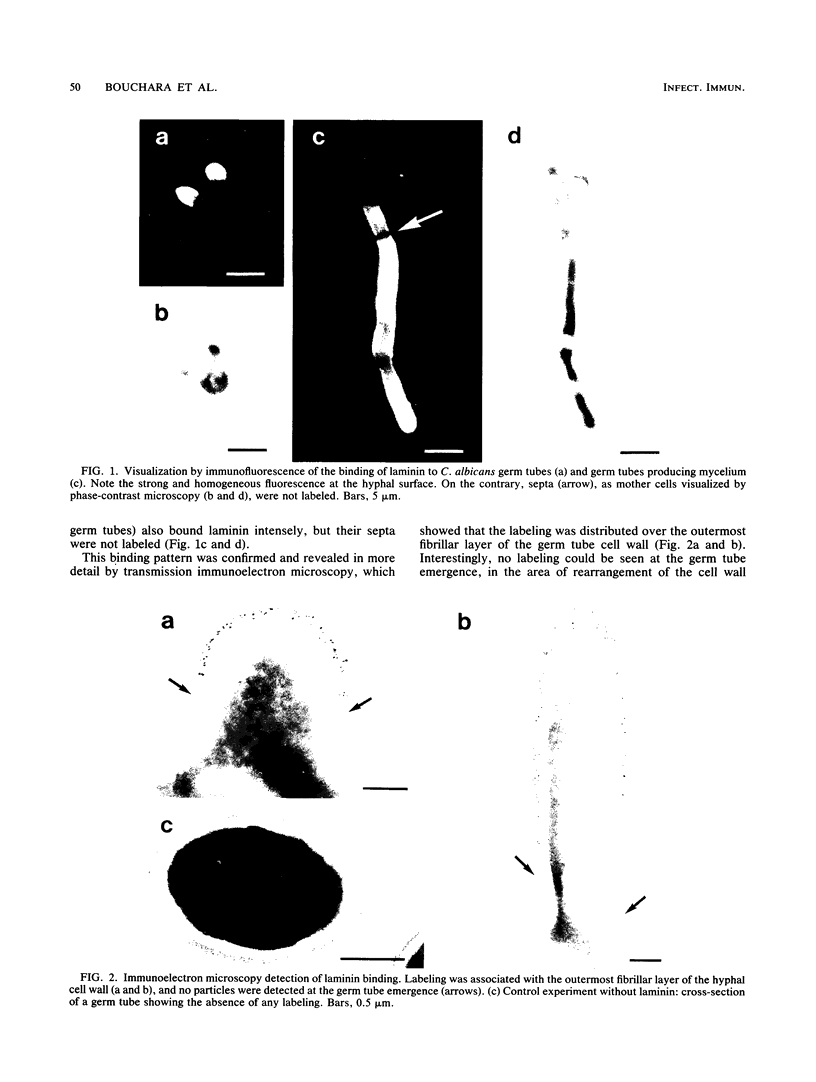
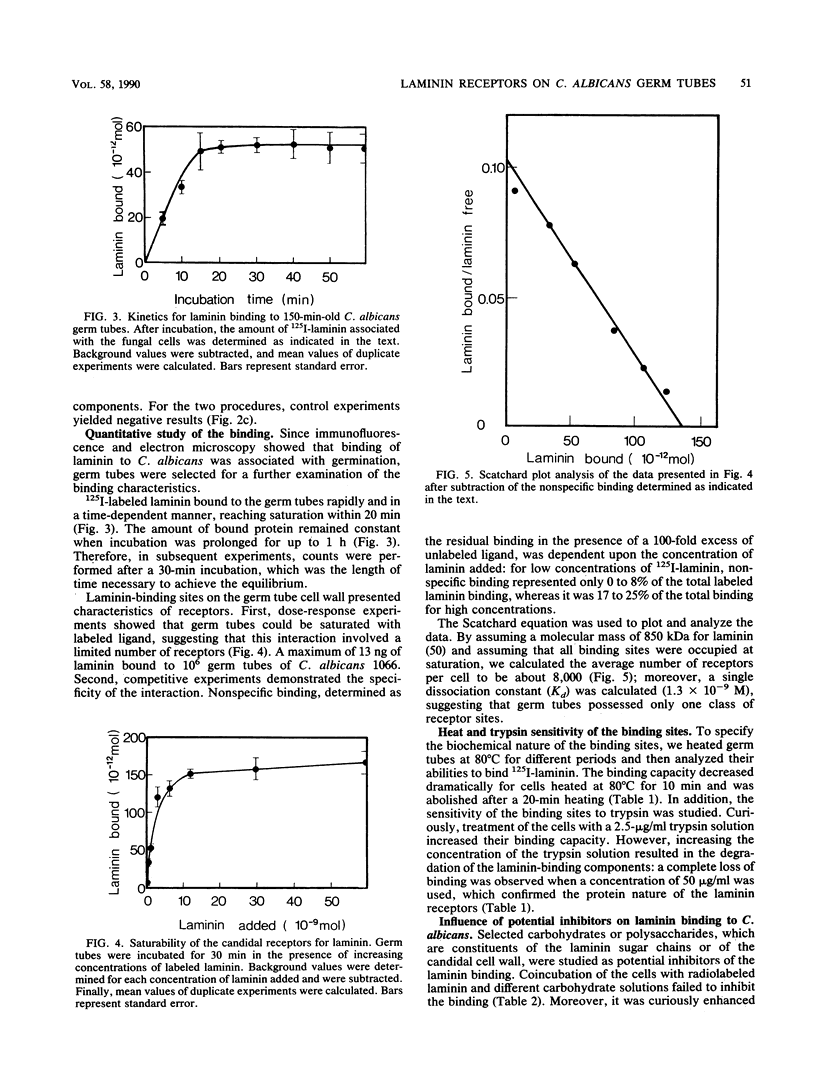
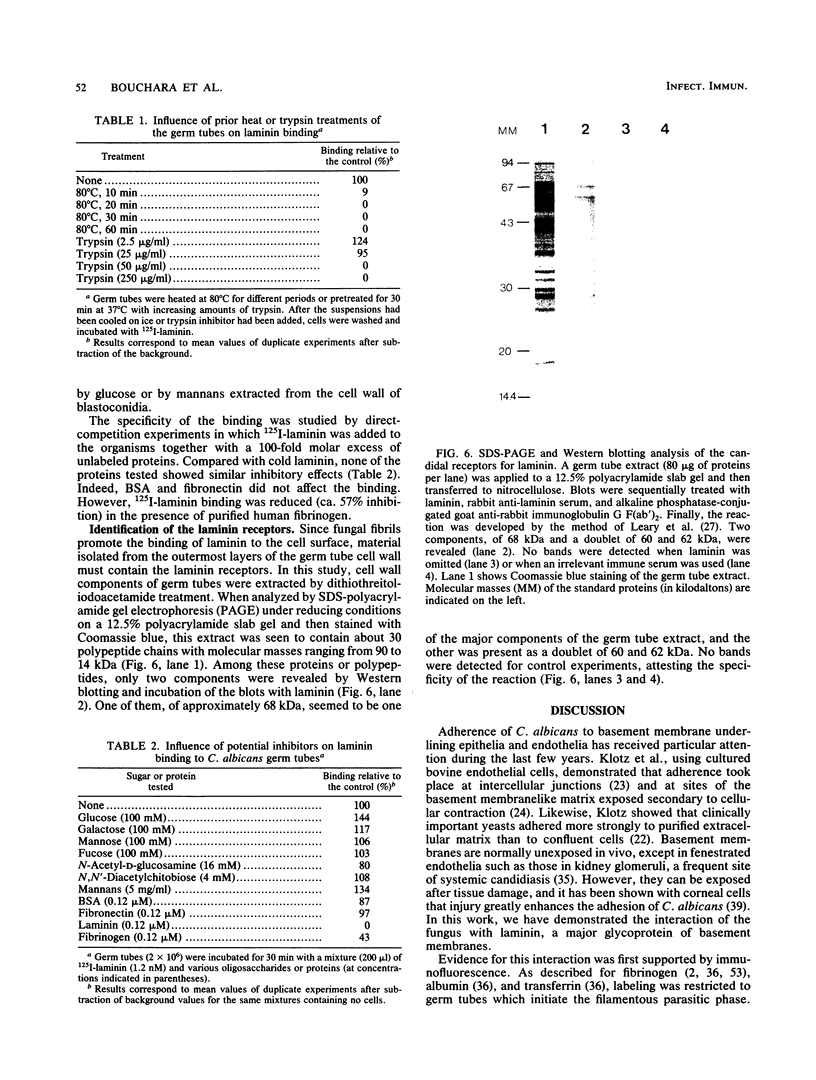
Abstract
Recent evidence for the role of laminin in cell adhesion and in the pathogenesis of several bacterial infections has led us to investigate the existence of receptors for this extracellular matrix component in Candida albicans. At first, immunofluorescence demonstrated the presence of laminin-binding sites at the surface of germ tubes. Electron microscopy confirmed this result and permitted precise localization of the binding sites on the outermost fibrillar layer of the germ tube cell wall. By using 125I-radiolabeled laminin, the binding was shown to be saturable and specific, hence demonstrating characteristics of true receptors. Analysis of the data by the Scatchard equation indicated that there were about 8,000 binding sites per cell, with a dissociation constant (Kd) of 1.3 x 10(-9) M. Binding was inhibited by prior heating or trypsinization of cells. Furthermore, of the different proteins and carbohydrates tested in competition experiments, only fibrinogen greatly reduced the laminin binding. Finally, dithiothreitol and iodoacetamide treatment of germ tubes allowed us to identify the laminin receptors through analysis of this extract by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblotting. Two components, of 68 kilodaltons and a doublet of 60 and 62 kilodaltons, were detected. Thus, C. albicans possesses germ tube-specific surface receptors for laminin which could mediate its attachment to basement membranes and so contribute to the establishment of candidiasis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsky S. H., Rao C. N., Hyams D., Liotta L. A. Characterization of a laminin receptor from human breast carcinoma tissue. Breast Cancer Res Treat. 1984;4(3):181–188. doi: 10.1007/BF01806483. [DOI] [PubMed] [Google Scholar]
- Bouali A., Robert R., Tronchin G., Senet J. M. Binding of human fibrinogen to Candida albicans in vitro: a preliminary study. J Med Vet Mycol. 1986 Aug;24(4):345–348. doi: 10.1080/02681218680000511. [DOI] [PubMed] [Google Scholar]
- Bouali A., Robert R., Tronchin G., Senet J. M. Characterization of binding of human fibrinogen to the surface of germ-tubes and mycelium of candida albicans. J Gen Microbiol. 1987 Mar;133(3):545–551. doi: 10.1099/00221287-133-3-545. [DOI] [PubMed] [Google Scholar]
- Brown S. S., Malinoff H. L., Wicha M. S. Connectin: cell surface protein that binds both laminin and actin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5927–5930. doi: 10.1073/pnas.80.19.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant G., Rao C. N., Brentani M., Martins W., Lopes J. D., Martin S. E., Liotta L. A., Schiffmann E. A role for the laminin receptor in leukocyte chemotaxis. J Leukoc Biol. 1987 Mar;41(3):220–227. doi: 10.1002/jlb.41.3.220. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Linehan L., Wadsworth E., Sandberg A. L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988 Jan;56(1):252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno A., Davis C. P., Cohen M. S., Warren M. M. Modulation of Candida albicans attachment to human epithelial cells by bacteria and carbohydrates. Infect Immun. 1983 Mar;39(3):1354–1360. doi: 10.1128/iai.39.3.1354-1360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley I. A., Douglas L. J. Role of glycosides as epithelial cell receptors for Candida albicans. J Gen Microbiol. 1987 Mar;133(3):637–643. doi: 10.1099/00221287-133-3-637. [DOI] [PubMed] [Google Scholar]
- Del Rosso M., Cappelletti R., Viti M., Vannucchi S., Chiarugi V. Binding of the basement-membrane glycoprotein laminin to glycosaminoglycans. An affinity-chromatography study. Biochem J. 1981 Dec 1;199(3):699–704. doi: 10.1042/bj1990699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L. J. Adhesion of Candida species to epithelial surfaces. Crit Rev Microbiol. 1987;15(1):27–43. doi: 10.3109/10408418709104446. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G. R., Kleinman H. K., Robey F. A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987 Mar 27;48(6):989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard T. K., Malinoff H. L., Wicha M. S. Macrophages express a plasma membrane receptor for basement membrane laminin. Am J Pathol. 1986 May;123(2):365–370. [PMC free article] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Harrison J. L., Huppert M. Adherence and penetration of vascular endothelium by Candida yeasts. Infect Immun. 1983 Oct;42(1):374–384. doi: 10.1128/iai.42.1.374-384.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Maca R. D. Endothelial cell contraction increases Candida adherence to exposed extracellular matrix. Infect Immun. 1988 Sep;56(9):2495–2498. doi: 10.1128/iai.56.9.2495-2498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Myhre E. B., Björck L., Berggård I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect Immun. 1978 Oct;22(1):136–142. doi: 10.1128/iai.22.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota G. F., Carneiro C. R., Gomes L., Lopes J. D. Monoclonal antibodies to Staphylococcus aureus laminin-binding proteins cross-react with mammalian cells. Infect Immun. 1988 Jun;56(6):1580–1584. doi: 10.1128/iai.56.6.1580-1584.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S., Odds F. C. Binding of plasma proteins to Candida species in vitro. J Gen Microbiol. 1988 Oct;134(10):2693–2702. doi: 10.1099/00221287-134-10-2693. [DOI] [PubMed] [Google Scholar]
- Rao N. A., Riggio D. W., Delmage J. M., Calandra A. J., Evans S., Lewis W. Adherence of Candida to corneal surface. Curr Eye Res. 1985 Aug;4(8):851–856. doi: 10.3109/02713688509095252. [DOI] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Calderone R. A., Edwards J. E., Jr Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986 Jan-Feb;8(1):73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Edwards J. E., Jr, Gibson T. R., Moore J. C., Cohen A. H., Green I. Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis. 1985 Dec;152(6):1264–1274. doi: 10.1093/infdis/152.6.1264. [DOI] [PubMed] [Google Scholar]
- Sandin R. L., Rogers A. L., Patterson R. J., Beneke E. S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982 Jan;35(1):79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Lehrer N., Ofek I. Adherence of Candida albicans to human vaginal epithelial cells: inhibition by amino sugars. Exp Cell Biol. 1982;50(1):13–17. doi: 10.1159/000163121. [DOI] [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Segal E., Sreevalsan T., Scheld W. M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984 Feb;30(2):221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Robert R., Senet J. M. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesins. Infect Immun. 1988 Aug;56(8):1987–1993. doi: 10.1128/iai.56.8.1987-1993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Robert R., Bouali A., Senet J. M. Immunocytochemical localization of in vitro binding of human fibrinogen to Candida albicans germ tube and mycelium. Ann Inst Pasteur Microbiol. 1987 Mar-Apr;138(2):177–187. doi: 10.1016/0769-2609(87)90194-3. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Liotta L. A., Jaye M., Ricca G. A., Drohan W. N., Claysmith A. P., Rao C. N., Wirth P., Coligan J. E., Albrechtsen R. Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7137–7141. doi: 10.1073/pnas.83.19.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J. R., John S. R., Kramer R. H., Hoover C. I., Murray P. A. Attachment of oral bacteria to a basement-membrane-like matrix and to purified matrix proteins. Infect Immun. 1987 Nov;55(11):2721–2726. doi: 10.1128/iai.55.11.2721-2726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannariello-Brown J., Wewer U., Liotta L., Madri J. A. Distribution of a 69-kD laminin-binding protein in aortic and microvascular endothelial cells: modulation during cell attachment, spreading, and migration. J Cell Biol. 1988 May;106(5):1773–1786. doi: 10.1083/jcb.106.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]