Abstract
BACKGROUND
Testicular germ cell tumours (TGCT) are thought to originate from fetal germ cells that fail to differentiate normally, but no animal model for these events has been described. We evaluated the marmoset (Callithrix jacchus) as a model by comparing perinatal germ cell differentiation with that in humans.
METHODS
Immunohistochemical profiling was used to investigate germ cell differentiation (OCT4, NANOG, AP-2γ, MAGE-A4, VASA, NANOS-1) and proliferation (Ki67) in fetal and neonatal marmoset testes in comparison with the human and, to a lesser extent, the rat.
RESULTS
In marmosets and humans, differentiation of gonocytes into spermatogonia is associated with the gradual loss of pluripotency markers such as OCT4 and NANOG, and the expression of germ cell-specific proteins such as VASA. This differentiation occurs asynchronously within individual cords during fetal and early postnatal life. This contrasts with rapid and synchronous germ cell differentiation within and between cords in the rat. Similarly, germ cell proliferation in the marmoset and human occurs throughout perinatal life, in contrast to rats in which proliferation ceases during this period.
CONCLUSIONS
The marmoset provides a good model for normal human germ cell differentiation and proliferation. The perinatal marmoset may be a useful model in which to establish factors that lead to failure of normal germ cell differentiation and the origins of TGCT.
Keywords: common marmoset, cell differentiation, germ cells, carcinoma in situ, testis
Introduction
Male fertility depends on the daily production of millions of sperm, and this is dependent upon the continuous, controlled proliferation of spermatogonia. Spermatogonia develop from relatively undifferentiated fetal germ cells (gonocytes), a process that is initiated in fetal life (Fukuda et al., 1975), and which involves loss of stem cell/embryonic cell characteristics (Gaskell et al., 2004; Honecker et al., 2004). If differentiation from a gonocyte to a spermatogonium does not occur, then this cell cannot contribute to spermatogenesis in adulthood. Additionally, persistence in the testis of an undifferentiated fetal germ cell with stem cell-like characteristics is potentially dangerous, as this is thought to be how carcinoma in situ (CIS) cells arise in humans (Rajpert-De Meyts, 2006), and it is these cells that ultimately give rise to testicular germ cell tumours (TGCT) in young men (Skakkebaek et al., 2001). The incidence of testicular cancer has increased progressively in Western countries, and it has been proposed that as yet unidentified environmental and lifestyle factors are responsible for this increase (Skakkebaek et al., 2007). It is unknown what perinatal factors might enable CIS cells to develop, and this ignorance is due in large part to the lack of an animal model in which to study the mechanistic origins of CIS/TGCT.
Rodent studies have provided some insight into the maturation of the germ cell lineage in fetal/neonatal life (Wilhelm et al., 2007), but their usefulness is limited as neither CIS nor TGCT occur spontaneously, nor can be induced, in the rodent testis. This could be due to inter-species differences in testis and/or germ cell development, as there are some fundamental differences in the ‘set-up’ of spermatogenesis between rodents and humans. For example, compared with the human, rodents exhibit a high efficiency of spermatogenesis as evidenced by a large germ cell to Sertoli cell ratio and a high level of daily sperm production per unit weight of testis (Sharpe, 1994), compared with some primates (Millar et al., 2000; Sharpe et al., 2000) and humans (Clermont, 1963). The ‘segmental’ arrangement of spermatogenic stages in the seminiferous tubules of rodents may be associated with more efficient spermatogenesis than the ‘mixed’ arrangement in the human. It is thought that this difference may itself arise because of the low number of spermatogonial mitoses that occur in the human when compared with the rat and with other animals with a segmental organization (Sharpe, 1994). Moreover, although differentiation of gonocytes into spermatogonia occurs during fetal life in both humans and rodents, the process appears less organized in the human, with gonocytes still detectable in early postnatal life. Evidence to support these conclusions has been established using a battery of germ cell-specific protein markers that enable sequential germ cell differentiation to be visualized (Gaskell et al., 2004; Honecker et al., 2004; Rajpert-De Meyts et al., 2004; Hoei-Hansen et al., 2005; Anderson et al., 2007). Such ‘organizational’ differences in germ cells might be a factor that predisposes towards development of CIS cells in the human but not in the rodent.
Based on previous studies it has been proposed that the common marmoset (Callithrix jacchus), a small New World primate, could potentially provide a better animal model for human germ cell development than rodents. In particular, the marmoset has many similarities to the human in terms of timing of testis development and function, in addition to the subsequent organization and efficiency of spermatogenesis. This includes a neonatal period of reproductive hormonal activity (neonatal testosterone rise or ‘mini-puberty’) (Mann and Fraser, 1996) followed by a period of ‘childhood’ quiescence (Kelnar et al., 2002) prior to the onset of puberty, which does not occur in rodents (Plant 2006). Then in adulthood, similar to the human, there is a ‘mixed’ organization of spermatogenic stages within the tubule, in contrast to rodents and other primate models in which there is a ‘segmental’ organization of the spermatogenic stages (Millar et al., 2000; Sharpe, 1994; Weinbauer et al., 2001). Additionally, the germ cell to Sertoli cell ratio is low in the marmoset, as in the human (Sharpe et al., 2000), indicating low efficiency of spermatogenesis when compared with rodents. These similarities led us to consider if the marmoset might also be a better model than rodents in terms of fetal germ cell development and differentiation, as this would have implications for studies into the origins of CIS/TGCT. However, a detailed analysis of testis development and germ cell maturation in the fetal marmoset testis has not previously been reported. Therefore the aim of the present study was to use a panel of germ cell differentiation and proliferation markers previously validated on human fetal testes (Gaskell et al., 2004; Honecker et al., 2004; Rajpert-De Meyts et al., 2004; Hoei-Hansen et al., 2005; Pauls et al., 2006; Anderson et al., 2007) to characterize the timing and organization of germ cell differentiation in the fetal and neonatal marmoset testis, and to undertake a direct comparison with the human, and to a lesser extent with the rat.
Materials and Methods
Tissue collection
Marmoset testes
Testes were obtained from the fetuses of elderly pregnant marmosets in the colony breeding stock that were being euthanized to make way for younger breeding animals. Gestation in the marmoset lasts for ∼20 weeks (144 days) (Windle et al., 1999). The gestation of pregnant females was assessed by palpation and confirmed by reference to published crown rump length data (Chambers and Hearn, 1985). Mothers and fetuses were euthanized by injection of an overdose of sodium pentobarbitone (Euthatal; Rhone Merieux Ltd, Harlow, Essex, UK). The fetuses (11 weeks, n = 1; 14 weeks, n = 2; 15 weeks, n = 1; 16 weeks, n = 2; 17 weeks, n = 2; 20 weeks, n = 2) were delivered by hysterotomy. Fetuses were fixed in Bouins for 6 h (larger fetuses were partially dissected prior to fixing) and then transferred to 70% ethanol. Sections of postnatal testes (aged 1 day, and 2, 6, 22, 35 or 48 weeks, adult; n = 3 for each age), were obtained from tissue remaining from previous studies, in order to reduce the animal numbers required (Kelnar et al., 2002; Sharpe et al., 2003), in addition to testes from animals euthanized for colony management purposes. All testes were fixed as described above, prior to processing.
First and second trimester human fetal testes
Human fetal testes were obtained following termination of pregnancy during the first (8–10 weeks, n = 4) or second trimester (14–19 weeks, n = 9). Women gave consent in accordance with national guidelines (Polkinghorne, 1989), and ethical approval was obtained from the Local Research Ethics Committee. Termination was induced with mifepristone (200 mg, orally), followed by misopristone (Pharmacia, Surrey, UK, 200 mg every 3 h per vaginam). No terminations were due to fetal abnormalities. In second trimester samples gestational age was determined initially by ultrasound examination, followed by direct measurement of foot length. The sex of first trimester samples was determined by PCR for the SRY gene as previously described (Gaskell et al., 2004). Testes were fixed for 2 h in Bouins and then transferred into 70% ethanol prior to processing.
Third trimester and postnatal human testes
Third trimester (n = 3) and postnatal human testes were obtained at autopsy with consent of their legal guardian (courtesy of K.M.) from boys who died from various causes (excluding reproductive and endocrine abnormalities). Testes were fixed in 10% neutral buffered formalin for at least 24 h and processed as below.
Rat testes
Wistar rats were maintained in our own animal facility according to U.K. Home Office guidelines. Pregnant dams were killed by inhalation of carbon dioxide when their fetuses had reached embryonic day 15.5 (e15.5, n = 5) or e19.5 (n = 5). Fetuses were removed, decapitated and placed in ice-cold phosphate-buffered saline (PBS) (Sigma, Poole, Dorset, UK). Testes were removed via microdissection and fixed for 1 h in Bouins followed by transfer into 70% ethanol.
Tissue processing
All fixed testes were embedded in paraffin using standard processes and sections of 5 µm thickness were prepared.
Immunohistochemistry
Details of antibodies, dilutions and requirement for antigen retrieval are shown in Table I. Sections were dewaxed in xylene, rehydrated in graded alcohols and washed in tap water. Sections requiring antigen retrieval were subjected to pressure cooking in 0.01 M citrate (pH 6.0) buffer as described previously (Norton et al., 1994). Sections were treated with 3% (v/v) H2O2 in methanol for 30 min and washed in water, followed by Tris-buffered saline (TBS, 0.05 M Tris and 0.85% NaCl, pH 7.6) for a further 5 min. Endogenous biotin was blocked using an avidin/biotin blocking kit (Vector Laboratories Inc., Peterborough, UK), according to the manufacturers instructions. Sections were incubated in appropriate normal serum (diluted 1:5 with TBS containing 5% (w/v) bovine serum albumin (BSA) (Sigma) for 30 min. Sections were incubated overnight with primary antibody diluted in serum at 4°C in a humidified chamber. Sections were washed in TBS for two 5 min periods and incubated for 30 min with the appropriate biotinylated secondary antibody (swine anti-rabbit, rabbit anti-mouse; both Dako, Cambridgeshire, UK or rabbit anti-goat; Vector Laboratories Inc.), diluted in normal serum. This was followed by two further 5 min washes in TBS and incubation for 30 min with Streptavidin–horse-radish peroxidase at 1:1000 (Dako), diluted in TBS.
Table I.
Antibodies and conditions for immunohistochemistry in the testes.
| Antigen | Source | Species | Dilution | Retrieval |
|---|---|---|---|---|
| AP-2γ | Santa Cruza | Mouse | 1:20 | Y |
| OCT 4 | Santa Cruza | Goat | 1:50 | Y |
| NANOG | R+Db | Goat | 1:50 | Y |
| VASA | Abcamc | Rabbit | 1:500 | Y |
| MAGE-A4 | Giftd | Mouse | 1:20 | N |
| NANOS-1 | Abcamc | Rabbit | 1:500 | Y |
| AMH | Santa Cruza | Rabbit | 1:500 | N |
| 3β-HSD | Gifte | Rabbit | 1:1000 | N |
| Ki67 | DAKOf | Mouse | 1:40 | Y |
All antibodies were raised against human peptide sequences. aSanta Cruz Biotechnology, CA, USA; bR+D Systems, MN, USA; cAbcam, Cambridge, UK; dDr Guilio Spagoli, University Hospital, Basel, Switzerland; eProf. Ian Mason, The Queen's Medical Research Institute, Edinburgh, UK; fDAKO, California, USA. OCT4 and AP-2γ: transcription factors; NANOG: homeobox domain protein; MAGE-A4: a cancer testis antigen; VASA and NANOS-1: RNA-binding proteins; 3β-HSD: 3β-hydroxysteroid dehydrogenase; Ki67: cell proliferation marker.
Visualization was performed using 3,3-diaminobenzidine tetrahydrochloride (Dako) and sections were counterstained with haematoxylin, dehydrated in graded alcohols and immersed in xylene before mounting in Pertex mounting medium (CellPath plc, Hemel Hempstead, UK). For each experiment a negative control in which the primary antibody had been replaced with the appropriate normal serum was included. Images were captured using an Olympus Provis microscope (Olympus Optical, London, UK) connected to a Canon DS126131 camera and images obtained using Canon EOS image capture software (Canon, Woodhatch, Surrey, UK).
For double immunohistochemistry, sections were stained for VASA (an RNA-binding protein) using Streptavidin–Alkaline phosphatase (Dako) at 1:400 for 30 min and visualization with 1 mg/ml Fast Blue (Sigma) in fast blue buffer, until desired staining was achieved (typically 10–15 min). Ki67 (marker of cell proliferation) primary antibody was then applied and subsequent steps were as described above for single immunostaining.
Immunofluorescence
Sections were initially treated as described for single staining as far as the primary antibody stage, with PBS (Sigma) washes between each step. Antigen retrieval was required for all experiments and normal goat (AP-2γ, VASA and NANOS) or chicken (OCT4) serum (1:5 with TBS and 5% BSA) was used for serum block and dilution of antibodies. Details of antibodies and the visualization method are listed in Table II.
Table II.
Antibodies and conditions used for immunofluorescence.
| Antigen 1 | Dilution | Secondary antibody | Visualization | Antigen 2 | Dilution | Secondary antibody | Visualization |
|---|---|---|---|---|---|---|---|
| AP-2γ | 1:60 | GAM-pa | Tyr-Cy3 (10 min)d | VASA | 1:200 | GAR-b | Str-488 |
| VASA | 1:200 | GAR(fab)-bb | Str-488 (1 h)e | NANOS | 1:60 | GAR(fab)-b | Str-546g |
| VASA | 1:200 | GAR-bc | Str-488 (1 h) | OCT4 | 1:50 | CAG-pf | Tyr-Cy3 |
aGAM-p, goat anti-mouse peroxidise; bGAR(fab)-b, goat anti-rabbit biotinylated fab fragment; cGAR-b, goat anti-rabbit biotinylated; dTyr-Cy3, tyramide-enhanced Cy3 (PerkinElmer, MA, USA); eStr-488, streptavidin 488 (Molecular Probes); fCAG-p, chicken anti-goat biotinylated; gStr-546, streptavidin 546 (Molecular Probes).
Following overnight incubation with primary antibody in goat serum, sections were incubated with secondary antibody for 30 min, followed by the first visualization reagent. For this and subsequent steps, the sections were kept in darkness. Sections were incubated for 30 min in goat serum, followed by a second avidin/biotin block. For NANOS-1 (an RNA binding protein) and VASA, rabbit immunoglobulin G (Sigma) at 1:2000 was applied for 30 min, followed by the second primary antibody in goat serum applied overnight at 4°C. Sections were incubated with secondary antibody for 30 min, followed by the second visualization reagent. To-pro-3 (Molecular Probes, Leiden, Netherlands) was applied at 1:1000 in PBS for 5 min as a nuclear counterstain and the slides were mounted using Permafluor (Immunotech, Marseille, France). Images were captured using an LSM 510 Confocal microscope (Carl Zeiss, Hertfordshire, UK).
Germ cell proliferation index
Ki67 immunohistochemistry (as described above) was used to determine which germ cells were proliferating in the marmoset testis. The fetal germ cells were identified based on morphology and position within the seminiferous cords. Sections were counted independently by two observers (R.M. and G.C.), to ensure accuracy of germ cell identification, while postnatal germ cells were identified using VASA/Ki67 co-staining. Cells were counted using an objective fitted to an Olympus BH2 microscope with Hitachi HVC20 Camera (Olympus Optical), and images were analysed using Image Pro-plus version 4.5.1 with Stereology Pro 5.0 (Media Cybernetics, Wokingham, Berkshire, UK). Excellent agreement between the two observers was found. The proliferation index was calculated by dividing the number of Ki67-positive germ cells by the total number of germ cells assessed in thirty randomly selected fields.
Results
Seminiferous cord structure and localization of cell types within the testis of the marmoset during fetal life
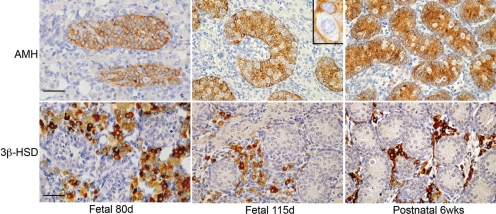
Testes obtained from fetal marmosets from 11 weeks until term all contained well-defined testicular cords surrounded by an interstitial compartment (Fig. 1). Sertoli cells were identified both histologically (small nuclei) and by the cytoplasmic localization of anti-Müllerian hormone (AMH), which was expressed in all the testes examined (Fig. 1). Germ cells were identified within the cords, because their nuclei had a larger diameter than the Sertoli cells, they expressed one or more germ cell markers (see below) and they lacked expression of AMH (Fig. 1). Within the interstitium, Leydig cells were identified by expression of 3β-hydroxysteroid dehydrogenase (Fig. 1). The general organization of the cell types within the fetal marmoset testis was comparable to that described in the human fetal testis (Gaskell et al., 2004).
Figure 1:
General structure and organization of the fetal and early postnatal marmoset (Callithrix jacchus) testis as illustrated by immunoexpression of anti-Müllerian hormone (AMH) in Sertoli cells within seminiferous cords (upper panels) and 3β-hydroxysteroid dehydrogenase (3β-HSD) in Leydig cells in the interstitium (lower panels).
In the top panels, the clear, unstained cytoplasm outlines the nuclei of germ cells within seminiferous cords (inset). Scale bar 50 µm. wks, weeks; d, days.
Expression of markers of pluripotency and early germ cell differentiation in the marmoset in perinatal life and comparison with the human
Marmoset
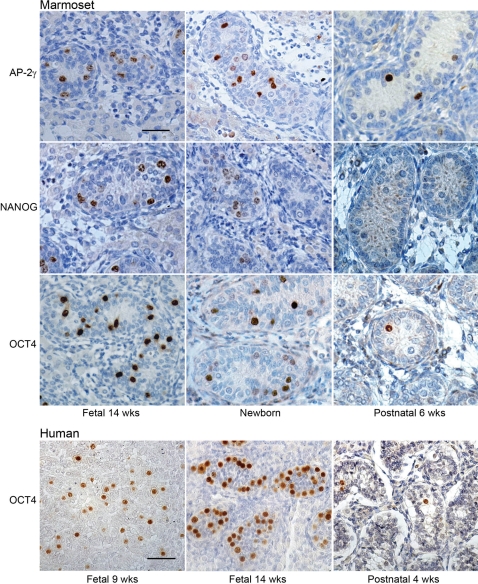
Expression of the transcription factors AP-2γ and OCT4, and NANOG (a homeobox domain protein) was germ cell-specific and was detected in a decreasing proportion of the total germ cell population during progression through fetal life into the early postnatal period (Fig. 2). All three proteins were expressed in the majority of germ cells at 11 weeks' gestation (not shown), but by the end of pregnancy the proteins were only immunolocalized to a small subset of germ cells. For NANOG, germ cell expression ceased between 2 and 6 weeks of postnatal age, while OCT4 and AP-2γ expression persisted in a few, scattered germ cells until 6 weeks postnatally (Fig. 2).
Figure 2:
Immunoexpression of pluripotency factors (AP-2γ, NANOG, OCT4) that are markers of undifferentiated germ cells (gonocytes) in the marmoset testis and comparison with the human.
Note that the proportions of germ cells expressing these markers decreases with increasing age. Note also the similar pattern of OCT4 expression in the marmoset compared to the human. Scale bar 50 µm.
Human
Consistent with previous reports, OCT4 (Fig. 2), NANOG and AP-2γ (not shown) were detected in most testicular germ cells of first trimester fetuses but the proportion of immunopositive germ cells decreased as gestation progressed (Looijenga et al., 2003; Gaskell et al., 2004; Honecker et al., 2004; Rajpert-De Meyts et al., 2004; Pauls et al., 2006; Kerr et al., 2008). A few OCT4-positive germ cells were still present at 4 weeks postnatally (Fig. 2) and occasionally at 6 months after birth (not shown) and similar results were obtained using the anti-AP-2γ antibody. NANOG was detected in occasional germ cells at 4 weeks of postnatal age but not in tissue from older individuals (not shown).
Expression of proteins associated with germ cell differentiation in the perinatal marmoset and comparison with the human
Marmoset
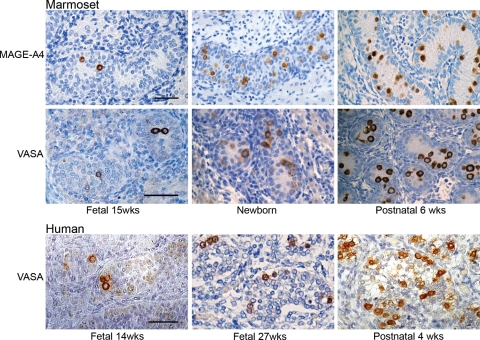
During fetal life there was an increase in the proportion of germ cells that were immunopositive for MAGE-A4 (a cancer testis antigen) and VASA as gestation progressed (Fig. 3). At 11 weeks of gestation none of the germ cells expressed VASA or MAGE-A4 (not shown) but at 15 weeks a small proportion of germ cells expressed these proteins within their cytoplasm and by birth the majority of germ cells were positive for VASA and similar results were obtained for MAGE-A4. By 6 weeks of postnatal age, only the occasional germ remained negative for these proteins.
Figure 3:
Immunoexpression of protein markers (MAGE-A4, VASA) of differentiated germ cells (pre-spermatogonia/spermatogonia) in the marmoset testis and comparison with the human.
Note the increasing proportion of germ cells expressing these markers in the marmoset with increasing age and the similarity with VASA expression in the human. Scale bar 50 µm.
Human
Consistent with a previous study (Anderson et al., 2007), no expression of VASA was detected in germ cells in testes in the first trimester (not shown), but VASA immunopositive germ cells were detected in second trimester testes, with increasing proportions of germ cells expressing VASA in the third trimester (Fig. 3). In the early postnatal period almost every testicular germ cell expressed VASA. A similar pattern of expression was also found for MAGE-A4 (not shown).
Co-localization of germ cell markers demonstrates asynchronous germ cell differentiation in the marmoset and human, in contrast to the rat
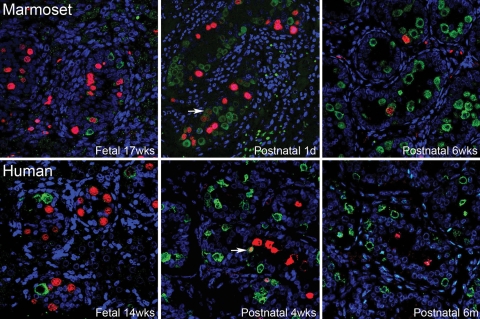
We used co-localization studies to investigate the relationship between germ cells expressing proteins previously localized to human gonocytes (OCT4, NANOG, AP-2γ) and those expressing VASA, MAGE-A4 or NANOS-1. These investigations revealed a distinct and only partially overlapping pattern of expression in germ cell subpopulations in both the marmoset and human. For example, co-localization of AP-2γ with VASA confirmed our earlier findings of decreasing proportions of AP-2γ-positive cells and increasing proportions of VASA-positive cells with increasing age (Fig. 4). In addition, a small proportion of cells were positive for both proteins at the same time. Notably, within a single testicular cross section we detected mixed populations of germ cells within individual cords, such that a single cord could contain AP-2γ+/VASA−, AP-2γ+/VASA+ or AP-2γ−/VASA+ germ cells; this pattern was equally evident in the marmoset and human. The existence of seminiferous cords containing a germ cell cohort that was predominantly AP-2γ+/VASA− and those that were mostly AP-2γ−/VASA+ appeared to be separated by a period of several months in both the marmoset and the human, and took place from fetal into early postnatal life. Germ cell expression of these proteins was not synchronized between or within the seminiferous cords of an individual fetus/neonate and there was also some variation in the timing of expression between individuals of the same age.
Figure 4:
Distinction of undifferentiated germ cells, immunoexpressing AP-2γ (red, nuclear localization), from differentiated germ cells, immunoexpressing VASA (green, cytoplasmic localization), in the marmoset and human perinatal testis using confocal microscopy.
Note that all germ cells are visualized with this combination of markers. A progressive increase with age in the proportion of germ cells expressing VASA is seen as the proportion of AP-2γ-positive germ cells decreases. A small proportion of germ cells co-expressed both markers (arrows) and are presumed to reflect a transition state between undifferentiated and differentiated. Sections conterstained with TO-PRO-3 (blue). m, months.
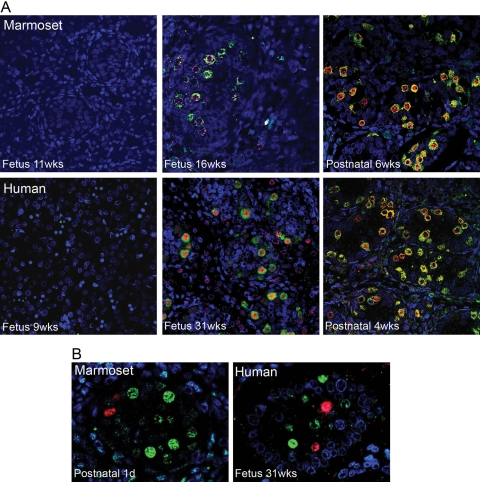
In the marmoset and human a similar expression profile was demonstrated for OCT4 and VASA (Fig. 5), with co-localization of both proteins in a small proportion of germ cells, resulting in three different subpopulations, namely OCT4+/VASA−, OCT4+/VASA+ and OCT4−/VASA+. However in the rat, there was no evidence for an OCT4+/VASA− population of germ cells and co-localization of OCT4 and VASA was detected in all germ cells at e15.5. A few days later (e19.5), no rat germ cells expressed OCT4, but all continued to express VASA (Fig. 5); the loss of OCT4 immunoexpression appeared to be synchronous in all germ cells in every cord over this relatively narrow time period.
Figure 5:
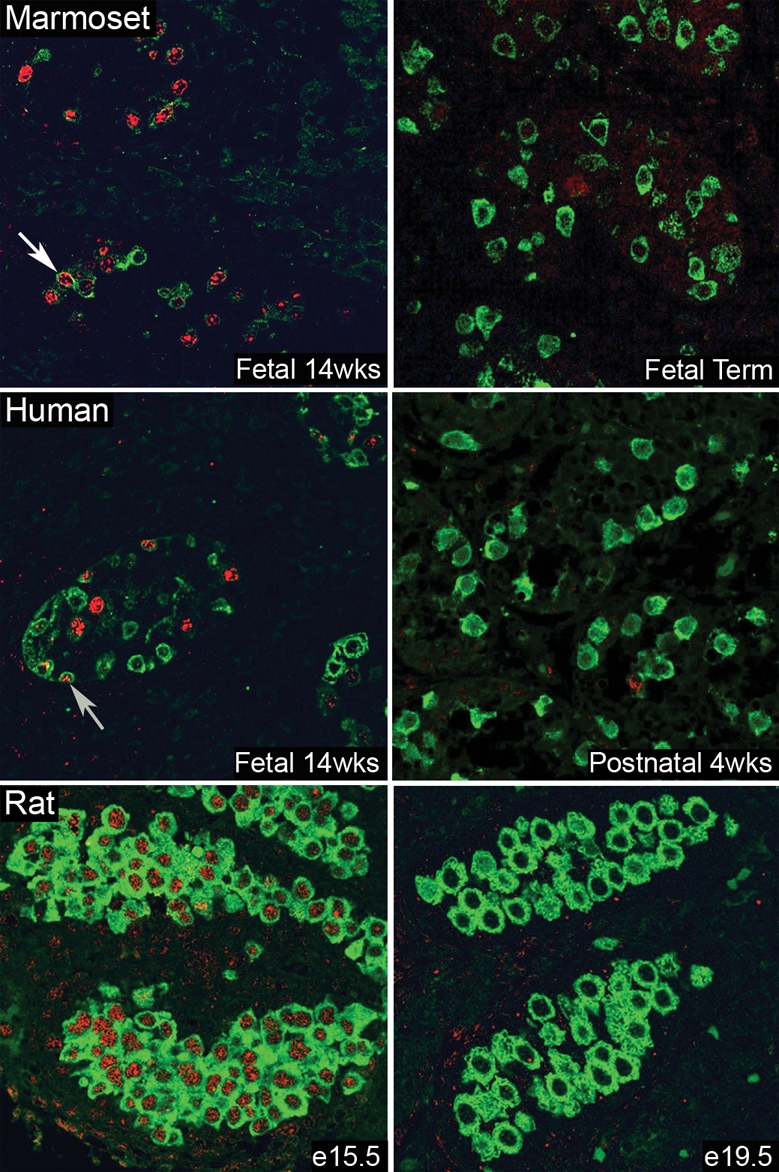
Comparison of perinatal germ cell differentiation in the marmoset, human and rat using protein markers of undifferentiated (OCT4: red, nuclear localization) and differentiated (VASA: green, cytoplasmic localization) germ cells and confocal microscopy.
Note that in seminiferous cords in both the marmoset and human at each age studied there is a mixed population of germ cells based on the expression of these two markers, including occasional cells in which the markers were co-localized (arrows). This is in contrast to the rat, in which OCT4 is co-expressed with VASA in all germ cells at embryonic day 15.5 (e15.5) but was no longer detected within any of the germ cells at e19.5 (and was largely absent at e17.5, not shown). wks, weeks.
Expression of NANOS-1 protein in fetal and postnatal marmosets and comparison with the human
In the marmoset at 11 weeks' gestation none of the germ cells expressed NANOS-1. Increasing proportions of germ cells contained immunopositive staining in their nucleus during the final third of gestation and by early postnatal life most of the germ cells contained NANOS-1, with localization of the protein to both the nucleus and peri-nuclear cytoplasm (Fig. 6). Human testes were immunonegative for NANOS-1 in the first trimester, but this protein was expressed in the nucleus of germ cells from the second trimester onwards with an increasing proportion of germ cells expressing NANOS-1 as gestation progressed. In postnatal life, NANOS-1 was also detected in the peri-nuclear cytoplasm of germ cells. Germ cells immunopositive for NANOS-1 also expressed VASA (Fig. 6A). However AP-2γ and NANOS-1 were not co-localized (Fig. 6B), which was in contrast to results obtained for AP-2γ and VASA co-staining (Fig. 4), when a proportion of germ cells were found to be immunopositive for both proteins. This suggests that expression of NANOS-1 occurs after VASA expression begins.
Figure 6:
(A) Comparison of the immunoexpression of NANOS-1 (red, nuclear and cytoplasmic localization) and VASA (green, cytoplasmic localization) in germ cells in the testis during fetal and postnatal life in the marmoset (top panel) and human (lower panel) using confocal microscopy.
Note that neither protein is expressed in early gestation in either species but begin to be co-expressed (yellow) in the final third of gestation in the marmoset and in the second trimester in the human testis. In postnatal life, these markers continue to be co-expressed and co-localization in the peri-nuclear region is seen in most germ cells. (B) Immunoexpression of NANOS-1 (green, nuclear and cytoplasmic localization) and AP-2γ (red, nuclear localization) in the marmoset and human testis for comparison with VASA/AP-2γ expression (Fig. 4). NANOS-1 is expressed in most of the germ cells in the 1-day-old marmoset and 31-week-gestation human testis, with the remainder expressing AP-2γ. However, in contrast to VASA/AP-2γ (Fig. 4), no germ cells showed co-localization of NANOS-1 and AP-2γ. All sections conterstained with TO-PRO-3 (blue).
Germ cell proliferation in the perinatal marmoset and human testis and comparison with the rat
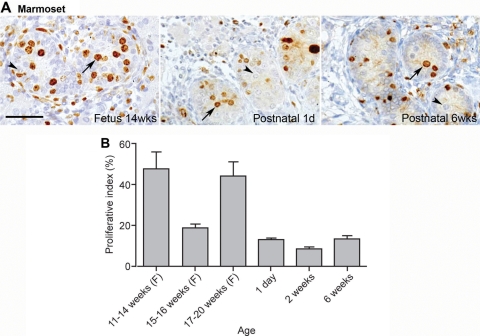
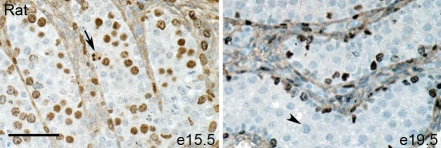
Immunoexpression of Ki67 was detected in the marmoset testis at all fetal ages investigated from 11 weeks' gestation until birth, with a reduction in the number of Ki67-positive germ cells during weeks 15–16 (Fig. 7). Ki67-positive germ cells were also found in postnatal life, though they were less frequent than during the majority of fetal life (Fig. 7). Proliferation occurred in a proportion of both undifferentiated (OCT4-positive, gonocytes) and differentiated (VASA-positive spermatogonia) germ cells (not shown). In the human fetal testis, proliferation was seen in a relatively high proportion of germ cells in the first and second trimester, similar to the proportions seen in the fetal marmoset (not shown). Unfortunately, lack of available good quality tissue prevented us from accurately determining the germ cell proliferation index in the third trimester. Previous studies have demonstrated Ki67 expression in germ cells in the third trimester and into early postnatal life in the human (Honecker et al., 2004). Ki67 expression in the rat was high at e15.5 with the majority of germ cells immunopositive, while at e19.5 none of the germ cells expressed this protein (Fig. 8).
Figure 7:
(A) Germ cell proliferation in the marmoset testis in fetal and early postnatal life as visualized by immunoexpression of Ki67 (brown) and (B) as quantified to generate a proliferation index. Germ cell proliferation (arrows) occurs in a proportion of germ cells throughout fetal (F) and postnatal life in the marmoset.
At all ages a variable proportion of germ cells are not proliferating (arrowheads). Note that some Sertoli cells are also proliferating in the sections in A. In (B), bars represent mean ± SEM (n = 3 for each age). Scale bar 50 µm.
Figure 8:
Germ cell proliferation in the fetal rat testis as visualized by immunoexpression of the proliferation marker, Ki67 (brown).
Germ cell proliferation (arrows) occurs in the majority of germ cells at e15.5 but has ceased completely by e19.5 when all germ cells are negative for Ki67 (arrowhead). Note that, at both ages, some Sertoli cells (peripherally located) are immunopositive for Ki67. Scale bar 50 µm.
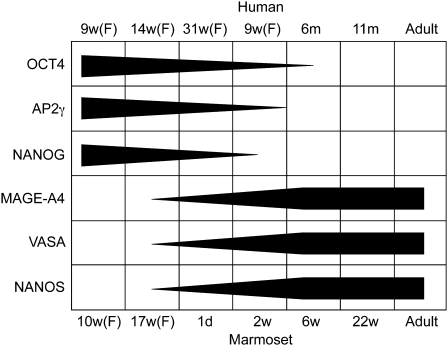
Germ cell proteins expressed during fetal and early postnatal life in the human testis were expressed over a similar age range in germ cells of the marmoset testis (Fig. 9).
Figure 9:
Comparison of germ cell protein expression in the marmoset and human from fetal (F) into postnatal life.
The dimensions of the grey bars illustrate the relative proportion of germ cells expressing an individual marker at a given age.
Discussion
Despite the similarities that exist between the marmoset and the human in terms of reproductive biology, little is known about germ cell differentiation and proliferation in the marmoset. We have demonstrated that seminiferous cords are present in the testes of the marmoset at 11 weeks' gestation and that there is a similar cellular organization compared with the human. In addition, germ cell proteins that are known to be expressed during fetal and early postnatal life in the human testis were also expressed over a comparable age range in germ cells of the marmoset testis. In common with the human we have also demonstrated evidence for mixed subpopulations of fetal and early postnatal germ cells within individual seminiferous cords in the marmoset. Based on our previous data we believe these represent gonocytes (expressing markers of pluripotency) and spermatogonia (expressing germ cell-specific markers), with an intermediate population that co-express proteins typical of both gonocytes and spermatogonia (Gaskell et al., 2004). The timing of expression of the germ cell-specific proteins during fetal life in the marmoset and human can be considered in relation to the differences in embryology between these two species. Fetal life in the marmoset is characterized by a relatively late implantation and a prolonged embryonic phase such that the fetal phase, during which most of the linear growth occurs, begins at 11/20 weeks gestation (Windle et al., 1999), compared with 7/40 weeks in the human (Phillips, 1976). This may explain why proteins that are first expressed in the human during the second trimester are not expressed in the marmoset until the final third of gestation.
OCT4, a transcription factor and NANOG, a homeobox domain protein which regulates self-renewal (Hoei-Hansen et al., 2005), are markers of pluripotency that are expressed in human gonocytes (Gaskell et al., 2004; Rajpert-De Meyts et al., 2004). These proteins are expressed in the nuclei of germ cells within the marmoset testis from fetal into early postnatal life. Within each seminiferous cord the proportion of germ cells immunopositive for these proteins decreased as gestation progressed, consistent with published findings for the human (Looijenga et al., 2003; Gaskell et al., 2004; Honecker et al., 2004; Rajpert-De Meyts et al. 2004; Kerr et al., 2008) and confirmed here. Our results, together with previous reports (Rajpert-De Meyts et al., 2004), show that OCT4 is still detected in occasional germ cells in the human at 4–6 months postnatally, while NANOG was no longer detected after 4 weeks. This is in keeping with previous suggestions of down-regulation of NANOG occurring earlier in development than OCT4 (Hoei-Hansen et al., 2005). AP-2γ, a DNA binding transcription factor, had a similar expression profile to OCT4 in germ cells of both the marmoset and human testis. In the marmoset, only occasional germ cells expressed this protein by 6 weeks postnatally, whereas in humans the protein was expressed until 4–6 months of age, in agreement with previous findings (Hoei-Hansen et al., 2004), but in contrast to one report that described no postnatal expression of this protein (Pauls et al., 2005).
As the proportion of germ cells expressing proteins such as OCT4, NANOG and AP-2γ decreased during gestation in the marmoset, we found an increase in the proportion of germ cells expressing germ cell-specific differentiation markers such as VASA, a member of the DEAD box family of RNA-binding proteins (Castrillon et al., 2000) and MAGE-A4, an X-encoded cancer testis antigen (Gjerstorff et al., 2007). VASA immunopositive germ cells were detected from 14 weeks' gestation in the marmoset and in an increasing proportion of germ cells as gestation progressed. In the fetal and early postnatal human testis the expression profile of these proteins was comparable to the marmoset, with expression from the second trimester onwards and the proportion of cells expressing these markers increasing with age, as has been reported for VASA (Castrillon et al., 2000) and MAGE-A4 (Aubry et al., 2001; Pauls et al., 2006), although one study did detect MAGE-A4 in very occasional germ cells in the first trimester (Gaskell et al., 2004). The frequency of cells expressing VASA during fetal life has been described as being constant from the second trimester onwards in the human (Honecker et al., 2004) or even reduced from high levels in the second trimester to a stable level around birth (Cools et al., 2006). However, these studies involved counting the total number of positive cells rather than the proportion of positive germ cells at each age.
NANOS-1, an RNA binding protein that is expressed in the adult human testis (Jaruzelska et al., 2003), is largely confined to the same germ cells that were immunopositive for VASA. In both the marmoset and human during fetal life, NANOS-1 was expressed in the nucleus. In the postnatal testis there was both nuclear and peri-nuclear cytoplasmic expression. This peri-nuclear expression of NANOS-1 has been described previously in human adult germ cells (Jaruzelska et al., 2003). A similar change in localization within germ cells has been reported for the RNA-binding protein DAZL, in which the protein is expressed in the nucleus of first trimester germ cells, but is expressed in both the nucleus and cytoplasm of second trimester germ cells (Anderson et al., 2007). The change in cellular localization of NANOS-1 suggests that this protein may have more than one function during germ cell development.
Co-localization of AP-2γ or OCT4 with VASA in the marmoset identified several different subpopulations of immature germ cells within each cord at any given stage of testicular development. Most germ cells within a cord expressed a single protein, however, there was also a population of cells that expressed both proteins. This suggests that there is a transition from germ cells expressing AP-2γ or OCT4 to germ cells expressing VASA. Similar subpopulations have also been described in the human for combinations of markers including OCT4/VASA (Honecker et al., 2004; Anderson et al., 2007), OCT4/MAGE-A4 (Gaskell et al., 2004; Pauls et al., 2006) and AP-2γ/MAGE-A4 (Pauls et al., 2006) and these subpopulations are associated with morphological changes that occur as gonocytes differentiate into spermatogonia (Fukuda et al., 1975). We would propose that in the marmoset, as in the human, the OCT4+/VASA− germ cells are gonocytes, with OCT4+/VASA+ cells being intermediate and OCT4−/VASA+ cells representing spermatogonia (Gaskell et al., 2004).
Differentiation of all the germ cells within an individual seminiferous cord occurred over a relatively long period of time and in an asynchronous fashion in both the marmoset and human, such that within a cord during fetal and early postnatal life, there are often germ cells expressing an early germ cell marker (e.g. OCT4+), a proportion of germ cells only expressing a later differentiation marker (e.g. VASA) and a small population of germ cells co-expressing both markers. Comparison with the rat, using OCT4 and VASA as an example, demonstrates a clear difference between the rat and marmoset/human. We have demonstrated that all germ cells within the fetal rat testis appear to be VASA-positive and there is no evidence of an OCT4+/VASA− population, even at e13.5 before the cords have formed (unpublished observation). This has also been demonstrated in the mouse, where VASA is expressed in the germ cells immediately after colonization of the gonad, but not in migratory primordial germ cells (Toyooka et al., 2000). In the rat, by e19.5 all of the germ cells in every cord no longer expressed OCT4 but remained VASA-positive. Indeed, in most germ cells, switching off of OCT4 is complete by as early as e17.5 (Ferrara et al., 2006).
In addition to germ cell differentiation, we have also demonstrated for the first time that proliferation of germ cells occurs in a variable proportion of these cells in the marmoset during fetal and early postnatal life, and that this proliferation occurs in germ cells at different stages of differentiation during this period. Proliferation of germ cells occurs in the human during the first and second trimester and has been reported to continue in the third trimester and into early postnatal life (Honecker et al., 2004). In contrast, the rat exhibits a synchronized pattern of germ cell proliferation in which a high proliferation index occurs at e15.5 followed by a complete cessation of proliferation for the remainder of gestation and until postnatal Days 4–6 (Ferrara et al., 2006). This proliferative quiescence covers the period of differentiation of germ cells from gonocytes into spermatogonia. A period when testicular germ cell proliferation has ceased has not been found in the marmoset or human and proliferation continues to occur during the transition of germ cells from gonocyte to spermatogonia.
The apparently gradual and asynchronous differentiation and proliferation of fetal germ cells in the marmoset and human is intriguing and may have implications regarding the development of CIS and TGCT. It is well recognized that CIS cells (the precursor lesion for TGCT), closely resemble human fetal germ cells in terms of both morphology (Gondos, 1993) and protein expression, including NANOG, OCT4, AP-2γ, VASA and MAGE-A4 (Rajpert-De Meyts, 2006). These expression patterns support the hypothesis that CIS cells arise from immature fetal germ cells that fail to differentiate completely into normal spermatogonia (Skakkebaek et al., 1987). Germ cell differentiation occurs over a prolonged period in humans and this may, given the right circumstances, predispose to the development of CIS (Rajpert-De Meyts, 2006). Indeed, in humans with some disorders of sex differentiation there is evidence of delayed germ cell differentiation, based on persistence of OCT4 expression, in addition to an increased risk of CIS and TGCT in later life (Cools et al., 2006). The presence of several subpopulations of germ cells at different stages of differentiation, all exposed to the same local environment within the seminiferous cords during fetal or early postnatal life may also increase the likelihood of incomplete differentiation of some of the more immature germ cells and thus increase the risk of developing CIS.
In humans, the development of CIS and TGCT has been proposed to be part of a testicular dysgenesis syndrome (TDS) (Skakkebaek et al., 2007). A combination of features similar to human TDS can be induced in the rat using phthalates (Fisher et al., 2003), but this does not include the development of CIS or TGCT. This failure to induce CIS in the rodent may be related to the way in which the fetal germ cells differentiate. If asynchronous and prolonged differentiation, with several subpopulations of germ cells co-existing in different stages of differentiation, predisposes to malignant transformation in the human (Rajpert-De Meyts, 2006), then CIS may also occur spontaneously or be inducible in the marmoset. However, there are no published reports of testicular tumours in the marmoset, which could suggest that other predisposing factors may be required. These factors may include exposure to environmental factors, such as phthalates. The effect of phthalates on marmoset germ cell differentiation is currently under investigation.
In summary, we have demonstrated that germ cell differentiation and proliferation in the marmoset is comparable to that of the human during fetal and early postnatal life. We have also demonstrated that the process of early germ cell differentiation takes place over a relatively prolonged period of time and is asynchronous, resulting in the presence of germ cells at several stages of differentiation within individual seminiferous cords during fetal and early postnatal life. In addition, proliferation occurs in a variable proportion of germ cells throughout perinatal life. This is in marked contrast to the rodent in which more synchronous germ cell differentiation occurs in late fetal and early postnatal life, during a relatively narrow time window and germ cell proliferation completely ceases during late gestation and early postnatal life. This pattern of germ cell differentiation and proliferation in the marmoset and human may result in an increased susceptibility to abnormal/incomplete germ cell differentiation and conditions that arise from this, such as CIS and TGCT. Further work is required to establish whether CIS can be induced in the marmoset.
Funding
This research has been funded by the Medical Research Council.
Acknowledgements
We are grateful to Sheila Macpherson for her assistance during the present study. The 3β-hydroxysteroid dehydrogenase antibody was a kind gift from Professor Ian Mason (University of Edinburgh, Edinburgh, UK). The MAGE-A4 antibody was a kind gift from Dr Giulio Spagoli (University Hospital, Basel, Switzerland).
References
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT. Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol. 2007;7:136. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry F, Satie AP, Rioux-Leclercq N, Rajpert-De Meyts E, Spagnoli GC, Chomez P, De Backer O, Jegou B, Samson M. MAGE-A4, a germ cell specific marker, is expressed differentially in testicular tumors. Cancer. 2001;92:2778–2785. doi: 10.1002/1097-0142(20011201)92:11<2778::aid-cncr10125>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci USA. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers PL, Hearn JP. Embryonic, foetal and placental development in the common marmoset monkey (Callithrix jacchus) J Zool Lond. 1985;207:545–561. [Google Scholar]
- Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat. 1963;112:35–51. doi: 10.1002/aja.1001120103. [DOI] [PubMed] [Google Scholar]
- Cools M, Stoop H, Kersemaekers AM, Drop SL, Wolffenbuttel KP, Bourguignon JP, Slowikowska-Hilczer J, Kula K, Faradz SM, Oosterhuis JW, et al. Gonadoblastoma arising in undifferentiated gonadal tissue within dysgenetic gonads. J Clin Endocrinol Metab. 2006;91:2404–2413. doi: 10.1210/jc.2005-2554. [DOI] [PubMed] [Google Scholar]
- Ferrara D, Hallmark N, Scott H, Brown R, McKinnell C, Mahood IK, Sharpe RM. Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology. 2006;147:5352–5362. doi: 10.1210/en.2006-0527. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Hedinger C, Groscurth P. Ultrastructure of developing germ cells in the fetal human testis. Cell Tissue Res. 1975;161:55–70. doi: 10.1007/BF00222114. [DOI] [PubMed] [Google Scholar]
- Gaskell TL, Esnal A, Robinson LL, Anderson RA, Saunders PT. Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod. 2004;71:2012–2021. doi: 10.1095/biolreprod.104.028381. [DOI] [PubMed] [Google Scholar]
- Gjerstorff MF, Kock K, Nielsen O, Ditzel HJ. MAGE-A1, GAGE and NY-ESO-1 cancer/testis antigen expression during human gonadal development. Hum Reprod. 2007;22:953–960. doi: 10.1093/humrep/del494. [DOI] [PubMed] [Google Scholar]
- Gondos B. Ultrastructure of developing and malignant germ cells. Eur Urol. 1993;23:68–74. doi: 10.1159/000474572. discussion 75. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Nielsen JE, Almstrup K, Sonne SB, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin Cancer Res. 2004;10:8521–8530. doi: 10.1158/1078-0432.CCR-04-1285. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Almstrup K, Nielsen JE, Brask Sonne S, Graem N, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology. 2005;47:48–56. doi: 10.1111/j.1365-2559.2005.02182.x. [DOI] [PubMed] [Google Scholar]
- Honecker F, Stoop H, de Krijger RR, Chris Lau YF, Bokemeyer C, Looijenga LH. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol. 2004;203:849–857. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- Jaruzelska J, Kotecki M, Kusz K, Spik A, Firpo M, Reijo Pera RA. Conservation of a Pumilio-Nanos complex from Drosophila germ plasm to human germ cells. Dev Genes Evol. 2003;213:120–126. doi: 10.1007/s00427-003-0303-2. [DOI] [PubMed] [Google Scholar]
- Kelnar CJ, McKinnell C, Walker M, Morris KD, Wallace WH, Saunders PT, Fraser HM, Sharpe RM. Testicular changes during infantile ‘quiescence’ in the marmoset and their gonadotrophin dependence: a model for investigating susceptibility of the prepubertal human testis to cancer therapy? Hum Reprod. 2002;17:1367–1378. doi: 10.1093/humrep/17.5.1367. [DOI] [PubMed] [Google Scholar]
- Kerr CL, Hill CM, Blumenthal PD, Gearhart JD. Expression of pluripotent stem cell markers in the human fetal testis. Stem Cells. 2008;26:412–421. doi: 10.1634/stemcells.2007-0605. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- Mann DR, Fraser HM. The neonatal period: a critical interval in male primate development. J Endocrinol. 1996;149:191–197. doi: 10.1677/joe.0.1490191. [DOI] [PubMed] [Google Scholar]
- Millar MR, Sharpe RM, Weinbauer GF, Fraser HM, Saunders PT. Marmoset spermatogenesis: organizational similarities to the human. Int J Androl. 2000;23:266–277. doi: 10.1046/j.1365-2605.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- Norton AJ, Jordan S, Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994;173:371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Pauls K, Jager R, Weber S, Wardelmann E, Koch A, Buttner R, Schorle H. Transcription factor AP-2gamma, a novel marker of gonocytes and seminomatous germ cell tumors. Int J Cancer. 2005;115:470–477. doi: 10.1002/ijc.20913. [DOI] [PubMed] [Google Scholar]
- Pauls K, Schorle H, Jeske W, Brehm R, Steger K, Wernert N, Buttner R, Zhou H. Spatial expression of germ cell markers during maturation of human fetal male gonads: an immunohistochemical study. Hum Reprod. 2006;21:397–404. doi: 10.1093/humrep/dei325. [DOI] [PubMed] [Google Scholar]
- Phillips IR. The embryology of the common marmoset (Callithrix jacchus) Adv Anat Embryol Cell Biol. 1976;52:3–47. [PubMed] [Google Scholar]
- Plant TM. The male monkey as a model for the study of the neurobiology of puberty onset in man. Mol Cell Endocrinol. 2006;254–255:97–102. doi: 10.1016/j.mce.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Polkinghorne J. London: Her Majesty's Stationary Office; 1989. Review of the Guidance on the Research Use of Fetuses and Fetal Material. [Google Scholar]
- Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum Reprod Update. 2006;12:303–323. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Hanstein R, Jorgensen N, Graem N, Vogt PH, Skakkebaek NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004;19:1338–1344. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Regulation of Spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- Sharpe RM, Walker M, Millar MR, Atanassova N, Morris K, McKinnell C, Saunders PT, Fraser HM. Effect of neonatal gonadotropin-releasing hormone antagonist administration on sertoli cell number and testicular development in the marmoset: comparison with the rat. Biol Reprod. 2000;62:1685–1693. doi: 10.1095/biolreprod62.6.1685. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Fraser HM, Brougham MF, McKinnell C, Morris KD, Kelnar CJ, Wallace WH, Walker M. Role of the neonatal period of pituitary-testicular activity in germ cell proliferation and differentiation in the primate testis. Hum Reprod. 2003;18:2110–2117. doi: 10.1093/humrep/deg413. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Berthelsen JG, Giwercman A, Muller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987;10:19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Jorgensen N, Main KM, Leffers H, Andersson AM, Juul A, Jensen TK, Toppari J. Testicular cancer trends as ‘whistle blowers’ of testicular developmental problems in populations. Int J Androl. 2007;30:198–204. doi: 10.1111/j.1365-2605.2007.00776.x. discussion 204–205. [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 2000;93:139–149. doi: 10.1016/s0925-4773(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Weinbauer GF, Aslam H, Krishnamurthy H, Brinkworth MH, Einspanier A, Hodges JK. Quantitative analysis of spermatogenesis and apoptosis in the common marmoset (Callithrix jacchus) reveals high rates of spermatogonial turnover and high spermatogenic efficiency. Biol Reprod. 2001;64:120–126. doi: 10.1095/biolreprod64.1.120. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Windle CP, Baker HF, Ridley RM, Oerke AK, Martin RD. Unrearable litters and prenatal reduction of litter size in the common marmoset (Callithrix jacchus) J Med Primatol. 1999;28:73–83. doi: 10.1111/j.1600-0684.1999.tb00254.x. [DOI] [PubMed] [Google Scholar]