Abstract
Amphibian metamorphosis serves as an excellent model to study T3 function during postembryonic development in vertebrate due to its total dependence on T3. Earlier molecular studies in the model species Xenopus laevis have led to a number of important in vivo findings on the function and mechanisms of T3 receptor (TR) action during vertebrate development. However, the lack of genomic sequence information, its tetraploid genome, and lengthy developmental cycle hinder further analyses on TR functions. In this regard, the highly related species, Xenopus tropicalis, is much more advantageous. Toward developing X. tropicalis for genome-wide and genetic studies of TR function, we analyzed the expression profiles of TRs and their heterodimerization partners, retinoid X receptors (RXRs) or 9-cis retinoic acid receptors. We show that their expression correlates with transformations in different organs and that TR/RXR heterodimers are capable of repressing and activating gene expression in vivo in the absence and presence of T3, respectively. We further demonstrate that TRs are bound to endogenous target genes in X. tropicalis tadpoles. Our results thus support a role of TRs in mediating the metamorphic effects of T3 in X. tropicalis. More importantly, the similarities in the expression and function between X. tropicalis and X. laevis TRs and RXRs as demonstrated by our study also pave the way to take advantages of existing morphological, molecular, and cellular knowledge of X. laevis development and the genetic and sequence superiority of X. tropicalis to dissect the molecular pathways governing tissue/organ-specific transformations during vertebrate postembryonic development.
T3 RECEPTORS (TRs) are ligand-dependent transcription factors. They form heterodimers with retinoid X receptors (RXRs) (1,2,3,4,5). The TR/RXR heterodimers repress and activate T3-inducible genes in the absence and presence of T3, respectively. In vitro and cell culture studies have shown that TRs function by recruiting corepressors when unliganded and coactivators when bound to T3.
T3 plays a critical role in adult organ function and development in mammals (2,3,6,7,8,9). High levels of T3 present during the last few months of fetal development and shortly after birth are critical for organ maturation, growth, and brain development in humans, and T3 deficiency causes a number of development abnormalities, including cretinism, which is characterized by extremely short stature and metal retardation. Unfortunately, the difficulty to manipulate the uterus-enclosed mammalian embryos has severely limited molecular and functional studies of T3 action during this critical postembryonic developmental period.
Amphibian metamorphosis shares many similarities with postembryonic development in mammals (6,7,8). This process is entirely controlled by T3. Inhibiting the synthesis of endogenous T3 leads to the formation of giant tadpoles failed to undergo natural metamorphosis but added physiological levels of exogenous T3 to the rearing water of premetamorphic tadpoles initiates precocious metamorphosis. This intriguing ability of a simple chemical controlling the transformation of essentially every organ/tissue of the animal has led to extensive morphological, biochemical, cellular, and molecular studies in the past century. Most of the molecular studies in the past 2 decades have been on the model species Xenopus laevis (7,10,11,12,13). These have led to the first systematic isolation and characterization of the T3 response genes during vertebrate development. More recently transgenic studies have allowed us and others to demonstrate that TRs are not only necessary but also sufficient to mediate the metamorphic effects of T3 in vivo (10,14,15,16,17). In addition, chromatin immunoprecipitation (ChIP) assays have demonstrated, again for the first time in vivo, the binding of TRs to target gene promoters in developing animals and T3-dependent, tissue- and gene-specific recruitment of cofactors in gene regulation and function by TR during metamorphosis (10,18,19,20,21,22,23,24,25,26).
Despite the enormous progress from the analyses in X. laevis, a few important limitations hamper further studies on TR function and mechanisms. Among them include the lack of genomic sequence information, its pseudotetraploid genome, and lengthy developmental cycle. These make it difficult to address some of the most important and urgent questions, such as genome-wide analysis of TR function to determine whether and how TRs regulate tissue/organ-specific target genes to induce tissue-specific metamorphic changes and genetic studies of TR and cofactor functions in vivo, etc. The highly related species, X. tropicalis, is much more superior in this regard (27). Its diploid genome has been sequenced, and it takes less than half of the time that X. laevis takes from fertilization to sexual maturity. Its smaller size also makes it easier to carry out genetic studies of gene function.
To take these advantages for studying the molecular mechanisms of the regulation of vertebrate development by T3, it is critical to determine whether and how TRs are regulated and function during X. tropicalis development. Here we studied the regulation and function of TRs and RXRs in X. tropicalis. We show that TR and RXR genes are expressed during metamorphosis. In general, high levels of their mRNAs are present at the stages in a given organ when it undergoes the most dramatic metamorphic changes, with TRβ strongly up-regulated by T3 in all organs analyzed. Furthermore, we demonstrate that X. tropicalis TR/RXR heterodimers also repress a T3-inducible promoter in vivo in the context of chromatin when T3 is absent and activate the promoter when T3 is present. Finally, we show by ChIP assay that X. tropicalis TRs are bound to its target promoters in metamorphosing tissues, supporting a direct role of TRs in mediating the metamorphic effects of T3 in X. tropicalis.
Materials and Methods
Experimental animals
All experiments involving X. tropicalis animals described here were conducted in accord with accepted standards of humane animal care and approved by Animal Use and Care Committee of National Institute of Child Health and Human Development, National Institutes of Health. X. tropicalis adults were purchased from NASCO (Fort Atkinson, WI). Tadpoles of X. tropicalis were produced and reared in the laboratory. They were staged according to other published reports (28). Animals at indicated stages were euthanized for RNA isolation or ChIP assays.
Antibodies
Two rabbit polyclonal anti-TR antibodies were used. The anti-TR (PB) antibody was made by immunizing a rabbit with polyacrylamide gels containing Escherichia coli produced, His-tagged X. laevis TRβ (29). Because very little of the antibody remained, the His-tagged X. laevis TRβ was produced in E. coli again and purified (ProteinOne, Inc., Bethesda, MD). The soluble protein was injected into rabbits to generate the anti-TR (new PB) antibody (Covance, Princeton, NJ). A polyclonal antibody against Id14, an extracellular protein (30), was used as a negative control for in vivo ChIP assays. The anti-FLAG M2 antibody was obtained from Sigma (St. Louis, MO).
TR and RXR cloning
Total RNA was extracted from the intestine of adult frog by TRIZOL (Invitrogen, Carlsbad, CA) and followed by deoxyribonuclease treatment with DNA-free (Ambion, Austin, TX) to remove any DNA contamination. The cDNA library was constructed by using SMART RACE cDNA amplification kit (BD Biosciences) and oligo (deoxythymidine) primers. Three pairs of primers (5′-TTCTCGAGATGGACCAGAATCTCAGCGGG-3′, forward and 5′-GGACTAGTTCAGACTTCCTGGTCCTCAAA-3′, reverse; 5′-TTCTCGAGATGCCAAGCAGTATGTCAGAG-3′, forward and 5′-GGGATATCCTAGTCCTCAAACACTTCCAA-3′, reverse; 5′-TTCTCGAGATGGGAGACAGTCGGGTATGC-3′, forward and 5′-GGACTAGTTCAAGATAGCTGGTGAGGTGC-3′, reverse) were used to clone the full-length X. tropicalis TRα, TRβ, and RXRβ, respectively. The PCR products were cloned into T7TS vector (31,32) to produce fusion proteins with three copies of the FLAG tag. For RXRβ, the FLAG tag was removed after cloning into T7TS vector.
RT-PCR
Total RNA was extracted from the brain, intestine, limb, and tail of X. tropicalis tadpoles at stages 52 (premetamorphosis) to 66 (no tail at this stage, end of metamorphosis) (staged according to Ref. 28) or after 10 nm T3 treatment for 0–3 d with TRIZOL (Invitrogen), and followed by deoxyribonuclease treatment with DNA-free (Ambion) to remove any DNA contamination. RT-PCR was performed by using SuperScript III one-step RT-PCR with PlatinumTaq (Invitrogen) with 200 ng total RNA as the template. Five pairs of primers, (5′-TCGCACCATCCAGAAGAACCTG-3′, forward and 5′-TTCCTACGCTGTTTCCAGTGGC-3′, reverse for TRα; 5′-TCAGAAGAACCTCCATCCGAGC-3′, forward and 5′-ATCCACTTTTCCACCCTCAGGC-3′, reverse for TRβ; 5′-CAAGCAGCAGACAAGCAACTCTTC-3′, forward and 5′-CAATGGCTCGTAAGCAACCG-3′, reverse for RXRα; 5′-CCTCTGCCCCTTTACATCCTTC-3′, forward and 5′-GCACACAGCCTCTTTCCACTAACTG-3′, reverse for RXRβ; and 5′-TGCCAGTATTGCCGCTATCAG-3′, forward and 5′-TTTGCCCATTCCACCAAGG-3′, reverse for RXRγ) were used to determine the relative expression levels of the five genes by RT-PCR. The expression of EF1α was analyzed similarly as an internal control for RNA quantity and quality by including the primer pair (5′-TGTCCACCGCCAAACATCTAAC-3′, forward and 5′-TCACCGTTCCATCCAGAAATAGG-3′, reverse) in the same PCR tubes. For RT-PCR analysis of the expression of TH/bZIP gene, the primers 5′-CTGCTGCCCATTCAGGCATC-3′ (forward) and 5′-CTTCTCATCTGGGGTGAATTCC-3′ (reverse) were used. The RT-PCR cycle number for TRα and TRβ and TH/bZIP was 25, and that for RXRα, -β, and -γ was 28. The cycle numbers were chosen empirically for each gene to ensure that the PCR did not reach saturation.
Quantitative real-time RT-PCR (qRT-PCR) with Taqman probes was carried out to quantify the expression levels of TRα and β on ABI 7000 (Applied Biosciences, Foster City, CA) as described previously (16). For the detection of TRα, forward primer 5′-CTGCTTGTCAGAGCCAGATGAA-3′, reverse primer 5′-TCCCATACATTGGCTGTTCTTTCTT-3′ and FAM-labeled Taqman probe ATCCGGCCACCTTTT were used. For the detection of TRβ, forward primer 5′-CAAGAGTTGTTGATTTTGCCAAAAAGC-3′, reverse primer 5′-ACATGATCTCCATACAACAGCCTTT-3′ and FAM-labeled Taqman probe CTGCCATGTGAAGACC were used. A set of primers/probe specific for ribosomal protein rpL8 (33) was used as the control for RNA input for each sample, and the expression level of the gene of interested in each sample was normalized to that of rpL8, which is essentially constant during development and independent of T3 treatment (34).
Transcription assay in X. laevis oocytes and Western blot analysis
The transcription assay in the X. laevis oocyte was done as described previously (29). To express proteins in the oocyte, expression plasmids for indicated genes were used to synthesize the corresponding mRNAs with T7 or SP6 in vitro transcription kits (mMESSAGE mMACHINE; Ambion). The mRNAs (1.15 ng/oocyte for X. tropicalis TRs or RXRβ) were microinjected into the cytoplasm. The firefly luciferase reporter plasmid TRE-Luc (0.33 ng/oocytes), under the control of the T3-regulated promoter of X. laevis TRβA gene, and the control Renilla luciferase vector phRG-TK (0.03 ng/oocytes) were coinjected into the oocyte nucleus after mRNA injection. After overnight incubation at 18 C in the absence or presence of 100 nm T3, oocyte lysates were prepared for luciferase assay with the dual-luciferase assay kit (Promega, Madison, WI) to determine the relative expression levels from the T3-regulated promoter over the control promoter. Fifteen identically injected oocytes for each sample were divided into three groups, and a luciferase assay was done for each group. Each data point represented the average of the three groups. The data shown here are representative of three independent experiments with similar results. A portion of the lysate was used for Western blotting with anti-TR and anti-FLAG antibodies.
ChIP assay
ChIP assay with oocytes was done as described previously (32). The mRNAs (5.75 ng/oocytes for TR or RXRβ) were injected into the cytoplasm of oocytes. After 2 h incubation at 18 C, the nucleus of the oocytes was then injected with the reporter DNA TRE-Luc (0.99 ng/oocyte) and phRG-TK (0.09 ng/oocyte). The oocytes were subsequently incubated at 18 C overnight with or without 100 nm T3 treatment, followed by treatment with 1% formaldehyde for 15 min. The oocytes were then washed twice with a solution of 10 mm HEPES (pH 7.6), 88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3 0.8 mm MgSO4, 0.4 mm CaCl2, 0.3 mm Ca(NO3)2 and incubated with 100 mm Tris-HCl (pH 9.4) and 10 mm dithiothreitol at 30 C for 15 min. After rinsing once with the homogenization buffer [20 mm Tris-HCl (pH 7.6), 60 mm KCl, 15 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, and Complete mini-EDTA-free (Roche, Indianapolis, IN; proteinase inhibitor cocktail tablets)], the oocytes were homogenized in the homogenization buffer. The homogenates were sonicated on ice and centrifuged at 13,000 rpm for 15 min at 4 C. The chromatin in the supernatant was diluted with equal volume of ChIP I buffer [0.1% sodium deoxycholate; 1% Triton X-100; 2 mm EDTA; 50 mm HEPES (pH 7.5); 150 mm NaCl, 0.4 mm phenylmethylsulfonyl fluoride, and Complete mini-EDTA-free (Roche; proteinase inhibitor cocktail tablets)] (Upstate Cell Signaling Solutions, Lake Placid, NY), quantitated, and frozen in aliquots at −80 C.
For immunoprecipitation, the DNA concentration of the chromatin was diluted to 10 ng/μl with a solution of equal volume of the homogenization buffer and ChIP Buffer I (Upstate Cell Signaling Solutions). After preclearing with salmon sperm DNA/protein A-agarose beads, input samples were taken, and 100 μl of each chromatin sample were added to tubes containing salmon sperm DNA/protein A-agarose beads and anti-TR (new PB) or anti-TR (PB) antibody. The mixture was incubated overnight at 4 C. The beads were then washed with 1 ml of ChIP Buffer I, ChIP Buffer II, ChIP Buffer III, and Tris/EDTA in succession (Upstate Cell Signaling Solutions). After the last wash, 100 μl of elution buffer were added to the samples as well as the input controls and incubated at 65 C for 6 h. The DNA was purified by QIAquick PCR purification kit (QIAGEN, Valencia, CA) and eluted with 40 μl EB buffer (QIAGEN).
The immunoprecipitated DNA was analyzed by quantitative PCR with Taqman probes on ABI 7000 (Applied Biosciences) by using gene-specific primers for the T3 response element (TRE) region of the reporter X. laevis TRβ promoter (32) and ampicillin resistance gene in the same reporter plasmid but far away from the TRE region (forward primer: 5′-GCCGAGCGCAGAAGTG-3′, reverse primer: 5′-TCTAGCTTCCCGGCAACAATTAA-3′, and FAM-labeled Taqman probe: 5′-CCGCCTCCATCCAGTCTA-3′).
ChIP assay on tadpole intestine was done as described previously (22) with anti-TR (PB) and anti-TR (new PB) antibodies. As a negative control, a polyclonal antibody against Id14, an extracellular protein (30), was also used. The immunoprecipitated DNA was analyzed by quantitative PCR with the gene-specific primers/probes for X. laevis TRβ promoter, TH/bZIP promoter, and exon 5 of TRβ (22) because the primer/probe sequences are conserved in X. tropicalis (data not shown). All ChIP experiments were done two to four times with similar results.
Statistics and sequence comparison
All of the data were presented as mean ± sem. Statistical significance for the difference between two groups was determined by Student’s t test. The level of significance was set for P < 0.05. The software MacVector version 8.0.1 was used to compare the amino acid sequence identities between X. laevis and X. tropicalis TRs and RXRs. The sequences used were full-length X. laevis TRs and RXRs (TRα: LOC397942; TRβ: LOC397734; RXRα: L11446; RXRβ: NM 00181088361; RXRγ: BC088915), full-length X. tropicalis TRs (accession no. EU723571 for TRα and EU723572 for TRβ), and RXRβ (NM_001015937) and partial sequences for RXRα (www.ensemble.org, Ensemble Transcript ID: ENSXETT00000042363, with the C-terminal 451 aa used for comparison) and RXRγ (BC121595, with the C-terminal 452 aa used for comparison).
Results
X. tropicalis and X. laevis TR and RXR genes are highly conserved
To investigate the possibility of using X. tropicalis as a model to study TR function during T3-dependent amphibian metamorphosis, we first compared the sequences of TRs and RXRs of X. tropicalis and X. laevis. Computer search of the databases for genomic sequences and expressed sequence tags identified the sequences of all TR and RXR genes in X. tropicalis. RT-PCR cloning was performed to clone the full-length TRα, TRβ, and RXRβ in X. tropicalis. Pair-wise sequence comparison revealed that X. tropicalis and X. laevis TR and RXR homologs shared over 96% identities (Tables 1 and 2). These homologies approach those observed between the two duplicated copies of TRα and TRβ genes in X. laevis (35), suggesting conserved functions across the two Xenopus species.
Table 1.
The amino sequence identities among TRs in X. laevis and X. tropicalis
| Identity (%) |
X. laevis
|
X. tropicalis
|
|||
|---|---|---|---|---|---|
| TRα | TRβ | TRα | TRβ | ||
| X. laevis | TRα | 100 | |||
| TRβ | 76.57 | 100 | |||
| X. tropicalis | TRα | 98.56 | 77.29 | 100 | |
| TRβ | 83.03 | 96.08 | 83.55 | 100 | |
Table 2.
The amino sequence identities among RXRs in X. laevis and X. tropicalis
| Identity (%) |
X. laevis
|
X. tropicalis
|
|||||
|---|---|---|---|---|---|---|---|
| RXRα | RXRβ | RXRγ | RXRα | RXRβ | RXRγ | ||
| X. laevis | RXRα | 100 | |||||
| RXRβ | 73.23 | 100 | |||||
| RXRγ | 67.45 | 67.70 | 100 | ||||
| X. tropicalis | RXRα | 96.90 | 71.62 | 69.18 | 100 | ||
| RXRβ | 74.83 | 96.66 | 68.37 | 73.27 | 100 | ||
| RXRγ | 70.35 | 65.93 | 96.02 | 69.84 | 67.04 | 100 | |
TR and RXR expression correlates with organ-specific metamorphosis
To investigate the roles of TR and RXR genes during metamorphosis, we analyzed the temporal regulation of these genes in several different organs during metamorphosis. Earlier studies in model species such as X. laevis and Rana catesbeiana have shown that the tail undergoes complete resorption through apoptosis and the limbs undergo de novo development, whereas brain and intestine undergo extensive remodeling involving both cell death and cell proliferation followed by differentiation (7,12,36). To determine whether X. tropicalis TR and RXR expression correlates with these changes, we analyzed TR and RXR mRNAs by RT-PCR across premetamorphic stages (stages 52–56), metamorphic climax (stages 58–64), and end of metamorphosis (stage 66). As shown in Fig. 1A, the mRNA level of TRβ increased dramatically in the brain at the climax of metamorphosis stages 61–64, whereas there was only a slight increase in TRα mRNA level during metamorphosis. The mRNA levels of RXR genes remained relatively constant in the brain during metamorphosis.
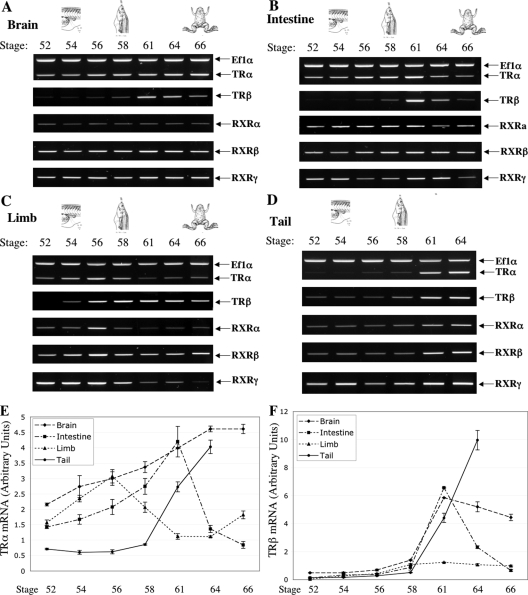
Figure 1.
Organ-specific expression of TRs and RXRs during spontaneous metamorphosis in X. tropicalis. RNAs were isolated from the brain (A), intestine (B), limb (C), and tail (D) of tadpoles at the indicated developmental stages and used for RT-PCR analysis of the expression of TRs and RXRs. In each RT-PCR, a primer set for Ef1α gene was also included to analyze the expression of Ef1α as an internal control, although Ef1α expression was shown only for TRα panel. Note that the TRβ panel for the limb was a longer exposure compared with other organs due to its weaker signal (for more quantitative comparison, see panel F). In general, the expression of TRs and RXRs peaks in individual organs at the climax of their metamorphic changes, i.e. around stage 61 in the brain and intestine, stage 56 in the limb, and stage 64 in the tail, and the expression of TRβ changes most dramatically during metamorphosis. E and F, The same RNA samples were analyzed by qRT-PCR to confirm the organ-specific changes observed by RT-PCR for TRα (E) and TRβ (F) during metamorphosis. The expression of the ribosomal protein rpL8 was also analyzed by qRT-PCR for the RNA samples, and the ratio of the TR signal to rpL8 signal was shown. For each stage, nine animals were used and all experiments were repeated twice with similar results.
In the animal intestine (Fig. 1B), the mRNA level of TRβ increased dramatically at stage 61 but was very low before and at the end of metamorphosis. Similarly, the expression of TRα increased gradually starting from stage 52, peaked at stage 61, and then reduced toward the end of metamorphosis, although overall magnitude of change was much smaller than that of TRβ. Again the mRNA levels of RXR genes were relatively constant during metamorphosis.
The limbs had different patterns of TR and RXR expression (Fig. 1C). The mRNA level of TRβ in limb increased gradually from stage 52 to 58 and then changed little toward the end of the metamorphosis. The mRNA levels of TRα and RXRs peaked at stage 56 and then decreased to lower levels. Whereas these patterns were different from those in the intestine and brain, higher levels of TR and RXR mRNAs were present in the stages when limb morphogenesis occurs (stages 54–58).
Finally, in the tail, the expression of all TRs and RXRs increased dramatically after stage 58, and high levels were present throughout tail resorption (stages 61–64, no tail remains by stage 66) (Fig. 1D).
To confirm the expression profiles, we carried out qRT-PCR analysis on TR genes. As shown in Fig. 1, E and F, and consistent with Fig. 1, A–D, TR expression correlated with metamorphosis in the different organs. The mRNA levels of both TR genes peaked at stage 61 in the intestine and increased after stage 58 in the brain and tail, although the changes were much more dramatic for TRβ (Fig. 1F) than TRα (Fig. 1E), just like that in Fig. 1, A, B, and D. In the limb, again, TRα expression peaked at stage 56, whereas TRβ expression increased gradually by stage 58 and stayed fairly constant afterward. In addition, qRT-PCR analysis also showed that the peak level of TRβ in the limb was much lower than those in other organs (Fig. 1F), consistent with the longer exposure needed for detection with RT-PCR analysis in Fig. 1C. (Note that the overall fold-increase in TRβ mRNA level in the limb during development was similar to those in other organs due to the very low levels in the limb at stage 52.)
Because T3 is known to induce gene expression and metamorphosis in premetamorphic tadpoles in X. laevis and R. catesbeiana (7,12), we carried out similar analyses to determine the expression profiles of TRs and RXRs during T3-induced metamorphosis in X. tropicalis tadpoles. We treated premetamorphic tadpoles at stage 54 with T3 and isolated total RNA from the brain, intestine, limb, and tail. RT-PCR analysis showed that T3 dramatically up-regulated the expression of TRβ. Lower levels of up-regulation were also observed for the TRα and RXRβ genes, whereas the expression of RXRα and RXRγ was not significantly affected by T3 treatment (Fig. 2). qRT-PCR analysis on TRα and TRβ expression confirmed the regulation profiles (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals. org). Thus, the TR and RXR expression profiles during T3 treatment mimic those during natural metamorphosis (note that the short period of T3 treatment mimic morphologically the period of development from stage 54 to around 56, when TR expression is also up-regulated in the limb, although not as dramatically compared with that during T3 treatment). These results suggest that TR and RXR expression pattern correlate temporally with organ-specific transformations, supporting their roles in mediating the metamorphic effects of T3.
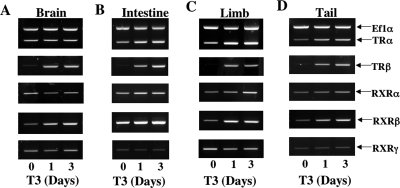
Figure 2.
Effects of T3 treatment of premetamorphic X. tropicalis tadpoles on TR and RXR expression in brain (A), intestine (B), limb (C), and tail (D). Tadpoles at stage 54 were exposed to 10 nm T3 for 0–3 d before tissue isolation and RNA extraction. RT-PCR analyses were done as in Fig. 1 for TR and RXR genes with Ef1α gene as an internal control. Note that TRβ was regulated most dramatically, followed by TRα and RXRβ, whereas RXRα and RXRγ was not significantly affected by T3 treatment, consistent with the developmental profiles in Fig. 1. For each time point, nine animals were used and all experiments were repeated two times with similar results.
X. tropicalis TR and RXR regulate transcription in a T3-dependent manner in vivo
The high degrees of identity between TRs and RXRs of X. laevis and X. tropicalis suggest that X. tropicalis TRs and RXRs also form heterodimers and repress or activate transcription from a target promoter in the absence or presence of T3, respectively. To investigate this possibility in vivo, we analyzed the effect of TR and RXR expression on the transcription of a target gene in the reconstituted X. laevis oocyte system, which provides a model for studying gene regulation in the context of chromatin (29). As a reporter for T3-dependent transcriptional activity, a plasmid containing the T3-dependent promoter of X. laevis TRβA gene driving the expression of firefly luciferase (TRE-Luc) was microinjected into the oocyte nucleus together with an internal control plasmid driving the expression of Renilla luciferase. Because the oocyte has little endogenous TR/RXR, in vitro-transcribed mRNAs encoding X. tropicalis TRα or TRβ, and RXRβ were coinjected into the cytoplasm. After overnight incubation in the presence or absence of T3, the oocytes were lysed and assayed for luciferase activities. The ratio of firefly luciferase activity to Renilla luciferase was determined as a measure for transcription level from the reporter gene. In the absence of T3, overexpression of either TRα or TRβ reduced the reporter gene transcription (Fig. 3). When T3 was added to the oocyte culture medium, the repression was relieved and the promoter was further activated (Fig. 3). As expected, coexpression of RXRβ further enhanced the effect of both TRs (Fig. 3).
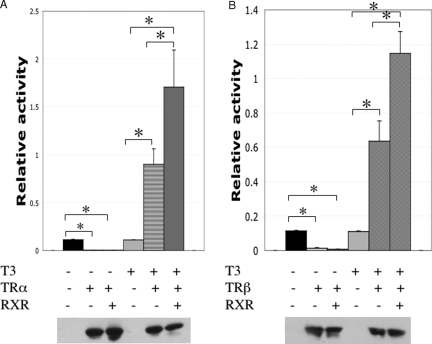
Figure 3.
Luciferase assays indicate that both X. tropicalis TRα (A) and TRβ (B) can repress and activate the activity of the T3-inducible promoter of X. laevis TRβA gene and RXR enhances the activities. X. laevis oocytes were injected into the cytoplasm with mRNAs encoding indicated proteins. Subsequently the reporter vector with the firefly luciferase under the control of the T3-inducible promoter of X. laevis TRβA gene and phRG-Tk Renilla luciferase control vector were coinjected into the nucleus of the oocytes. The oocytes were then incubated with or without T3 before harvesting for luciferase assays. The ratio of the reporter firefly luciferase activity to the control Renilla luciferase activity was plotted (top panels). Western blot analysis was also done on the same the lysate with anti-FLAG antibody, demonstrating the expression of the FLAG-tagged TRα and TRβ (bottom panels). In a separate experiment using mRNA encoding FLAG-tagged RXRβ, Western blot analysis with the anti-FLAG antibody showed the expected expression of RXRβ in the injected oocytes (data not shown).
To show that X. tropicalis TRs bind to the TRE in chromatin in vivo, we carried out ChIP assays. Two different rabbit polyclonal antibodies had been made against X. laevis TRβ, one using TRβ in polyacrylamide gel slices as the antigen [anti-TR (PB)] (29), whereas the other using purified TRβ in solution as the antigen [anti-TR (new PB)]. As shown in Fig. 4A, anti-TR (PB) recognized X. tropicalis TRα and TRβ similarly, whereas anti-TR (new PB) preferentially recognized TRβ in the Western blot assay. Both antibodies were then used to immunoprecipitate chromatin isolated from oocytes injected with the reporter plasmids and mRNAs for TR/RXR. Quantitative PCR analysis of the precipitated DNA with the anti-TR (PB) showed that overexpression of TRα (Fig. 4, B and C) or TRβ (Fig. 4, B and D) and RXRβ led to the binding of either TR to the TRE region of the reporter, whereas only background signal was detected in the coding region of the ampicillin resistance gene on the same TRE plasmid. Similar results were obtained with the anti-TR (new PB) antibody (supplemental Fig. 2). These results indicate that X. tropicalis TRs regulates target gene transcription in a liganded-dependent manner by binding to the TRE in chromatin, similar to TRs in other species.
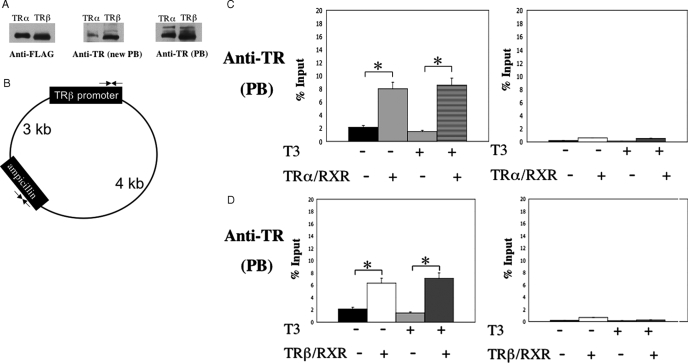
Figure 4.
Both TRs bind to the TRE in the chromatin in the frog oocytes. A, Western blot analysis shows that two independently generated anti-Xenopus laevis TRβ antibodies (PB and new PB) recognize both X. tropicalis TRα and TRβ, although the anti-TR (new PB) recognizes X. tropicalis TRβ a fewfold better than TRα. In vitro-translated FLAG-tagged X. tropicalis TRα and TRβ were subjected to Western blot analysis with the anti-FLAG, anti-TR (new PB), or anti-TR (PB) antibodies. B, Schematic diagram of the reporter plasmid containing the T3-dependent promoter of the Xenopus TRβΑ gene. Two sets (opposing arrows) of forward and reverse primers were designed for quantitative PCR analysis of the promoter region containing the TRE and a region in the ampicillin resistance gene, respectively. The primer sets are about 3 and 4 kb away from each other on the two sides of the plasmid, respectively. C and D, ChIP assays with anti-TR (PB) antibody show that chromatin-bound X. tropicalis TRα (C) and TRβ (D). Oocytes were injected with the reporter DNA and indicated mRNAs. After overnight incubation in the presence and absence of T3, the oocytes were isolated and subjected to ChIP assay with anti-TR (PB) antibody. The precipitated DNA was amplified for detection of the TRE region of the X. laevis TRβ promoter or the ampicillin resistance gene in the reporter vector. Note the enhanced signal of the promoter region but not the ampicillin resistance gene in the presence of TR/RXR both in the presence and absence of T3, showing the constitutive recruitment of TRs to the promoter.
X. tropicalis TRs are bound to TREs of endogenous target genes during tadpole development
To further investigate the involvement of X. tropicalis TRs in metamorphosis, we analyzed the binding of the TRs to endogenous TREs in tadpole intestine in vivo. We have shown previously that in X. laevis TRs are bound to the TREs in TRβ and TH/bZIP (T3-responsive basic leucine zipper transcription factor) genes, both of which are direct target genes of TR and contain functional TREs (37,38), and T3 treatment led to enhanced binding of TRs to both promoters (22,23). As shown above, X. tropicalis TRβ gene is up-regulated by T3. To determine whether X. tropicalis TH/bZIP is also regulated by T3, we analyzed its expression in premetamorphic tadpoles treated with T3. As shown in Fig. 5A, TH/bZIP was strongly induced in all organs after 1–3 d of T3 treatment. Furthermore, analysis of the genomic sequences of both TRβ and TH/bZIP genes in X. tropicalis revealed the TRE regions were identical with those in X. laevis (Fig. 5B), suggesting that both TRβ and TH/bZIP genes are direct target genes of TR.
Figure 5.
TRs are bound to target genes during T3-induced X. tropicalis metamorphosis. A, RT-PCR analysis shows that T3 induces the expression of X. tropicalis TH/bZIP gene. The RNA samples in Fig. 2 were analyzed for the expression of TH/bZIP. Again, the expression of Ef1α gene was analyzed in the same PCR as an internal control. Note that X. tropicalis TH/bZIP was induced by T3 ubiquitously, just like the X. laevis TH/bZIP gene. The experiment was repeated twice with similar results. B, The TRE regions of the T3-target genes TRβ and TH/bZIP are conserved between X. laevis and X. tropicalis. The sequences flanking the TRE regions used for quantitative PCR analysis of ChIP DNA from the intestine were aligned between the X. tropicalis TRβ (genomic scaffold_26:1899211–1899280) and X. laevis TRβ (38) (top panel) and between X. tropicalis TH/bZIP (genomic scaffold_448:1011455–1011524) and X. laevis TH/bZIP (37) (bottom panel), respectively. Note that there are 96 and 100% identities for TRβ and TH/bZIP genes, respectively. The TREs are shown in bold letters and the primers/probe for qPCR analysis are also indicated. C, ChIP assays shows that TRs are bound to endogenous target genes TRβ and TH/bZIP in the intestine of X. tropicalis. Tadpoles at stage 54 were treated with or without T3 for 2 d, and the intestine was isolated for ChIP assay with the anti-TR (new PB), anti-TR (PB), or anti-Id14 (extracellular protein, as a negative control) antibody. The precipitated DNA was analyzed by quantitative PCR for the presence of the TRE regions of the TRβ and TH/bZIP promoters. A region of exon 5 of TRβ gene was also analyzed as a negative control. Note that TR was bound to both promoters in the intestine of stage 54 tadpoles, and T3 treatment led to enhanced binding, whereas only background signal was detected for exon 5.
ChIP assay was then carried out to investigate the binding of TRs to the TREs of these two genes. We used both anti-TR (PB) and anti-TR (new PB) to independently determine the binding of TR to the TREs. For this purpose, we treated premetamorphic tadpoles with T3 and isolated the animal intestine for ChIP assay. The results from both antibodies showed that TRs were bound to the TREs in both genes in the absence of T3 and T3 treatment enhanced the binding (Fig. 5C). [Note that although anti-TR (new PB) preferentially recognized TRβ in Western blot assay (Fig. 4A), both anti-TR (new PB) and anti-TR (PB) antibodies yielded similar results in the ChIP assays with either the frog oocyte samples (above) or tadpole intestine, suggesting that antigen-recognition efficiency/preference by the antibodies may differ under Western blot and ChIP assay conditions. This could be due to the differences in the antibody recognition of native vs. denatured TRs and/or free TRs vs. TRs cross-linked to DNA and other proteins]. In contrast to the TRE regions, little signal was detected at exon 5 in the coding region of TRβ gene with either antibody in the presence or absence of T3. In addition, ChIP assay with an antibody against ID14, an extracellular protein (30), gave only background signals at the TREs (Fig. 5C). These results demonstrate that TR binds to target genes in developing X. tropicalis tadpoles to regulate their expression during development.
Discussion
Because of its total dependence on T3 and manipulability in the absence of maternal influence, amphibian metamorphosis has long served as a model to study postembryonic organ development and tissue remodeling (7,12,36). In the past 2 decades, with the cloning of TRs and identification and characterization of T3 target genes, amphibian metamorphosis, especially that of X. laevis, has made it possible the in vivo studies of TR function and the underlying mechanisms. X. tropicalis offers a number of advantages for further analyses of TR and TR-interacting cofactors in vivo, especially on their roles in tissue- and gene-specific functions during development. Our studies here strongly argue that TRs mediate the metamorphic effects of T3 during X. tropicalis metamorphosis.
Our expression analyses indicate that TR and to some extent RXR genes are regulated in an organ-dependent manner during X. tropicalis development. In particularly, high levels of their mRNAs are present in a given organ at the stages when the organ undergoes the most dramatic metamorphic changes, consistently with the role of TR/RXR heterodimers in mediating the metamorphic effects of T3 in different organs. Thus, in the brain and intestine, high levels of TR and RXR are present at the climax of metamorphosis (stages 58–64) as these organs remodel into the adult forms (7,12,36,39). Higher levels of TR/RXR occur earlier in the limb, around stage 54–56, which is consistent with the fact that limb morphogenesis takes place around these stages and after stage 57, limbs undergo growth with few morphological changes (28). Finally, tail resorption mainly occurs after stage 62 (28), which coincides with the high levels of TR and RXR mRNAs. Furthermore, this organ-dependent, temporal correlation is also observed during T3-induced metamorphosis. T3 treatment of premetamorphic tadpoles leads to strong induction of TRβ gene and, to a lesser degree, TRα and RXRβ genes, similar to the regulation of these genes in X. laevis (7,12,29,40,41,42,43,44,45,46,47).
As in other vertebrate species, X. tropicalis TRs bind to TREs in the context of chromatin constitutively and repress a target promoter in the absence of T3 and activates it when bound by T3. More importantly, our ChIP assays on the intestine of premetamorphic tadpoles indicate that TR is bound to endogenous target genes in premetamorphic tadpoles. T3 treatment leads to enhanced binding of TRs to the target genes, accompanying the activation of the genes. These results thus suggest that unliganded TRs bind to TREs in premetamorphic tadpoles to repress gene expression during tadpole growth period to prevent precocious metamorphosis and that during metamorphosis, T3 binding allows the chromatin bound TR to activate these target genes to initiate the metamorphic process.
In general, our findings on X. tropicalis TRs and RXRs are similar to those reported for X. laevis TRs and RXRs. Although not surprisingly, such conservations indicate that we can combine the existing knowledge on the morphological, histological, molecular, and cellular changes associated with X. laevis metamorphosis with the advantages of genomic sequences information, diploid genome, and shorter life cycle of X. tropicalis to dissect the molecular pathways governing the distinct morphological transformations in different organs at different developmental stages, a fascinating complex developmental process controlled by a simple chemical, the thyroid hormone.
Supplementary Material
Footnotes
This work was supported by the Intramural Research Program of National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 17, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; PB, anti-TR; qRT-PCR, quantitative real-time RT-PCR; RXR, retinoid X receptor; TR, T3 receptor; TRE, T3 response element.
References
- Evans RM 1988 The steroid and thyroid hormone receptor superfamily. Science 240:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA 1993 Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193 [DOI] [PubMed] [Google Scholar]
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P 1995 The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- Tata JR 1993 Gene expression during metamorphosis: an ideal model for post-embryonic development. Bioessays 15:239–248 [DOI] [PubMed] [Google Scholar]
- Shi Y-B 1999 Amphibian metamorphosis: from morphology to molecular biology. New York: John Wiley, Sons, Inc. [Google Scholar]
- Atkinson BG 1994 Metamorphosis: model systems for studying gene expression in postembryonic development. Dev Genet 15:313–319 [Google Scholar]
- Hetzel BS 1989 The story of iodine deficiency: an international challenge in nutrition. Oxford: Oxford University Press [Google Scholar]
- Buchholz DR, Paul BD, Fu L, Shi YB 2006 Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol 145:1–19 [DOI] [PubMed] [Google Scholar]
- Denver RJ 1998 The molecular basis of thyroid hormone-dependent central nervous system remodeling during amphibian metamorphosis. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 119:219–228 [DOI] [PubMed] [Google Scholar]
- Gilbert LI, Tata JR, Atkinson BG 1996 Metamorphosis: post-embryonic reprogramming of gene expression in amphibian and insect cells. New York: Academic Press [Google Scholar]
- Furlow JD, Neff ES 2006 A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis. Trends Endocrinol Metab 17:38–45 [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yaoita Y 2003 Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev Dyn 227:246–255 [DOI] [PubMed] [Google Scholar]
- Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD 2001 Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci USA 98:10739–10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Tomita A, Fu L, Paul BD, Shi Y-B 2004 Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol 24:9026–9037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Hsia VS-C, Fu L, Shi Y-B 2003 A dominant negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol 23:6750–6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Shi Y-B 2003 Distinct expression profiles of transcriptional coactivators for thyroid hormone receptors during Xenopus laevis metamorphosis. Cell Res 13:459–464 [DOI] [PubMed] [Google Scholar]
- Paul BD, Fu L, Buchholz DR, Shi Y-B 2005 Coactivator recruitment is essential for liganded thyroid hormone receptor to initiate amphibian metamorphosis. Mol Cell Biol 25:5712–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Buchholz DR, Fu L, Shi Y-B 2005 Tissue- and gene-specific recruitment of steroid receptor coactivator-3 by thyroid hormone receptor during development. J Biol Chem 280:27165–27172 [DOI] [PubMed] [Google Scholar]
- Havis E, Sachs LM, Demeneix BA 2003 Metamorphic T3-response genes have specific coregulator requirements. EMBO Rep 4:883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz DR, Paul BD, Shi YB 2005 Gene-specific changes in promoter occupancy by thyroid hormone receptor during frog metamorphosis. Implications for developmental gene regulation. J Biol Chem 280:41222–41228 [DOI] [PubMed] [Google Scholar]
- Sachs LM, Shi Y-B 2000 Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc Natl Acad Sci USA 97:13138–13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ 2008 A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor β. J Biol Chem 283:2275–2285 [DOI] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Shi Y-B 2004 Recruitment of N-CoR/SMRT-TBLR1 corepressor complex by unliganded thyroid hormone receptor for gene repression during frog development. Mol Cell Biol 24:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs LM, Jones PL, Havis E, Rouse N, Demeneix BA, Shi Y-B 2002 N-CoR recruitment by unliganded thyroid hormone receptor in gene repression during Xenopus laevis development. Mol Cell Biol 22:8527–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E, Offield MF, Grainger RM 1998 Frog genetics: Xenopus tropicalis jumps into the future. Trends Genet 14:253–255 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J 1956 Normal table of Xenopus laevis. 1st ed. Amsterdam: North Holland Publishing [Google Scholar]
- Wong J, Shi Y-B 1995 Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem 270:18479–18483 [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Ishizuya-Oka A, Shi YB 2004 Spatial and temporal expression pattern of a novel gene in the frog Xenopus laevis: correlations with adult intestinal epithelial differentiation during metamorphosis. Gene Expr Patterns 4:321–328 [DOI] [PubMed] [Google Scholar]
- Tomita A, Buchholz DR, Obata K, Shi Y-B 2003 Fusion protein of retinoic acid receptor a with promeyelocytic leukaemia protein or promyelocytic leukaemia zinc-finger protein recruits N-CoR-TBLR1 corepressor complex to repress transcription in vivo. J Biol Chem 278:30788–30795 [DOI] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Shi Y-B 2007 Contrasting effects of two alternative splicing forms of coactivator-associated arginine methyltransferase 1 on thyroid hormone receptor-mediated transcription in Xenopus laevis. Mol Endocrinol 21:1082–1094 [DOI] [PubMed] [Google Scholar]
- Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B 2007 Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol 303:576–590 [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Liang VC-T 1994 Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta 1217:227–228 [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Shi YB, Brown DD 1990 Xenopus laevis α and β thyroid hormone receptors. Proc Natl Acad Sci USA 87:7090–7094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd MHI, Dodd JM 1976 The biology of metamorphosis. In: Lofts B, ed. Physiology of the amphibia. New York: Academic Press; 467–599 [Google Scholar]
- Furlow JD, Brown DD 1999 In vitro and in vivo analysis of the regulation of a transcription factor gene by thyroid hormone during Xenopus laevis metamorphosis. Mol Endocrinol 13:2076–2089 [DOI] [PubMed] [Google Scholar]
- Ranjan M, Wong J, Shi YB 1994 Transcriptional repression of Xenopus TRβ gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem 269:24699–24705 [PubMed] [Google Scholar]
- Shi Y-B, Ishizuya-Oka A 1996 Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Topics Dev Biol 32:205–235 [DOI] [PubMed] [Google Scholar]
- Helbing CC, Gergely C, Atkinson BG 1992 Sequential up-regulation of thyroid hormone β receptor, ornithine transcarbamylase, and carbamyl phosphate synthetase mRNAs in the liver of Rana catesbeiana tadpoles during spontaneous and thyroid hormone-induced metamorphosis. Dev Gen 13:289–301 [DOI] [PubMed] [Google Scholar]
- Iwamuro S, Tata JR 1995 Contrasting patterns of expression of thyroid hormone and retinoid X receptor genes during hormonal manipulation of Xenopus tadpole tail regression in culture. Mol Cell Endocrinol 113:235–243 [DOI] [PubMed] [Google Scholar]
- Fairclough L, Tata JR 1997 An immunocytochemical analysis of the expression of thyroid hormone receptor α and β proteins during natural and thyroid hormone-induced metamorphosis in Xenopus. Dev Growth Differ 39:273–283 [DOI] [PubMed] [Google Scholar]
- Kawahara A, Baker BS, Tata JR 1991 Developmental and regional expression of thyroid hormone receptor genes during Xenopus metamorphosis. Development 112:933–943 [DOI] [PubMed] [Google Scholar]
- Yaoita Y, Brown DD 1990 A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Genes Dev 4:1917–1924 [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Liang VC-T, Parkison C, Cheng S-Y 1994 Tissue-dependent developmental expression of a cytosolic thyroid hormone protein gene in Xenopus: its role in the regulation of amphibian metamorphosis. FEBS Lett 355:61–64 [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Galton VA 1991 Regulation of c-erbA-messenger RNA species in tadpole erythrocytes by thyroid hormone. Mol Endocrinol 5:201–208 [DOI] [PubMed] [Google Scholar]
- Davey JC, Schneider MJ, Galton VA 1994 Cloning of a thyroid hormone-responsive Rana catesbeiana c-erbA-β gene. Dev Genet 15:339–346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.