Abstract
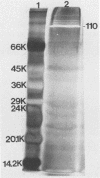
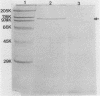
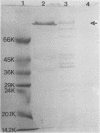
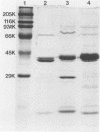
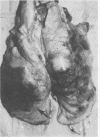
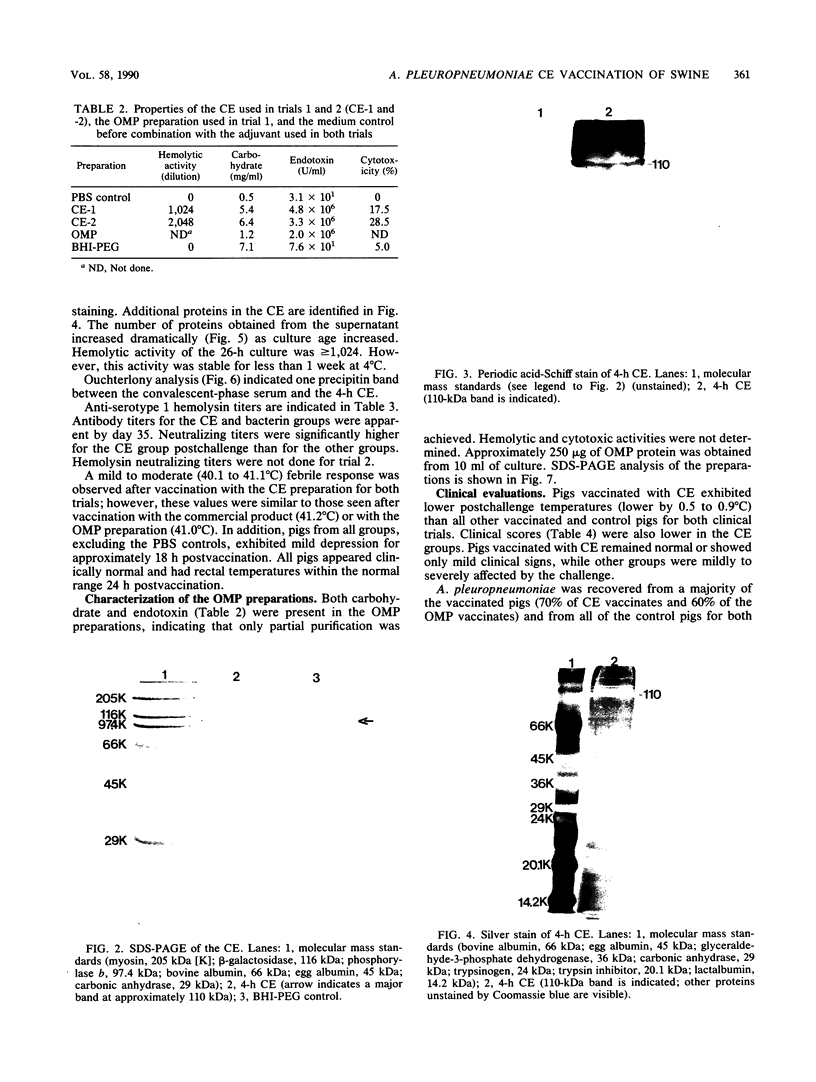
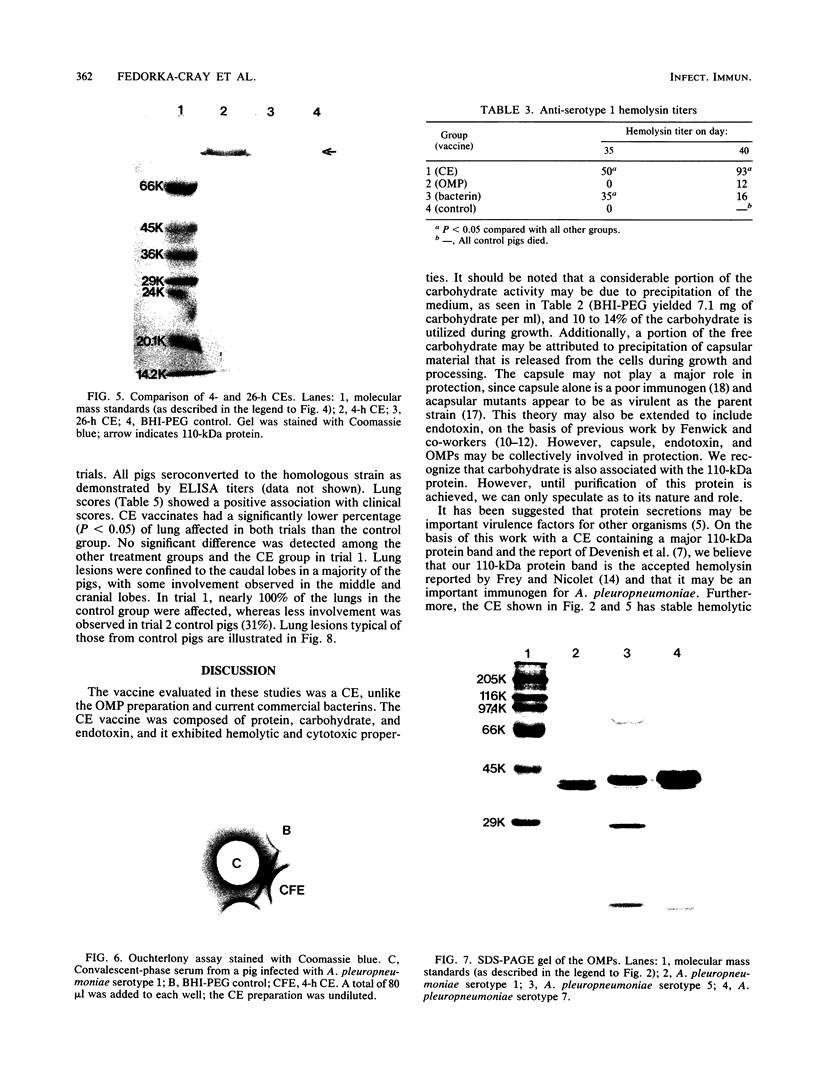
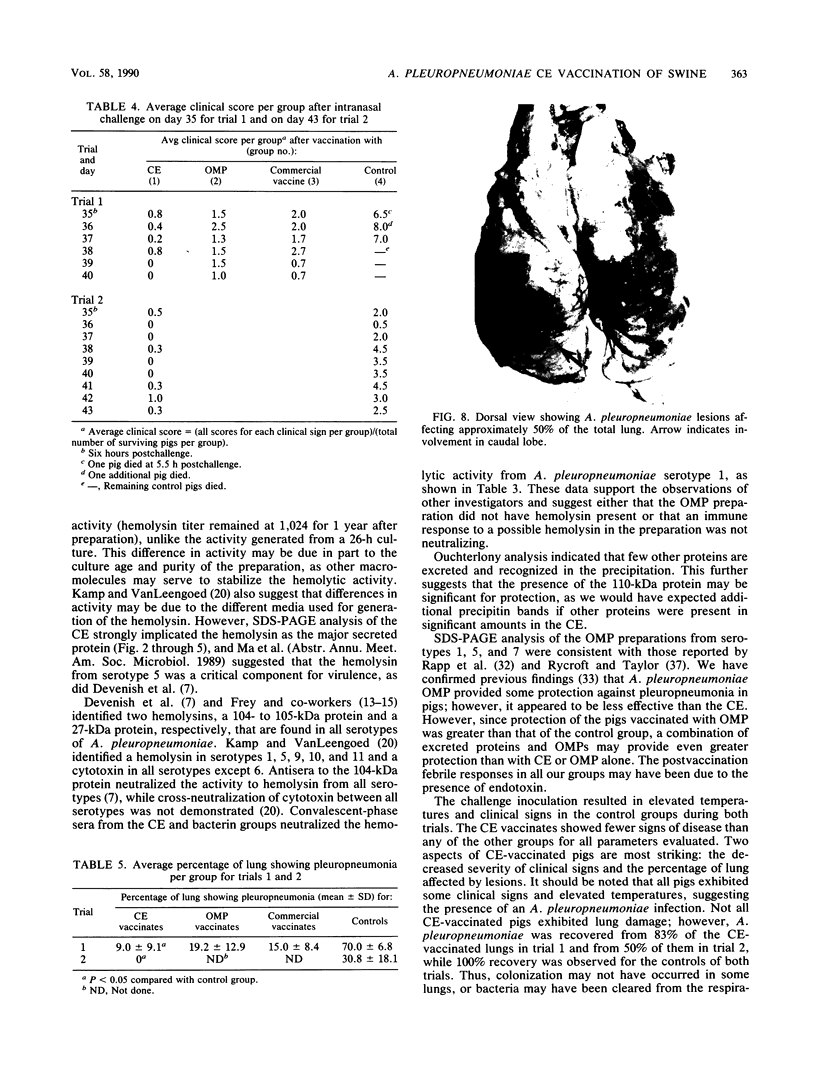
We partially characterized a cell extract (CE) from Actinobacillus pleuropneumoniae serotype 1 and used the CE to test the efficacy of secreted proteins against disease. Secreted products from 4-h culture supernatants were precipitated with 20% polyethylene glycol. Analysis of the CE indicated the presence of protein, endotoxin, and carbohydrate. Hemolytic activity to bovine erythrocytes and cytotoxic activity to porcine mononuclear leukocytes was also demonstrated. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the CE from a 4-h culture showed a major band at 110 kilodaltons (kDa), while a CE of a 26-h culture indicated the presence of a number of additional proteins, including the 110-kDa protein. The 110-kDa protein was also identified as a glycoprotein by periodic acid-Schiff and silver staining. A single band precipitated against convalescent-phase pig antiserum when the polyethylene glycol precipitate was used in an Ouchterlony plate. Vaccination with CE conferred greater protection against challenge with the homologous serotype than either a commercial bacterin or an outer membrane protein vaccine. Hemolysin-neutralizing titers were higher both pre- and postchallenge in the group vaccinated with the CE compared with in all other groups. We believe that this demonstrates the importance of secreted factors in protection against disease and suggests that the 110-kDa protein is an important immunogen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlone G. M., Thomas M. L., Rumschlag H. S., Sottnek F. O. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986 Sep;24(3):330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer A. W., Panciera R. J., Mosier D. A. Bovine pneumonic pasteurellosis: immunity to Pasteurella haemolytica. J Am Vet Med Assoc. 1988 Nov 15;193(10):1308–1316. [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun. 1989 Mar;57(3):798–804. doi: 10.1128/iai.57.3.798-804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Rosendal S., Johnson R., Hubler S. Immunoserological comparison of 104-kilodalton proteins associated with hemolysis and cytolysis in Actinobacillus pleuropneumoniae, Actinobacillus suis, Pasteurella haemolytica, and Escherichia coli. Infect Immun. 1989 Oct;57(10):3210–3213. doi: 10.1128/iai.57.10.3210-3213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier P. J., Perino L., Urbance J. Porcine Haemophilus pleuropneumonia: microbiologic and pathologic findings. J Am Vet Med Assoc. 1984 Mar 15;184(6):716–719. [PubMed] [Google Scholar]
- Fenwick B. W., Cullor J. S., Osburn B. I., Olander H. J. Mechanisms involved in protection provided by immunization against core lipopolysaccharides of Escherichia coli J5 from lethal Haemophilus pleuropneumoniae infections in swine. Infect Immun. 1986 Aug;53(2):298–304. doi: 10.1128/iai.53.2.298-304.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect Immun. 1986 Nov;54(2):575–582. doi: 10.1128/iai.54.2.575-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Vaccine potential of Haemophilus pleuropneumoniae oligosaccharide-tetanus toxoid conjugates. Infect Immun. 1986 Nov;54(2):583–586. doi: 10.1128/iai.54.2.583-586.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Perrin J., Nicolet J. Cloning and expression of a cohemolysin, the CAMP factor of Actinobacillus pleuropneumoniae. Infect Immun. 1989 Jul;57(7):2050–2056. doi: 10.1128/iai.57.7.2050-2056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins R., Larivière S., Mittal K. R., Martineau G. P., Rousseau P., Cameron J. Evaluation of a Killed Vaccine Against Porcine Pleuropneumonia Due to Haemophilus pleuropneumoniae. Can Vet J. 1985 Feb;26(2):86–89. [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Ma J., Workman T., Gogolewski R. P., Anderson P. Virulence properties and protective efficacy of the capsular polymer of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5. Infect Immun. 1988 Aug;56(8):1880–1889. doi: 10.1128/iai.56.8.1880-1889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., Popma J. K., Van Leengoed L. A. Serotyping of Haemophilus pleuropneumoniae in the Netherlands: with emphasis on heterogeneity within serotype 1 and (proposed) serotype 9. Vet Microbiol. 1987 Mar;13(3):249–257. doi: 10.1016/0378-1135(87)90087-3. [DOI] [PubMed] [Google Scholar]
- Kamp E. M., van Leengoed L. A. Serotype-related differences in production and type of heat-labile hemolysin and heat-labile cytotoxin of Actinobacillus (Haemophilus) pleuropneumoniae. J Clin Microbiol. 1989 Jun;27(6):1187–1191. doi: 10.1128/jcm.27.6.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski C., Callewaert D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983 Nov 25;64(3):313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Kume K., Nakai T., Sawata A. Efficacy of Haemophilus pleuropneumoniae vaccine in pigs. Nihon Juigaku Zasshi. 1985 Apr;47(2):201–206. doi: 10.1292/jvms1939.47.201. [DOI] [PubMed] [Google Scholar]
- Kume K., Nakai T., Sawata A. Interaction between heat-stable hemolytic substance from Haemophilus pleuropneumoniae and porcine pulmonary macrophages in vitro. Infect Immun. 1986 Feb;51(2):563–570. doi: 10.1128/iai.51.2.563-570.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liggett A. D., Harrison L. R., Farrell R. L. Sequential study of lesion development in experimental haemophilus pleuropneumonia. Res Vet Sci. 1987 Mar;42(2):204–212. [PubMed] [Google Scholar]
- Martin P. G., Lachance P., Niven D. F. Production of RNA-dependent haemolysin by Haemophilus pleuropneumoniae. Can J Microbiol. 1985 May;31(5):456–462. doi: 10.1139/m85-085. [DOI] [PubMed] [Google Scholar]
- Nakai T., Sawata A., Kume K. Characterization of the hemolysin produced by haemophilus pleuropneumoniae. Am J Vet Res. 1983 Feb;44(2):344–347. [PubMed] [Google Scholar]
- Nakai T., Sawata A., Kume K. Pathogenicity of Haemophilus pleuropneumoniae for laboratory animals and possible role of its hemolysin for production of pleuropneumonia. Nihon Juigaku Zasshi. 1984 Dec;46(6):851–858. doi: 10.1292/jvms1939.46.851. [DOI] [PubMed] [Google Scholar]
- Pijoan C. Effect of Pasteurella multocida and Haemophilus pleuropneumoniae toxins on swine alveolar macrophages. Vet Immunol Immunopathol. 1986 Sep;13(1-2):141–149. doi: 10.1016/0165-2427(86)90055-3. [DOI] [PubMed] [Google Scholar]
- Rapp V. J., Munson R. S., Jr, Ross R. F. Outer membrane protein profiles of Haemophilus pleuropneumoniae. Infect Immun. 1986 May;52(2):414–420. doi: 10.1128/iai.52.2.414-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F. Immunogenicity of Outer Membrane Components of Haemophilus (Actinobacillus) pleuropneumoniae. Can Vet J. 1988 Jul;29(7):585–587. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A., Gilbride K. A. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med. 1985 Jan;49(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Carpenter D. S., Mitchell W. R., Wilson M. R. Vaccination against pleuropneumonia of pigs caused by Haemophilus pleuropneumoniae. Can Vet J. 1981 Feb;22(2):34–35. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Miniats O. P., Sinclair P. Protective efficacy of capsule extracts of Haemophilus pleuropneumoniae in pigs and mice. Vet Microbiol. 1986 Sep;12(3):229–240. doi: 10.1016/0378-1135(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Rycroft A. N., Taylor D. J. Preparation and characterisation of envelope proteins from Haemophilus pleuropneumoniae. Vet Microbiol. 1987 Dec;15(4):303–314. doi: 10.1016/0378-1135(87)90018-6. [DOI] [PubMed] [Google Scholar]
- Sanford S. E., Josephson G. K. Porcine Haemophilus pleuropneumonia epizootic in southwestern Ontario: clinical, microbiological, pathological and some epidemiological findings. Can J Comp Med. 1981 Jan;45(1):2–7. [PMC free article] [PubMed] [Google Scholar]
- Schultz R. A., Young T. F., Ross R. F., Jeske D. R. Prevalence of antibodies to Haemophilus pleuropneumoniae in Iowa swine. Am J Vet Res. 1982 Oct;43(10):1848–1851. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Udeze F. A., Latimer K. S., Kadis S. Role of haemophilus pleuropneumoniae lipopolysaccharide endotoxin in the pathogenesis of porcine Haemophilus pleuropneumonia. Am J Vet Res. 1987 May;48(5):768–773. [PubMed] [Google Scholar]