Abstract
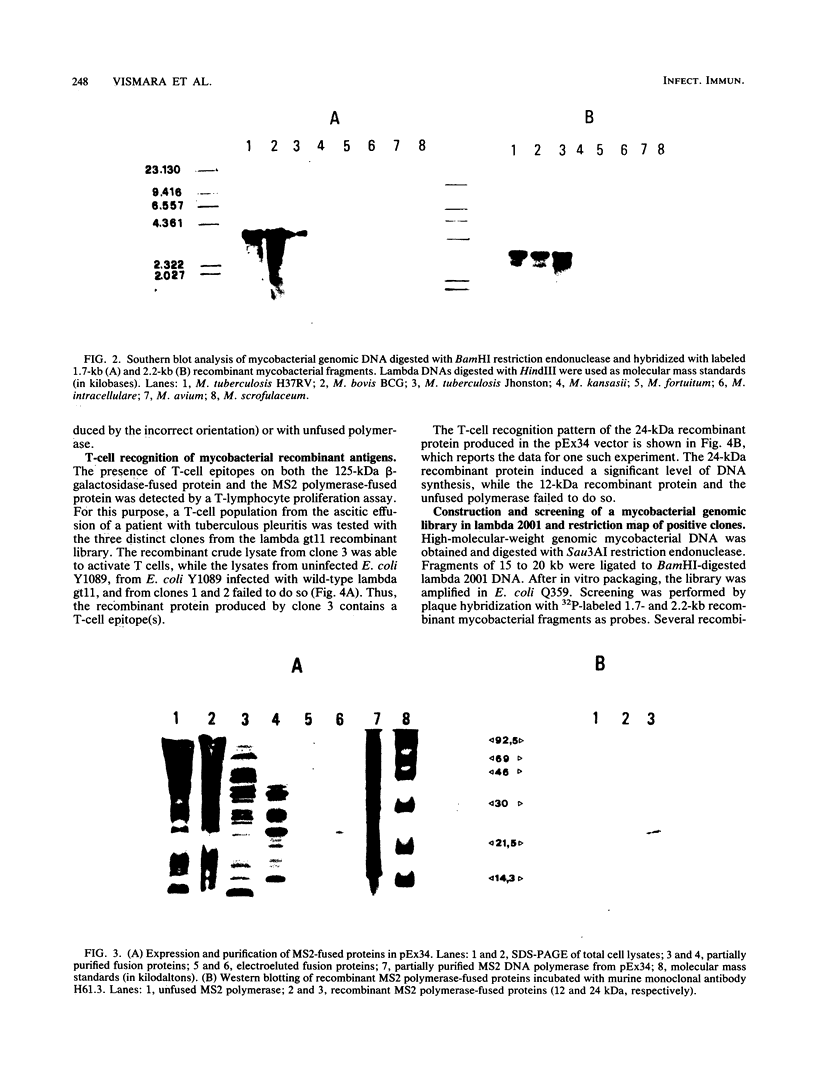
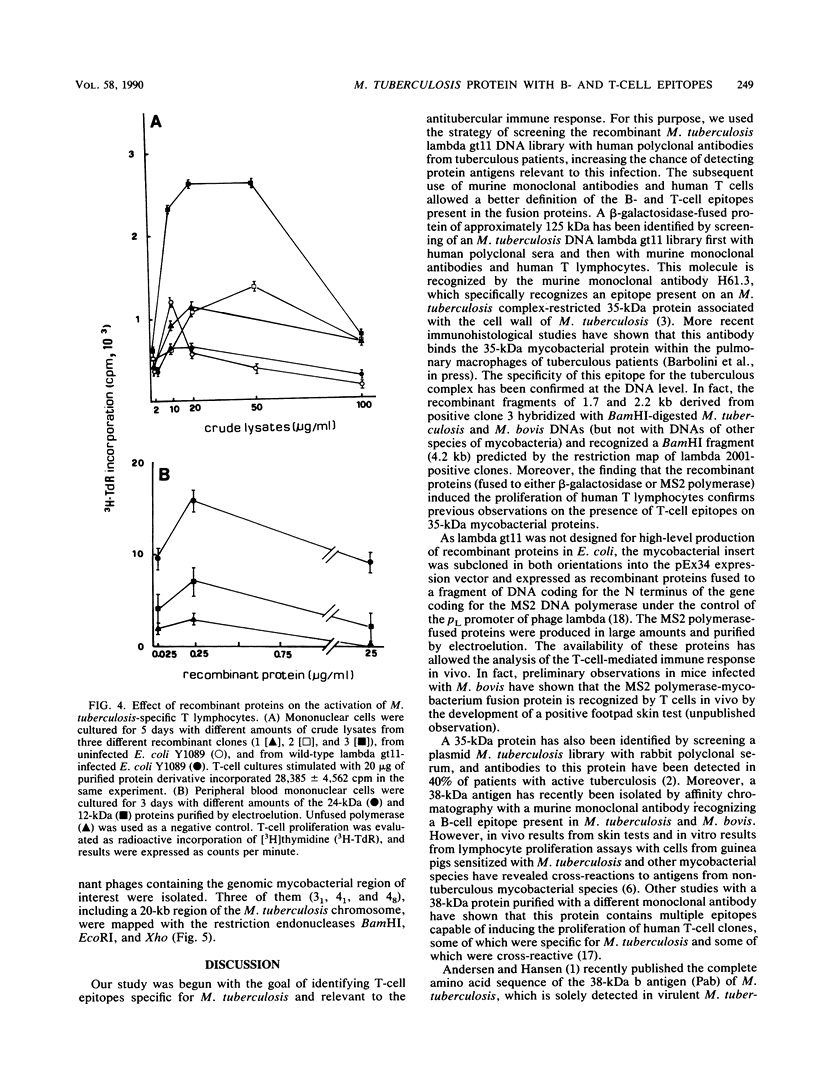
Screening of a Mycobacterium tuberculosis genomic DNA library in the lambda gt11 expression vector was carried out by using, as probes, sera from tuberculous patients and murine monoclonal antibody H61.3 recognizing a mycobacterial 35-kilodalton protein present only on the M. tuberculosis complex. The recombinant beta-galactosidase-fused protein present in the crude lysate induced the proliferation of T lymphocytes from patients with tuberculous pleuritis. As the recombinant insert contains an internal EcoRI restriction site, it was possible to identify two fragments, one proximal to the lacZ gene and 1.7 kilobases (kb) in size and the other distal to the lacZ gene and 2.2 kb in size. Southern blot analysis showed that both of them hybridized with the genomic DNA from M. tuberculosis and M. bovis but not with the DNA from other mycobacterial species. To perform extensive immunological studies, the amount of beta-galactosidase-fused protein being very low, we fused the 1.7-kb fragment to the N-terminal part of the gene coding for the DNA polymerase of bacteriophage MS2 in the expression vector pEx34. The fusion protein was partially purified, and subsequent Western blotting (immunoblotting) and T-cell proliferation experiments confirmed the presence of B- and T-cell mycobacterial epitopes. Furthermore, to isolate the chromosomal region containing the 35-kilodalton gene, we constructed another mycobacterial genomic library in the lambda 2001 vector by cloning 15 to 20 kb of foreign DNA. Screening of this library was carried out by using 1.7- and 2.2-kb recombinant fragments as probes. Restriction maps of some clones isolated were determined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen M. L., Mayer L. W., Rumschlag H. S., Yakrus M. A., Jones W. D., Jr, Good R. C. Expression of proteins of Mycobacterium tuberculosis in Escherichia coli and potential of recombinant genes and proteins for development of diagnostic reagents. J Clin Microbiol. 1987 Jul;25(7):1176–1180. doi: 10.1128/jcm.25.7.1176-1180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani G., Biano A., Beltrame A., Vismara D., Mezzopreti M. F., Colizzi V., Young D. B., Bloom B. R. Generation and characterization of monoclonal antibodies to 28-, 35-, and 65-kilodalton proteins of Mycobacterium tuberculosis. Infect Immun. 1988 May;56(5):1281–1287. doi: 10.1128/iai.56.5.1281-1287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. New bacteriophage lambda vectors with positive selection for cloned inserts. Methods Enzymol. 1983;101:3–19. doi: 10.1016/0076-6879(83)01004-6. [DOI] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity against intracellular bacteria: biological effector functions and antigen specificity of T lymphocytes. Curr Top Microbiol Immunol. 1988;138:141–176. [PubMed] [Google Scholar]
- Kingston A. E., Salgame P. R., Mitchison N. A., Colston M. J. Immunological activity of a 14-kilodalton recombinant protein of Mycobacterium tuberculosis H37Rv. Infect Immun. 1987 Dec;55(12):3149–3154. doi: 10.1128/iai.55.12.3149-3154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A., Young R. A., Young D. B. The identification of T cell epitopes in Mycobacterium tuberculosis using human T lymphocyte clones. Lepr Rev. 1986 Dec;57 (Suppl 2):131–137. [PubMed] [Google Scholar]
- Lamb J. R., Rees A. D., Bal V., Ikeda H., Wilkinson D., De Vries R. R., Rothbard J. B. Prediction and identification of an HLA-DR-restricted T cell determinant in the 19-kDa protein of Mycobacterium tuberculosis. Eur J Immunol. 1988 Jun;18(6):973–976. doi: 10.1002/eji.1830180623. [DOI] [PubMed] [Google Scholar]
- Matthews R., Scoging A., Rees A. D. Mycobacterial antigen-specific human T-cell clones secreting macrophage activating factors. Immunology. 1985 Jan;54(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Bartoloni A., Perugini M., Rappuoli R. Expression and immunological properties of the five subunits of pertussis toxin. Infect Immun. 1987 Apr;55(4):963–967. doi: 10.1128/iai.55.4.963-967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Husson R., Young R. A., Godal T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J Immunol. 1987 Feb 1;138(3):927–931. [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Shinnick T. M., Houghten R. A., Kvalheim G., Degre M., Lundin K. E., Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J Immunol. 1988 Oct 15;141(8):2749–2754. [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strebel K., Beck E., Strohmaier K., Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986 Mar;57(3):983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara D., Lombardi G., Piccolella E., Colizzi V. Dissociation between interleukin-1 and interleukin-2 production in proliferative response to microbial antigens: restorative effect of exogenous interleukin-2. Infect Immun. 1985 Aug;49(2):298–304. doi: 10.1128/iai.49.2.298-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kent L., Young R. A. Screening of a recombinant mycobacterial DNA library with polyclonal antiserum and molecular weight analysis of expressed antigens. Infect Immun. 1987 Jun;55(6):1421–1425. doi: 10.1128/iai.55.6.1421-1425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]