection. J Viral Hepat. 2004;11:251–256. doi: 10.1111/j.1365-2893.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 26.Tarantino M. Recent advances in the treatment of childhood immune thrombocytopenic purpura. Semin Hematol. 2006;43:S11–S17. doi: 10.1053/j.seminhematol.2006.04.008. discussion S18-9. [DOI] [PubMed] [Google Scholar]
- 27.Prytz H, Bjorneboe M, Christoffersen P, Poulsen H, Orskov F. Correlation between hepatic morphology and immunoglobulins and antibodies to Escherichia coli in cirrhosis. Gut. 1977;18:28–32. doi: 10.1136/gut.18.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45:645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naber AH, Van Haelst U, Yap SH. Nodular regenerative hyperplasia of the liver: an important cause of portal hypertension in non-cirrhotic patients. J Hepatol. 1991;12:94–99. doi: 10.1016/0168-8278(91)90916-y. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien K, Hussain N, Warady BA, Kleiner DE, Kleta R, Bernardini I, Heller T, Gahl WA. Nodular regenerative hyperplasia and severe portal hypertension in cystinosis. Clin Gastroenterol Hepatol. 2006;4:387–394. doi: 10.1016/j.cgh.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Colina F, Pinedo F, Solis JA, Moreno D, Nevado M. Nodular regenerative hyperplasia of the liver in early histological stages of primary biliary cirrhosis. Gastroenterology. 1992;102:1319–1324. [PubMed] [Google Scholar]
- 32.Ueno S, Tanabe G, Sueyoshi K, Yoshinaka H, Yamamoto S, Kurita K, Yoshidome S, Nuruki K, Aikou T. Hepatic hemodynamics in a patient with nodular regenerative hyperplasia. Am J Gastroenterol. 1996;91:1012–1015. [PubMed] [Google Scholar]
- 33.Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, Vierling JM, Geller SA, Targan SR, Poordad FF. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125:298–303. doi: 10.1016/s0016-5085(03)00938-7. [DOI] [PubMed] [Google Scholar]
- 34.Battista S, Bar F, Mengozzi G, Zanon E, Grosso M, Molino G. Hyperdynamic circulation in patients with cirrhosis: direct measurement of nitric oxide levels in hepatic and portal veins. J Hepatol. 1997;26:75–80. doi: 10.1016/s0168-8278(97)80012-8. [DOI] [PubMed] [Google Scholar]
- 35.Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003;139:186–193. doi: 10.7326/0003-4819-139-3-200308050-00008. [DOI] [PubMed] [Google Scholar]
- 36.Rizvi MR, Omanwar S, Fahim M, Sarin SK. Altered alpha adrenergic vasoresponsiveness in a non-cirrhotic portal hypertension model of E. coli injection. J Gastroenterol Hepatol. 2007;22:870–876. doi: 10.1111/j.1440-1746.2006.04440.x. [DOI] [PubMed] [Google Scholar]
- 37.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taura P, Fuster J, Garcia-Valdecasas JC, Lacy A, Suarez MJ, Rimola A, Rodes J. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 38.Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17:397–425. doi: 10.1016/s1521-6918(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 39.Davis ID, Ho M, Hupertz V, Avner ED. Survival of childhood polycystic kidney disease following renal transplantation: the impact of advanced hepatobiliary disease. Pediatr Transplant. 2003;7:364–369. doi: 10.1034/j.1399-3046.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 40.Ferraz AA, Campos JM, Junior JG, De Albuquerque AC, Ferraz EM. Gut bacterial translocation and postoperative infections: a prospective study in schistosomotic patients. Surg Infect (Larchmt) 2005;6:197–201. doi: 10.1089/sur.2005.6.197. [DOI] [PubMed] [Google Scholar]
- 41.Kashtan CE, Primack WA, Kainer G, Rosenberg AR, McDonald RA, Warady BA. Recurrent bacteremia with enteric pathogens in recessive polycystic kidney disease. Pediatr Nephrol. 1999;13:678–682. doi: 10.1007/s004670050680. [DOI] [PubMed] [Google Scholar]