Abstract
Maternal antibody to an outer membrane 68-kilodalton (kDa) protein of Bordetella bronchiseptica was shown to be protective in experiments on specific-pathogen-free piglets. After challenge with B. bronchiseptica, 100% (n = 19) control piglets from nonimmunized sows developed pneumonia, coughing, and sneezing, and 74% of the animals developed severe atrophic rhinitis. In 12 piglets from a sow immunized with 68-kDa protein, pneumonia occurred only in 34% of offspring, coughing was reduced, the duration of coughing bouts was shortened, and severe atrophic rhinitis occurred in one animal only (8%). The difference in the occurrence of atrophic rhinitis and of pneumonia in immunized and nonimmunized offspring was statistically significant (P less than 0.05). Sera of protected piglets had high titers (enzyme-linked immunosorbent assay) of antibodies that showed a high specificity for the 68-kDa protein isolated from B. bronchiseptica, whereas their reactivity with an analogous 69-kDa protein isolated from Bordetella pertussis was low or absent. The 68-kDa protein of B. bronchiseptica appeared to be the major protective antigen in B. bronchiseptica infection; however, isolated protein alone did not induce such a solid protection, as observed in a previous study after the application of an effective whole cell vaccine.
Full text
PDF

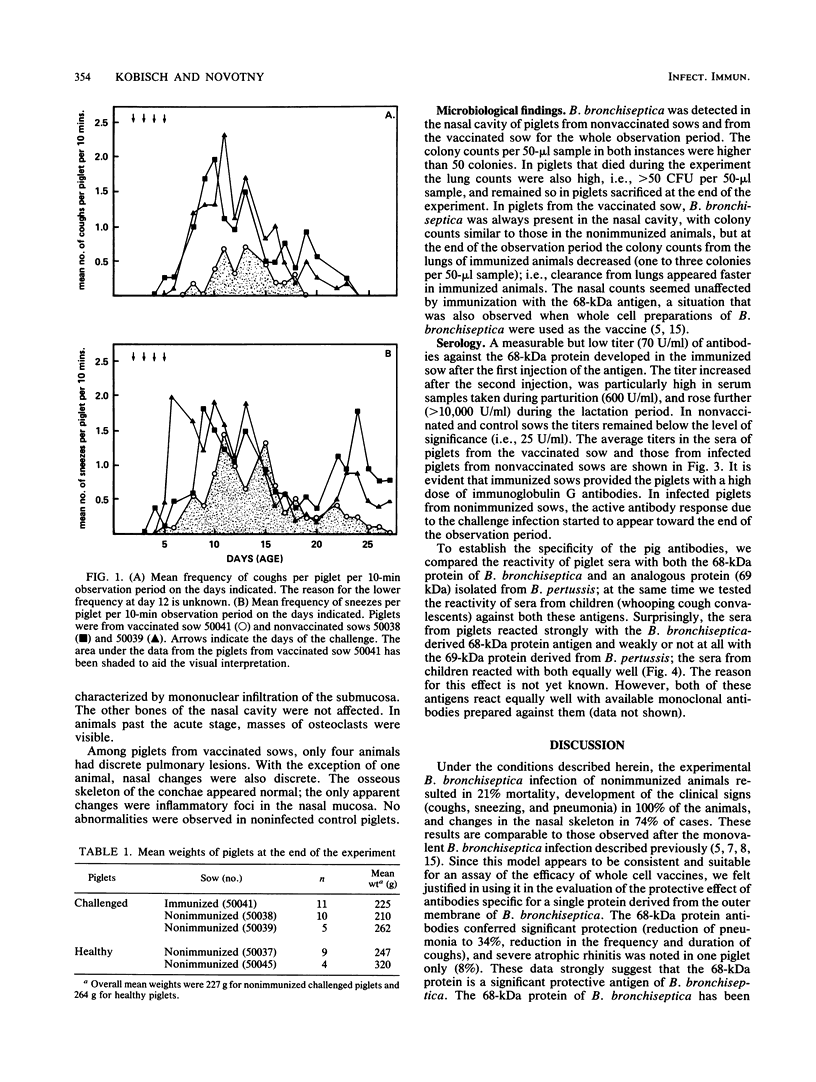
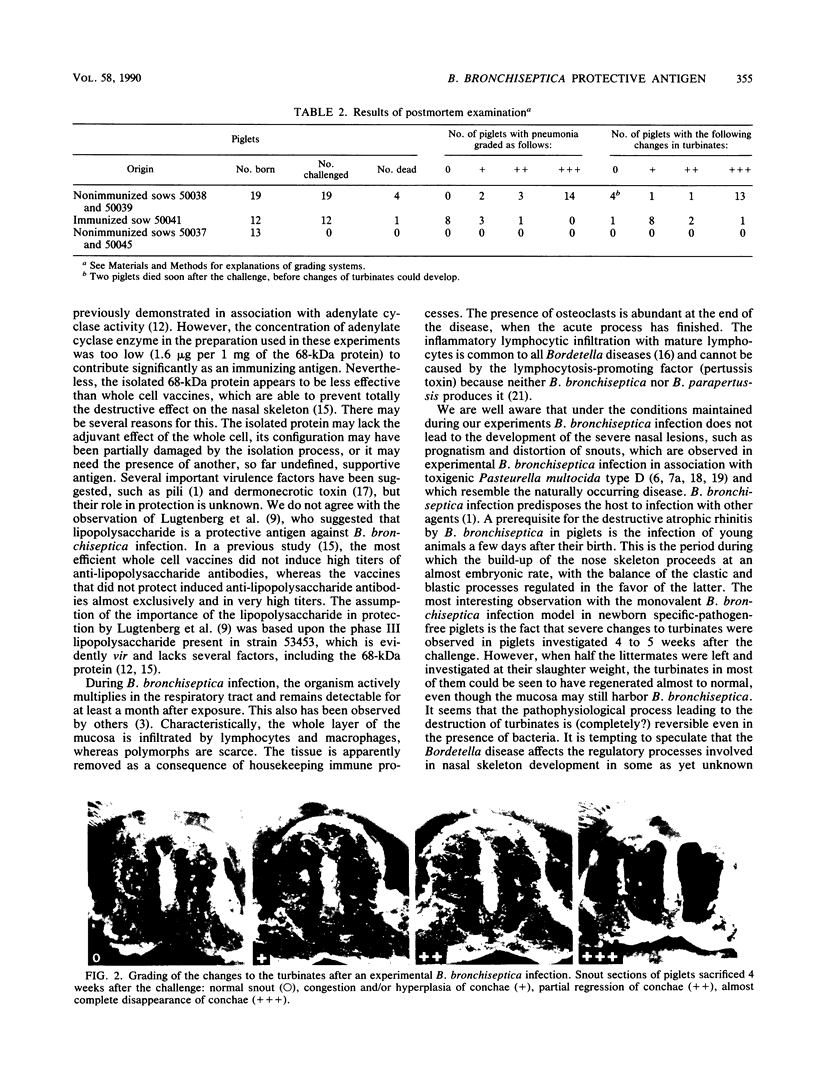
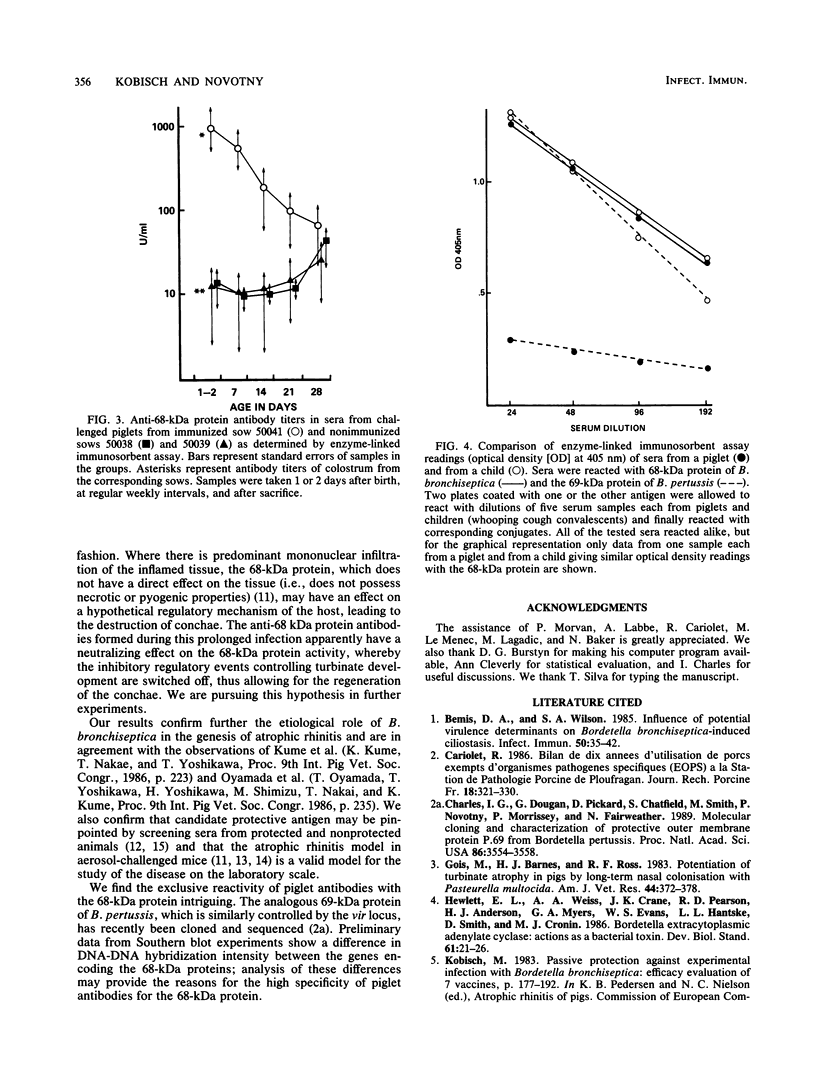

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bemis D. A., Wilson S. A. Influence of potential virulence determinants on Bordetella bronchiseptica-induced ciliostasis. Infect Immun. 1985 Oct;50(1):35–42. doi: 10.1128/iai.50.1.35-42.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Dougan G., Pickard D., Chatfield S., Smith M., Novotny P., Morrissey P., Fairweather N. F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gois M., Barnes H. J., Ross R. F. Potentiation of turbinate atrophy in pigs by long-term nasal colonization with Pasteurella multocida. Am J Vet Res. 1983 Mar;44(3):372–378. [PubMed] [Google Scholar]
- Hewlett E. L., Weiss A. A., Crane J. K., Pearson R. D., Anderson H. J., Myers G. A., Evans W. S., Hantske L. L., Kay H. D., Cronin M. J. Bordetella extracytoplasmic adenylate cyclase: actions as a bacterial toxin. Dev Biol Stand. 1985;61:21–26. [PubMed] [Google Scholar]
- Kobisch M., Pennings A. An evaluation in pigs of Nobi-Vac AR and an experimental atrophic rhinitis vaccine containing P multocida DNT-toxoid and B bronchiseptica. Vet Rec. 1989 Jan 21;124(3):57–61. doi: 10.1136/vr.124.3.57. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., van Boxtel R., van den Bosch R., de Jong M., Storm P. Biochemical and immunological analyses of the cell surface of Bordetella bronchiseptica isolates with special reference to atrophic rhinitis in swine. Vaccine. 1984 Dec;2(4):265–273. doi: 10.1016/0264-410x(84)90042-2. [DOI] [PubMed] [Google Scholar]
- Montaraz J. A., Novotny P., Ivanyi J. Identification of a 68-kilodalton protective protein antigen from Bordetella bronchiseptica. Infect Immun. 1985 Mar;47(3):744–751. doi: 10.1128/iai.47.3.744-751.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Chubb A. P., Cownley K., Montaraz J. A. Adenylate cyclase activity of a 68,000-molecular-weight protein isolated from the outer membrane of Bordetella bronchiseptica. Infect Immun. 1985 Oct;50(1):199–206. doi: 10.1128/iai.50.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Chubb A. P., Cownley K., Montaraz J. A., Beesley J. E. Bordetella adenylate cyclase: a genus specific protective antigen and virulence factor. Dev Biol Stand. 1985;61:27–41. [PubMed] [Google Scholar]
- Novotny P., Kobisch M., Cownley K., Chubb A. P., Montaraz J. A. Evaluation of Bordetella bronchiseptica vaccines in specific-pathogen-free piglets with bacterial cell surface antigens in enzyme-linked immunosorbent assay. Infect Immun. 1985 Oct;50(1):190–198. doi: 10.1128/iai.50.1.190-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. C. Pertussis. Medicine (Baltimore) 1975 Nov;54(6):427–469. doi: 10.1097/00005792-197511000-00001. [DOI] [PubMed] [Google Scholar]
- Roop R. M., 2nd, Veit H. P., Sinsky R. J., Veit S. P., Hewlett E. L., Kornegay E. T. Virulence factors of Bordetella bronchiseptica associated with the production of infectious atrophic rhinitis and pneumonia in experimentally infected neonatal swine. Infect Immun. 1987 Jan;55(1):217–222. doi: 10.1128/iai.55.1.217-222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M. Virulence of Pasteurella multocida in atrophic rhinitis of gnotobiotic pigs infected with Bordetella bronchiseptica. Res Vet Sci. 1983 May;34(3):287–295. [PubMed] [Google Scholar]
- Shattuck R. L., Oldenburg D. J., Storm D. R. Purification and characterization of a calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1985 Nov 5;24(23):6356–6362. doi: 10.1021/bi00344a006. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]