Abstract
All surgical disciplines encounter planned and unplanned ischemic events that may ultimately lead to cellular dysfunction and death. Stem cell therapy has shown promise for the treatment of a variety of ischemic and inflammatory disorders where tissue damage has occurred. As stem cells have proven beneficial in many disease processes, important opportunities in the future treatment of gastrointestinal disorders may exist. Therefore, this manuscript will serve to: review the different types of stem cells that may be applicable to the treatment of gastrointestinal disorders, review the mechanisms suggesting that stem cells may work for these conditions; discuss current practices for harvesting and purifying stem cells; and provide a concise summary of a few of the pediatric intestinal disorders that could be treated with cellular therapy.
INTRODUCTION
Pediatric gastrointestinal disorders such as inflammatory bowel disease (IBD) and necrotizing enterocolitis (NEC) are concerning sources of patient morbidity and mortality within the pediatric surgical community. Medical management of these diseases is often suboptimal, and surgical resection of the diseased intestine may be warranted1. In many cases, however, surgical resection leaves the patient with an inadequate length of small intestine that precludes normal nutrient and fluid absorption. These patients may therefore require long term parenteral nutritional support due to short bowel syndrome (SBS).
Research is underway to understand the mechanisms associated with the intestinal ischemia and inflammation, bacterial translocation, sepsis, and organ failure that frequently go hand-in-hand with these disorders2-7. In addition, studies continue in the fields of small bowel transplantation, tissue engineering, and operative intervention, with the hopes of finding a means to replace or supplement the absorptive properties of the native intestine8-10. In this regard, stem cells represent a novel treatment modality with increasing therapeutic potential11-14. The extensive proliferation and differentiation capacities of stem cells make them optimal for seeding tissue engineered grafts15. In addition, the release of protective factors (paracrine effects) has also been shown to be beneficial to ischemic tissues11, 16-20.
Surgeons are best-positioned to take optimal advantage of stem cell therapy for their patients because they encounter tissue loss and dysfunction in everyday practice. In our ongoing investigational efforts to replace, regrow, and protect damaged or threatened tissue, a thorough understanding of the potential of stem cell therapy is paramount. Therefore, this manuscript will serve to: 1) review the different types of stem cells that may be applicable to the treatment of intestinal disorders, 2) review the mechanisms suggesting that stem cells may work for these conditions; 3) discuss current practices for harvesting and purifying stem cells and 4) provide a concise summary of a few of the pediatric intestinal disorders that could be treated with cellular therapy. It is our hope that the contents of this review will provide an overview of stem cell biology, and spur continued research into stem cell applications for the treatment of gastrointestional disorders.
STEM CELLS: CHARACTERIZATION AND ISOLATION
Embryonic, Bone Marrow Derived, and Umbilical Stem Cells
Stem cells are defined as unspecialized or undifferentiated precursor cells with the capacity for self-renewal and the power to differentiate into multiple different specialized cell types12. Grossly, stem cells are divided into either embryonic stem cells (ESCs) or adult stem cells, with adult stem cells being further divided into specific tissue stem cells, umbilical stem cells, or bone marrow stem cells. Adult stem cells are certainly the most commonly studied, as embryonic stem cells (ESCs) are present only during fetal development.
In 1998, James Thompson and his colleagues reported the establishment of human ESC lines that were extracted from embryos created by in-vitro fertilization. These cells, which form the inner cell mass at day 5−7 after fertilization, were transferred to a culture dish with feeder cells and allowed to replicate. In theory, these cells could retain their self-renewing capabilities without differentiating, and could give rise to cells with indefinite replicative properties. Thus ESCs have the potential to develop into most, if not all cell types within the body21-23. However, due to ethical, legal, and political issues, government funding agencies are only permitted to fund studies using ESC lines derived prior to August 9, 200124. Due to this mandate, other sources of stem cells have been investigated.
The adult body has a limited number of somatic stem cells in certain tissues and organs25-27. These adult stem cells possess the ability to regenerate the tissue from which they are derived. For example, hematopoietic stem cells constantly regenerate the circulating blood cells and cells of the immune system. Adult stem cells though, have a limited differentiation capability, whereas ESCs have the potential to form most, if not all cells over long periods of in-vitro culture.
Several studies have confirmed that adult stem cells participate in tissue regeneration. This was first seen by Ferraris and colleagues after the injection of bone marrow stem cells into injured muscle tissue28. Progenitor cell therapy was also shown to aid ischemic tissue by increasing angiogenesis in ischemic hind limb models29. Furthermore, multiple studies continue to support the notion that stem cell therapy improves cardiac function during conditions of ischemia30-32, including recently published human trials examining stem cell transplantation following myocardial infarction33, 34. Therefore, it is likely that stem cells can be utilized for a variety of ischemic and inflammation induced diseases, including those of the intestine.
Each stem cell, whether derived from the bone marrow, or from a specific tissue, has a unique manner of isolation. Bone marrow stem cells are typically divided into hematopoietic or mesenchymal stem cells based on cell surface markers. Hematopoietic cells are CD34+ and CD44-, whereas mesenchymal stem cells are negative for hematopoietic markers such as CD34, CD45, CD117, and CD11b, but positive for mesenchymal stem cell markers including CD44, Sca-1, and CD9035-37. Mesenchymal stem cells can be purified and isolated from the marrow quite easily due to their ability to bind to plastic38. Both cell lines can also be isolated and purified via fluorescent activated cell sorting, which makes use of the cell surface markers that are unique to each line. In addition, mesenchymal stem cells have the ability to suppress the immune response, which may become clinically applicable during stem cell transplantation39.
Umbilical cord stem cells are isolated in a manner similar to that of bone marrow stem cells. Umbilical cord derived mesenchymal stem cells are found in lower frequency in the umbilical cord and are negative for hematopoietic antigens. Umbilical cord mesenchymal stem cells express CD29, CD44, CD49, CD51, and CD105. They also have a high potential for proliferation, and are capable of differentiating into a number of tissues40. Due to the existence of umbilical cord blood banks and their high degree of proliferation, these cells may be an abundant source of mesenchymal cells for therapeutic use.
Gastrointestinal Stem Cells
Gastrointestinal stem cells possess the ability to differentiate into all cells that occupy the villus, including enterocytes, endocrine cells, and goblet cells41. Under normal conditions, these stem cells may divide asymmetrically, giving rise to one stem cell and one cell that differentiates into the different types of epithelial cells. Under conditions of stress though, these stem cells may divide symmetrically, giving rise to two progenitors that replace injured stem cells42.
Although it has been proven that the intestinal crypts harbor the intestinal stem cells, their exact location within the crypts is controversial. Previously, intestinal stem cells had been proposed to lie, on average, four cells up from the base of the crypts, immediately above the Paneth cells. These cells were conventionally named +4 label-retaining cells (+4 LRCs) based on studies demonstrating that they maintained a quiescent state, were slow cycling, and therefore retained chemiluminescent labels (Figure 1)43, 44. Recent studies have proposed a second group of intestinal stem cells that also maintain specific cellular markers, divide rapidly, and give rise to all cells of the intestine. These cells are referred to as crypt-based columnar cells (CBCs), and are located at the base of the crypts interspersed between the Paneth cells45.
Figure 1. Schematic view of a Crypt of Lieberkuhn.
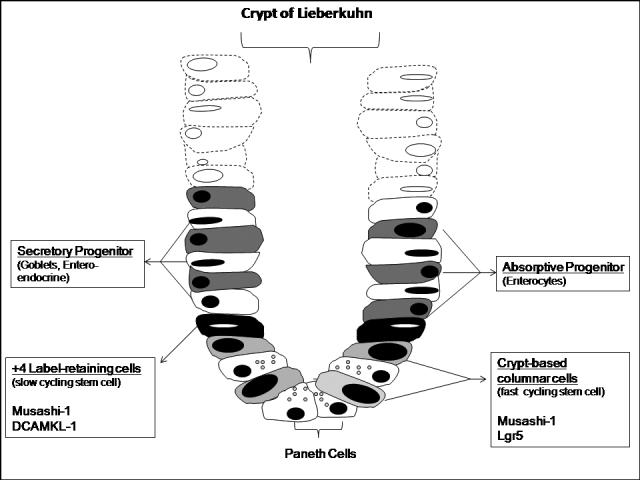
Intestinal stem cells reside at the bases of the Crypts of Lieberkuhn. Two different intestinal stem cell populations are thought to exist, including a slow cycling cell located at the +4 crypt position. In addition, a faster cycling stem cell known as a crypt-based columnar cell resides at the base of the crypt between the Paneth cells . As more superficial cells slough into the intestinal lumen, the stem cells divide and differentiate into absorptive and secretory progenitors, which then further differentiate into mature intestinal cells that migrate to the top of the villus.
Until recently, there had not been consistently reliable markers to identify these intestinal stem cells. However, with ongoing study, several intestinal stem cell markers including Musashi 1, Lgr5 (leucine-rich-repeat-containing G-protein-coupled receptor 5, also known as Gpr49), and DCAMKL-1 (doublecortin and CaM kinase-like-1) have been identified41, 46, 47. Musashi 1, an RNA-binding protein, was originally thought to be a neural stem cell marker48, 49. However, subsequent studies demonstrated that Musashi-1 was also present on intestinal and colonic stem cells 41, 50, 51. Utilizing this marker, the isolation of an unpure culture of intestinal stem cells from the jejunum was achieved52. However, additional markers were clearly needed to purify this culture.
Lgr5 has recently been discovered and has been shown to exist exclusively in crypt base cells within the intestine. Cells expressing this marker were found to exhibit stem cell characteristics, persist for at least 60 days, and were resistant to irradiation46. Similarly, antibodies directed against DCAMKL-1, a microtubule associated kinase, revealed single cell staining in crypt bases at or near the +4 position. These cells were resistant to irradiation, and immunohistocehmical analysis showed co-localization of DCAMKL-1 positive cells with Mushashi-147. Therefore, the combined use of these markers may allow for the isolation of purified intestinal specific stem cells within this protected stem cell niche.
Studies examining intestinal stem cell signaling have also suggested that Wnt/Ephrin, BMP (bone morphogenic protein), Notch, and PI3K/PTEN (P-phosphatase and tensin homologue) signaling cascades are dramatically involved with intestinal stem cell proliferation and lineage commitment53-56. The “on-off” signals provided by these pathways may work to convert quiescent stem cells to their active forms, much like a combination on a lock. These cascades have also been shown to play a role in the migratory process of the cells out of the crypt, as a decreasing concentration gradient of Wnt/Ephrin-B2 receptors, and an increasing gradient of BMP toward the top of the villus drives crypt cells upward. This differential expression ultimately specifies crypt cell position. Thus, as cells migrate upward along the crypt and farther from the Wnt source at the crypt base, Ephrin-B receptor expression decreases and Ephrin-B ligand expression increases, thereby preventing downward migration. In addition, because Paneth cells do not express Ephrin-B ligands and only express Ephrin-B3 receptors, their upward migration is prohibited, and, subsequently, they are forced to remain at the crypt base.
Experiments designed to further purify intestinal stem cells are certainly required prior to their widespread use. Furthermore, study of these purified cells under conditions of stress will allow for the understanding of their various intracellular signaling cascades. Once these mechanisms are elucidated, intestinal stem cells may be deemed the most optimal stem cell to seed tissue engineered grafts or to apply to injured tissues during therapy.
STEM CELLS AS A THERAPEUTIC TREATMENT OPTION
Several recent studies have suggested that higher levels of circulating stem cells may be an important aspect of native tissue protection57-60. Premature neonates have remarkably high levels of hematopoietic stem cells in their peripheral blood at birth61. Therefore, if stem cells are a potent source of protection for injured tissues, it is plausible that those patients who develop IBD or NEC may have lower numbers of functional native stem cells or may not be able to mobilize stem cells to the areas of injury. Furthermore, recent studies have suggested that intestinal stem cell activity is upregulated following massive intestinal loss62. In this regard, supplying an adequate number of functional stem cells to affected patients, either through tissue engineered neointestine, or via stem cell transplantation, may increase overall enteric function, promote intestinal restitution, and relieve disease symptoms. Although the optimum method of stem cell delivery has yet to be determined, many options have been tested including intravascular delivery and direct infusion into ischemic tissue63. Other methods such as enteral delivery via oral or transrectal approaches have yet to be addressed.
Tissue Engineering
The engineering of neointestine dates back to 1988, when Vacanti and his colleagues from Boston attempted to grow enterocytes on biodegradeable polymers64. The concept of tissue engineering surrounds three principles, namely the cell source, the biomaterial, and the biomolecules that integrate the cells and the matrix65. Cells can be of multiple different sources, including differentiated progenitor cells, stem cells, or tissue/organ specific cells. Furthermore, the biomaterials and biomolecules can be natural or synthetic, and can be made of varying compounds.
The cell source is a very important component of engineering neointestine, as the cells should possess the ability to differentiate into all aspects of the intestine, including absorptive and secretory cells, as well as cells to supply the nearby vasculature and physical support. In this regard, stem cells, with their multilineage capabilities, are probably most ideal to differentiate into all the necessary cells of the bowel. Finding the stem cell with optimum characteristics for tissue regeneration and ischemic protection, including optimal age, gender, and host source, therefore becomes paramount66-68.
Bone marrow derived progenitor cells have the ability to regenerate multiple mesenchymal tissues including that of bone, muscle, cartilage, and stroma, and have recently been shown to repopulate the intestine after injury65, 69. Specifically, mesenchymal stem cells have been shown to develop into hepatocytes70, muscle cells28, neurons71, renal tubular epithelial cells72, and intestinal cells73. Circulating hematopoietic stem cells have also been shown to differentiate into mature hepatocytes and epithelial cells of skin and the gastrointestinal tract. This was determined by analyzing biopsy specimens from subjects who received hematopoietic stem cell transplantation for the treatment of leukemia74. As bone marrow stem cells are easily harvested and isolated, it seems reasonable to utilize them as the cellular source for the construction of tissue engineered grafts.
Elevations in stem cell quantity appear to be protective to the intestine, as increased numbers of bone marrow derived cells were closely related to recovery after ulcerative endothelial damage, as measured by immunohistochemistry57. Despite that other studies have also seen improvements in disease pathology with increasing numbers of circulating stem cells75, some studies have seen no improvement76. This may suggest that elevations of specific stem cell lines have implications in providing tissue protection from only certain diseases.
Isolation of enterocytes and intestinal stem cells is also of primary interest for potential therapy of injured intestine. Maintaining primary enterocyte cultures, however, has proven difficult, as these are fully differentiated, non-replicating cells. Methods have been established to isolate the three dimensional structure of the villus, termed the intestinal organoid. Intestinal organoid units are multicellular units derived from neonatal small intestine which contain a mesenchymal core surrounded by a polarized intestinal epithelium. These organoid units contain all the cells within a full-thickness section of bowel77, 78. Isolation of organoids was first achieved by Weiser, and has been modified to involve a Collagenase/Dispase method of digestion of the intestinal lumen77.
Once the isolation of intestinal organoid units was achieved, biological engineers began to utilize them as the cellular source for small intestinal grafts. Vacanti's group transplanted small intestinal organoid units on a polymer scaffold onto rat omentum, and noticed that the grafts formed cystic structures of neointestine. Other investigators have implanted scaffolds, followed by seeding with intestinal stem cells, and have found that the neointestine maintains similar characteristics of native intestine up to three months after implantation. Immunofluoresence analysis demonstrated that crypt cells continued to divide, and that massive small bowel resections seemed to increase the stimuli for growth and incorporation of the transplanted tissue engineered grafts79, 80. Furthermore, organoid units harvested from areas of peak absorption, such as where bile acids are absorbed in the terminal ileum, were shown to express these absorptive properties even when grown on a scaffold in the jejunum81.
Intestinal organoids that grew on scaffolds not only repopulated the intestine, but also resulted in vascular and lymphatic proliferation, predominantly through VEGF production82, 83. When the neointestinal cysts were anastomosed to the native intestine, the neointestine developed the dynamic features of the native intestine. Recent work in rats has demonstrated that implantation of tissue engineered small intestine following massive small bowel resection increased postoperative weight and serum B12 levels compared to those animals without tissue engineered small intestinal implantation84. These data suggest that the neointestine is not only anatomically intact, but is also functioning to absorb calorically dense nutrients. These studies give promising hope to future human applications of tissue engineered intestine. Further studies designed to evaluate the long term effects of neointestinal implantation, as well as investigation into nano- and microfabrication techniques to evaluate the individual cellular response to the microenvironment are warranted15, 85 .
Intestinal cell engineering groups continue to focus on identifying the most appropriate polymer scaffold on which to seed new cells. Small intestinal submucosa (SIS) has been evaluated as a new scaffolding polymer but has achieved mixed reviews on its efficacy86, 87. Additionally, SIS is a natural matrix which makes large scale and homogenous preparation difficult. Alternative scaffolds that have many of the properties of SIS, such as chitosan and polyester matrices, are therefore being evaluated with modest success88-91.
Paracrine Effects
A variety of cellular growth factors have been previously shown to play key roles in maintaining gut mucosal barrier function92-95. Therefore, increased local concentrations of these factors may promote healing and gut restitution following injury. For example, hepatocyte growth factor (HGF) may work to decrease cellular apoptosis and increase angiogenesis while also improving rat small intestinal function, mucosal mass, and substrate absorption96-100. Similarly, vascular endothelial growth factor (VEGF) has been shown to inhibit leukocyte/epithelial cell adherence and the effects of chronic inflammation101. VEGF also appears to enhance angiogenesis during acute inflammation and ischemia, and may enhance stem cell survival during transplantation102-104. Other growth factors, such as keratinocyte growth factor (KGF), have been shown to inhibit intestinal ulceration and to promote protective prostaglandin release, while glucagon-like protein 2 (GLP-2) and epidermal growth factor (EGF) may promote intestinal restitution following injury94, 105, 106. Finally, elevated levels of insulin-like growth factor-1 (IGF-1) have been shown to decrease pro-apoptotic signaling and increase cellular proliferation107.
Stem cells have previously been thought to aid injured tissue via several different mechanisms. Some feel that stem cells differentiate into specific end organ cells, which then become incorporated into the tissue to increase post-injury functional recovery108. However, others have shown in cardiac, pulmonary, and renal tissue that protection from injury can be achieved in the absence of stem cell differentiation, thereby suggesting that stem cells aid native tissues via cell-cell interactions or via the release of protective paracrine substances during their transit through injured tissue31, 109-111.
Stem cell paracrine properties likely aid in the recovery of injured tissue via a variety of mechanisms (Figure 2). These include the production of antioxidants such as catalase, glutathione peroxidase, and manganese superoxide dismutase, which work to decrease the number of damaging oxygen free radicals present in ischemic tissue112. In addition, stem cells have been shown to produce growth factors, such as VEGF, HGF, KGF, EGF, and IGF-1, which may enhance stem cell survival during transplantation, and are believed to protect ischemic tissues via the promotion of angiogenesis, the inhibition of apoptosis, and the promotion of cellular proliferation113, 114.
Figure 2. Stem Cell Paracrine Properties.
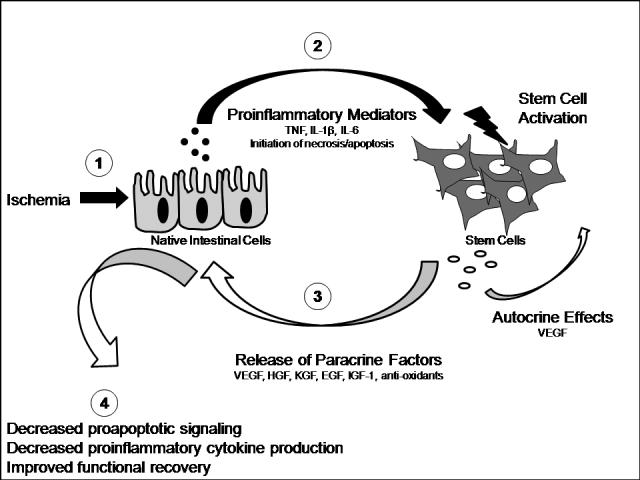
As a result of ischemia or other noxious insult, native stem cells release multiple inflammatory mediators that activate stem cells. These stem cells, in turn, release a number of protective paracrine substances, which may work to decrease inflammation, apoptosis, and intestinal dysfunction.
The release of stem cell paracrine mediators are triggered by a number of factors, including endotoxin and hypoxia115. These noxious stimuli may activate stem cells directly, causing them to release beneficial paracrine mediators via the activation of a variety of intracellular signaling cascades113, 115. Conversely, proinflammatory mediators, such as IL-6, TNF, and IL-1βthat are released from native tissue after injury116-120 may subsequently trigger stem cell activation and the release of beneficial mediators to enhance native tissue recovery.
Through the release of these complex paracrine factors, stem cells collectively work to enhance neovascularization and perfusion to injured tissues. Indeed, studies on intestinal vascular neogenesis noted that non-bone marrow-derived circulating progenitor cells increased liver and intestinal vasculogenesis by 6.3% and 4.7% respectively121. Enhanced neovascularization after stem cell therapy has also been demonstrated in inflammatory bowel disease11, as well as in other tissues, including adipose29 and cardiac tissue122. Previous investigators who have studied the molecular mechanisms of ulcer healing have noted that VEGF, KGF, and HGF are essential to the re-epithelialization, muscle restoration, epithelial cell proliferation, and reconstruction of gastric glands after ulcer injury105, 123. Furthermore, the application of mesenchymal stem cells to these ulcers was shown to increase ulcer healing via a VEGF dependent mechanism124.
Stem cell paracrine mechanisms also work to salvage tenuous native cells125, alter the extracellular matrix126, and activate resident stem cells24. These characteristics become important during intestinal inflammation in that stem cell transplant may work to preserve the absorptive and barrier functions of marginally damaged intestinal cells. Furthermore, activation and recruitment of native intestinal stem cells may facilitate enhanced intestinal restitution and cellular repair, thereby decreasing disease symptoms.
In an attempt to design stem cells that produce maximum amounts of protective factors, it becomes essential to understand the intracellular signaling associated with stem cell paracrine effects. Several studies have confirmed enhanced stem cell growth factor production and enhanced post ischemic protection associated with female gender17, 67, 127, 128, TNF receptor inhibition129, as well as p38 MAPK and STAT 3 activation130. Indeed, many others have recognized positive effects associated with estrogen131-133 and TNF inhibition134-139 in other tissues, and therefore, it stands to reason that these would also be beneficial in stem cell signaling. Further studies designed to elucidate the mechanisms of stem cell activation and their associated intracellular signaling cascades are certainly warranted before widespread human application can take place.
PEDIATRIC INTESTINAL DISORDERS WHERE STEM CELLS MAY SHOW PROMISE
Stem cells have been relatively understudied for the treatment of pediatric disorders. However, investigations into the potential use of stem cells in pediatric cardiac140, neurological141, perinatal142, and pulmonary diseases143, 144 have been recently undertaken. Stem cell therapy has not yet been adopted for routine use in the treatment of pediatric intestinal disorders, however, a few disorders, such as IBD, NEC and short bowel syndrome, may be amenable to stem cell therapy. Cellular therapy for the treatment of inflammatory bowel diseases and tissue engineering studies to counter short bowel syndrome have already been initiated, and have been met with modest success. Cellular therapy for the treatment of necrotizing enterocolitis has not yet been addressed. Tremendous potential exists in the treatment of these disorders with stem cells, and a review of the current and potential role of cellular therapy for them follows.
Inflammatory bowel disease
Crohn's disease (CD) and ulcerative colitis (UC) are heterogeneous chronic inflammatory bowel disorders (IBD)145. The prevalence of CD and UC in children younger than 20 years is 43 and 28 per 100,000, respectively. Under normal circumstances, injury to the intestinal mucosa promotes a process of wound healing that restores normal tissue architecture and function. In IBD, and particularly in CD, the transmural inflammation and tissue damage elicit an excessive wound-healing response that leads to a distortion of tissue architecture, fibrosis, and subsequent stenosis. These complications, therefore, are some of the major reasons for surgical intervention146.
Stem cell therapy has recently been found to benefit patients suffering from IBD. Positive effects were noted in several studies which examined IBD patients undergoing allogenic stem cell transplantation for leukemia57. Patient IBD symptoms improved after transplant, and follow-up colonoscopy revealed complete resolution of pathologic findings147, 148. Animal studies examining the role of embryonic stem cells on small intestinal and colonic repair after IL-10 induced colitis demonstrated that embryonic stem cells homed to areas of injury in the small intestine and colon, promoted repair, decreased tissue inflammation, and restored balance to the immune system149. In addition, other studies have demonstrated that the levels of circulating endothelial progenitor cells were decreased or depleted in those with IBD150. These data would suggest that there is an inherent error in the stem cell homing or regenerative capacities in patients with IBD, and that improving these characteristics via stem cell therapy may improve intestinal cell function and/or restitution, and subsequently, may ameliorate patient symptoms.
The European experience for stem cell therapy in IBD was recently published, and showed sustained remission of IBD symptoms in approximately one third of cases151. A prospective randomized trial of stem cell therapy for IBD treatment in Europe (ASTIC-Autologous Stem Cell Transplantation International Crohn's Disease) is currently underway146. The results of this study should provide tremendous insight into the clinical utility of stem cell therapy for IBD.
Necrotizing Enterocolitis and Short Bowel Syndrome
NEC is one of the most devastating intra-abdominal emergencies of the newborn infant. Approximately 1−7% of all neonatal ICU admissions, and up to 10% of very low birth weight infants are affected1. Due to the severity of the disease process, resection of ischemic and necrotic intestine is often required152. Outcomes studies have noted that infants with NEC have not only increased long-term intestinal morbidity including increased rates of stricture, abscess, and short gut syndrome153, 154, but also increased likelihood of neurodevelopmental impairment, especially if they have undergone surgical intervention155.
Stem cell therapy has yet to be studied in human cases or animal models of NEC, but could certainly be useful for limiting the degree of intestinal ischemia and the subsequent peritonitis associated with perforation. Stem cells have already been shown to accelerate the healing of gastric ulcers as well as colonic perforations due to diverticulitis156, 157. Stem cell therapy might protect injured native intestine by promoting neovascularization, while also facilitating intestinal restitution following the removal of the injuring stimulus121. Stem cells could be delivered through multiple mechanisms, but may be most effective at the time of surgery. Stem cell delivery via open surgical intervention or to-be-developed minimally invasive approaches may work to halt the progression to frank perforation, gross intraperitoneal contamination, sepsis, and subsequent death.
Stem cells may also play a powerful role in the treatment of short bowel syndrome, which may result from massive intestinal resection for Hirschprung's disease158, NEC9, severe intestinal atresia, or volvulus159. SBS is the most common cause of protracted intestinal failure mandating long term dependence on parenteral nutrition for survival159. Moreover, parenteral nutrition increases the risk for developing parenteral nutrition-associated liver failure160. Pediatric surgeons, therefore, play a vital role in influencing the morbidity and mortality of SBS patients by optimizing bowel preservation at the time of resective surgery, using gut lengthening procedures to optimize the residual small bowel's functional surface area, and facilitating timely consideration for intestinal transplantation161.
Experimental studies designed to increase intestinal absorptive capacity, such as growth hormone, epidermal growth hormone, recombinant glucagon-like-peptide 2, or glutamine therapy, as well as operative gut lengthening procedures, have been met with modest success162-164. In this regard, stem cell engineered bioprosthetic neointestine may prove beneficial in conjunction with these techniques to increase the absorptive capacity of the intestine. Such procedures have already shown benefit in animal models, and may decrease the need for long-term parenteral nutrition or multivisceral organ transplantation84.
CONCLUSION
Stem cell research has made tremendous advancements over the past decade. Through multiple studies, it has become clear that stem cells possess tremendous therapeutic potential in protecting native tissues from ischemia and inflammation induced injury. It is our hope that this review will spur interest in cellular therapy, and encourage research in utilizing stem cells for the treatment of pediatric intestinal diseases. Further insight into the mechanisms of stem cells may provide better ways of utilizing progenitor cell therapy for maximizing therapeutic potential during the treatment of ischemic and inflammation induced intestinal disorders.
Acknowledgments
This work was supported in part by NIH R01GM070628, NIH R01HL085595, NIH K99/R00 HL0876077-01, NIH F32 HL085982, AHA Grant in aid, and AHA Post-doctoral Fellowship 0526008Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006;25(4):329–337. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 2.Nussler NC, Muller AR, Weidenbach H, Vergopoulos A, Platz KP, Volk HD, Neuhaus P, Nussler AK. IL-10 increases tissue injury after selective intestinal ischemia/reperfusion. Ann Surg. 2003;238(1):49–58. doi: 10.1097/01.sla.0000074962.26074.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford HR. Mechanism of nitric oxide-mediated intestinal barrier failure: insight into the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 2006;41(2):294–299. doi: 10.1016/j.jpedsurg.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176(5):3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 5.Ayala A, Song GY, Chung CS, Redmond KM, Chaudry IH. Immune depression in polymicrobial sepsis: the role of necrotic (injured) tissue and endotoxin. Crit Care Med. 2000;28(8):2949–2955. doi: 10.1097/00003246-200008000-00044. [DOI] [PubMed] [Google Scholar]
- 6.Cetin S, Leaphart CL, Li J, Ischenko I, Hayman M, Upperman J, Zamora R, Watkins S, Ford HR, Wang J, Hackam DJ. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2007;292(5):G1347–1358. doi: 10.1152/ajpgi.00375.2006. [DOI] [PubMed] [Google Scholar]
- 7.Tadros T, Traber DL, Heggers JP, Herndon DN. Effects of interleukin-1alpha administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Ann Surg. 2003;237(1):101–109. doi: 10.1097/00000658-200301000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SF, Che XM, Chen JC, Lu SY, Fan L, Wang R, Li GW. Treatment of short gut syndrome with early living related small bowel transplantation. Transplant Proc. 2005;37(10):4461–4463. doi: 10.1016/j.transproceed.2005.10.092. [DOI] [PubMed] [Google Scholar]
- 9.Petty JK, Ziegler MM. Operative strategies for necrotizing enterocolitis: The prevention and treatment of short-bowel syndrome. Semin Pediatr Surg. 2005;14(3):191–198. doi: 10.1053/j.sempedsurg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Sola A, De Oca J, Gonzalez R, Prats N, Rosello-Catafau J, Gelpi E, Jaurrieta E, Hotter G. Protective effect of ischemic preconditioning on cold preservation and reperfusion injury associated with rat intestinal transplantation. Ann Surg. 2001;234(1):98–106. doi: 10.1097/00000658-200107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S, Mutschler WE, Siebeck M, Huss R. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132(3):944–954. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Nagy RD, Tsai BM, Wang M, Markel TA, Brown JW, Meldrum DR. Stem cell transplantation as a therapeutic approach to organ failure. J Surg Res. 2005;129(1):152–160. doi: 10.1016/j.jss.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Haider H, Ashraf M. Bone marrow cell transplantation in clinical perspective. J Mol Cell Cardiol. 2005;38(2):225–235. doi: 10.1016/j.yjmcc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Raeburn CD, Zimmerman MA, Arya J, Banerjee A, Harken AH. Stem cells and myocardial repair. J Am Coll Surg. 2002;195(5):686–693. doi: 10.1016/s1072-7515(02)01309-1. [DOI] [PubMed] [Google Scholar]
- 15.Satija NK, Gurudutta GU, Sharma S, Afrin F, Gupta P, Verma YK, Singh VK, Tripathi RP. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16(1):7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42(2):441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol. 2007;42(1):142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 19.Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Surgically relevant aspects of stem cell paracrine effects. Surgery. 2008;143(5):577–581. doi: 10.1016/j.surg.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Crisostomo PR, Wang M, Weil B, Abarbanell A, Poynter J, Manukyan MC, Meldrum DR. No Suppresses the Secretion of Vascular Endothelial Growth Factor and Hepatocyte Growth Factor from Human Mesenchymal Stem Cells. Shock. 2008 doi: 10.1097/SHK.0b013e31816f1ec9. [DOI] [PubMed] [Google Scholar]
- 21.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 22.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70(3):837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 23.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20(9):933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 24.Crisostomo PR, Wang M, Markel TA, Lahm T, Abarbanell AM, Herrmann JL, Meldrum DR. Stem cell mechanisms and paracrine effects: potential in cardiac surgery. Shock. 2007;28(4):375–383. doi: 10.1097/shk.0b013e318058a817. [DOI] [PubMed] [Google Scholar]
- 25.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 26.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287(5457):1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 27.Ho AD, Punzel M. Hematopoietic stem cells: can old cells learn new tricks? J Leukoc Biol. 2003;73(5):547–555. doi: 10.1189/jlb.0902458. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 29.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 30.Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, Peng H, Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50(17):1677–1684. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock. 2006;25(5):454–459. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 32.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28(21):2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 33.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 34.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355(12):1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Huang Z, Lazzarini P, Wang Y, Di A, Chen M. A unique human blood-derived cell population displays high potential for producing insulin. Biochem Biophys Res Commun. 2007;360(1):205–211. doi: 10.1016/j.bbrc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 36.Zhang SJ, Zhang H, Hou M, Zheng Z, Zhou J, Su W, Wei Y, Hu S. Is it possible to obtain “true endothelial progenitor cells” by in vitro culture of bone marrow mononuclear cells? Stem Cells Dev. 2007;16(4):683–690. doi: 10.1089/scd.2006.0062. [DOI] [PubMed] [Google Scholar]
- 37.Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51(8):723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 38.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 39.Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, Dahlke MH. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81(11):1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 40.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 41.Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H, Chiba T. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535(13):131–135. doi: 10.1016/s0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 42.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105(11):1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115(Pt 11):2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 44.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24(1):91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 45.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134(3):849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 46.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 47.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26(3):630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura M, Okano H, Blendy JA, Montell C. Musashi, a neural RNA-binding protein required for Drosophila adult external sensory organ development. Neuron. 1994;13(1):67–81. doi: 10.1016/0896-6273(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 49.Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306(2):349–356. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura S, Wakabayashi N, Toyoda K, Kashima K, Mitsufuji S. Expression of Musashi-1 in human normal colon crypt cells: a possible stem cell marker of human colon epithelium. Dig Dis Sci. 2003;48(8):1523–1529. doi: 10.1023/a:1024763723240. [DOI] [PubMed] [Google Scholar]
- 51.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71(1):28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 52.Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology. 2005;129(5):1567–1580. doi: 10.1053/j.gastro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24(11):2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 54.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307(5717):1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 55.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306(2):357–363. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 56.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39(2):189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8(9):1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 59.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 60.Shimura K, Ashihara E, Shimazaki C, Matsunaga S, Taniguchi K, Uchiyama H, Matsumoto Y, Kimura S, Matsubara H, Taniwaki M, Maekawa T. Circulating endothelial progenitor cells decreased in patients with sclerodermatous chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(4):426–437. doi: 10.1016/j.bbmt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Bizzarro MJ, Bhandari V, Krause DS, Smith BR, Gross I. Circulating stem cells in extremely preterm neonates. Acta Paediatr. 2007;96(4):521–525. doi: 10.1111/j.1651-2227.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 62.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1013–1022. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 63.Crisostomo PR, Meldrum DR. Stem cell delivery to the heart: clarifying methodology and mechanism. Crit Care Med. 2007;35(11):2654–2656. doi: 10.1097/01.CCM.0000288086.96662.40. [DOI] [PubMed] [Google Scholar]
- 64.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23(1 Pt 2):3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 65.Gupta A, Dixit A, Sales KM, Winslet MC, Seifalian AM. Tissue engineering of small intestine--current status. Biomacromolecules. 2006;7(10):2701–2709. doi: 10.1021/bm060383e. [DOI] [PubMed] [Google Scholar]
- 66.Crisostomo PR, Wang M, Wairiuko GM, Morrell ED, Terrell AM, Seshadri P, Nam UH, Meldrum DR. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26(6):575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 67.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007;142(2):215–221. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Markel TA, Wang M, Crisostomo PR, Manukyan MC, Poynter JA, Meldrum DR. Neonatal stem cells exhibit specific characteristics in function, proliferation, and cellular signaling that distinguish them from their adult counterparts. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1491–1497. doi: 10.1152/ajpregu.00031.2008. [DOI] [PubMed] [Google Scholar]
- 69.Okamoto R, Matsumoto T, Watanabe M. Regeneration of the intestinal epithelia: regulation of bone marrow-derived epithelial cell differentiation towards secretory lineage cells. Hum Cell. 2006;19(2):71–75. doi: 10.1111/j.1749-0774.2006.00010.x. [DOI] [PubMed] [Google Scholar]
- 70.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406(6793):257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 71.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195(2):229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Nakamura T, Eguchi H, Kawano S. The transdifferentiation of bone-marrow-derived cells in colonic mucosal regeneration after dextran-sulfate-sodium-induced colitis in mice. Pharmacology. 2007;80(4):193–199. doi: 10.1159/000104148. [DOI] [PubMed] [Google Scholar]
- 74.Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346(10):738–746. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 75.Sobrino T, Hurtado O, Moro MA, Rodriguez-Yanez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Davalos A, Lizasoain I, Castillo J. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38(10):2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 76.Ripa RS, Haack-Sorensen M, Wang Y, Jorgensen E, Mortensen S, Bindslev L, Friis T, Kastrup J. Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulation. 2007;116(11 Suppl):I24–30. doi: 10.1161/CIRCULATIONAHA.106.678649. [DOI] [PubMed] [Google Scholar]
- 77.Weiser MM. Intestinal epithelial cell surface membrane glycoprotein synthesis. II. Glycosyltransferases and endogenous acceptors of the undifferentiated cell surface membrane. J Biol Chem. 1973;248(7):2542–2548. [PubMed] [Google Scholar]
- 78.Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101(Pt 1):219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- 79.Kim SS, Kaihara S, Benvenuto M, Choi RS, Kim BS, Mooney DJ, Taylor GA, Vacanti JP. Regenerative signals for tissue-engineered small intestine. Transplant Proc. 1999;31(1−2):657–660. doi: 10.1016/s0041-1345(98)01737-0. [DOI] [PubMed] [Google Scholar]
- 80.Kim SS, Kaihara S, Benvenuto MS, Choi RS, Kim BS, Mooney DJ, Taylor GA, Vacanti JP. Regenerative signals for intestinal epithelial organoid units transplanted on biodegradable polymer scaffolds for tissue engineering of small intestine. Transplantation. 1999;67(2):227–233. doi: 10.1097/00007890-199901270-00007. [DOI] [PubMed] [Google Scholar]
- 81.Avansino JR, Chen DC, Hoagland VD, Woolman JD, Stelzner M. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery. 2006;140(3):423–434. doi: 10.1016/j.surg.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 82.Duxbury MS, Grikscheit TC, Gardner-Thorpe J, Rocha FG, Ito H, Perez A, Ashley SW, Vacanti JP, Whang EE. Lymphangiogenesis in tissue-engineered small intestine. Transplantation. 2004;77(8):1162–1166. doi: 10.1097/01.tp.0000121506.34924.3c. [DOI] [PubMed] [Google Scholar]
- 83.Rocha FG, Sundback CA, Krebs NJ, Leach JK, Mooney DJ, Ashley SW, Vacanti JP, Whang EE. The effect of sustained delivery of vascular endothelial growth factor on angiogenesis in tissue-engineered intestine. Biomaterials. 2008;29(19):2884–2890. doi: 10.1016/j.biomaterials.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grikscheit TC, Siddique A, Ochoa ER, Srinivasan A, Alsberg E, Hodin RA, Vacanti JP. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann Surg. 2004;240(5):748–754. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park H, Cannizzaro C, Vunjak-Novakovic G, Langer R, Vacanti CA, Farokhzad OC. Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Eng. 2007;13(8):1867–1877. doi: 10.1089/ten.2006.0198. [DOI] [PubMed] [Google Scholar]
- 86.Lee M, Chang PC, Dunn JC. Evaluation of Small Intestinal Submucosa as Scaffolds for Intestinal Tissue Engineering. J Surg Res. 2008 doi: 10.1016/j.jss.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 87.Gilbert TW, Stewart-Akers AM, Sydeski J, Nguyen TD, Badylak SF, Woo SL. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Eng. 2007;13(6):1313–1323. doi: 10.1089/ten.2006.0318. [DOI] [PubMed] [Google Scholar]
- 88.Verma V, Verma P, Ray P, Ray AR. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed Mater. 2008;3(2):25007. doi: 10.1088/1748-6041/3/2/025007. [DOI] [PubMed] [Google Scholar]
- 89.Lawrence BJ, Maase EL, Lin HK, Madihally SV. Multilayer composite scaffolds with mechanical properties similar to small intestinal submucosa. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.31903. [DOI] [PubMed] [Google Scholar]
- 90.Chen DC, Avansino JR, Agopian VG, Hoagland VD, Woolman JD, Pan S, Ratner BD, Stelzner M. Comparison of polyester scaffolds for bioengineered intestinal mucosa. Cells Tissues Organs. 2006;184(3−4):154–165. doi: 10.1159/000099622. [DOI] [PubMed] [Google Scholar]
- 91.Yixiang D, Yong T, Liao S, Chan CK, Ramakrishna S. Degradation of Electrospun Nanofiber Scaffold by Short Wave Length Ultraviolet Radiation Treatment and Its Potential Applications in Tissue Engineering. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0395. [DOI] [PubMed] [Google Scholar]
- 92.Griga T, Tromm A, Schmiegel W, Pfisterer O, Muller KM, Brasch F. Collagenous colitis: implications for the role of vascular endothelial growth factor in repair mechanisms. Eur J Gastroenterol Hepatol. 2004;16(4):397–402. doi: 10.1097/00042737-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Jeschke MG, Bolder U, Finnerty CC, Przkora R, Muller U, Maihofer R, Thompson JC, Wolf SE, Herndon DN. The effect of hepatocyte growth factor on gut mucosal apoptosis and proliferation, and cellular mediators after severe trauma. Surgery. 2005;138(3):482–489. doi: 10.1016/j.surg.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 94.Bulut K, Pennartz C, Felderbauer P, Meier JJ, Banasch M, Bulut D, Schmitz F, Schmidt WE, Hoffmann P. Glucagon like peptide-2 induces intestinal restitution through VEGF release from subepithelial myofibroblasts. Eur J Pharmacol. 2008;578(2−3):279–285. doi: 10.1016/j.ejphar.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 95.Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V, Hsueh W, Raymond AS, Shur BD, Tan XD. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117(12):3673–3683. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yo Y, Morishita R, Nakamura S, Tomita N, Yamamoto K, Moriguchi A, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Potential role of hepatocyte growth factor in the maintenance of renal structure: anti-apoptotic action of HGF on epithelial cells. Kidney Int. 1998;54(4):1128–1138. doi: 10.1046/j.1523-1755.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 97.Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio PM. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. Embo J. 1996;15(22):6205–6212. [PMC free article] [PubMed] [Google Scholar]
- 98.Kosai K, Matsumoto K, Nagata S, Tsujimoto Y, Nakamura T. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem Biophys Res Commun. 1998;244(3):683–690. doi: 10.1006/bbrc.1998.8293. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol. 2004;37(5):1041–1052. doi: 10.1016/j.yjmcc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz MZ, Kato Y, Yu D, Lukish JR. Growth-factor enhancement of compromised gut function following massive small-bowel resection. Pediatr Surg Int. 2000;16(3):174–175. doi: 10.1007/s003830050716. [DOI] [PubMed] [Google Scholar]
- 101.Scalia R, Booth G, Lefer DJ. Vascular endothelial growth factor attenuates leukocyte-endothelium interaction during acute endothelial dysfunction: essential role of endothelium-derived nitric oxide. Faseb J. 1999;13(9):1039–1046. doi: 10.1096/fasebj.13.9.1039. [DOI] [PubMed] [Google Scholar]
- 102.von Dobschuetz E, Meyer S, Thorn D, Marme D, Hopt UT, Thomusch O. Targeting vascular endothelial growth factor pathway offers new possibilities to counteract microvascular disturbances during ischemia/reperfusion of the pancreas. Transplantation. 2006;82(4):543–549. doi: 10.1097/01.tp.0000229434.92523.99. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Haider HK, Ahmad N, Xu M, Ge R, Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol. 2006;40(5):736–745. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 104.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417(6892):954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 105.Han DS, Li F, Holt L, Connolly K, Hubert M, Miceli R, Okoye Z, Santiago G, Windle K, Wong E, Sartor RB. Keratinocyte growth factor-2 (FGF-10) promotes healing of experimental small intestinal ulceration in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279(5):G1011–1022. doi: 10.1152/ajpgi.2000.279.5.G1011. [DOI] [PubMed] [Google Scholar]
- 106.Dise RS, Frey MR, Whitehead RH, Polk DB. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G276–285. doi: 10.1152/ajpgi.00340.2007. [DOI] [PubMed] [Google Scholar]
- 107.Zimmermann EM, Li L, Hou YT, Mohapatra NK, Pucilowska JB. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G1022–1029. doi: 10.1152/ajpgi.2001.280.5.G1022. [DOI] [PubMed] [Google Scholar]
- 108.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, Wu JC. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116(11 Suppl):I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel KM, Crisostomo P, Lahm T, Markel T, Herring C, Wang M, Meldrum KK, Lillemoe KD, Meldrum DR. Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction by a paracrine mechanism. J Surg Res. 2007;143(2):281–285. doi: 10.1016/j.jss.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 111.Wairiuko GM, Crisostomo PR, Wang M, Morrell ED, Meldrum KK, Lillemoe KD, Meldrum DR. Stem cells improve right ventricular functional recovery after acute pressure overload and ischemia reperfusion injury. J Surg Res. 2007;141(2):241–246. doi: 10.1016/j.jss.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 112.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104(12):3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 113.Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R880–884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 114.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-{alpha}, LPS, or hypoxia produce growth factors by an NF{kappa}B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294(3):C675–682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 116.Yang S, Hu S, Choudhry MA, Rue LW, 3rd, Bland KI, Chaudry IH. Anti-rat soluble IL-6 receptor antibody down-regulates cardiac IL-6 and improves cardiac function following trauma-hemorrhage. J Mol Cell Cardiol. 2007;42(3):620–630. doi: 10.1016/j.yjmcc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 117.Matsutani T, Kang SC, Miyashita M, Sasajima K, Choudhry MA, Bland KI, Chaudry IH. Liver cytokine production and ICAM-1 expression following bone fracture, tissue trauma, and hemorrhage in middle-aged mice. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G268–274. doi: 10.1152/ajpgi.00313.2006. [DOI] [PubMed] [Google Scholar]
- 118.Ayala A, Perrin MM, Meldrum DR, Ertel W, Chaudry IH. Hemorrhage induces an increase in serum TNF which is not associated with elevated levels of endotoxin. Cytokine. 1990;2(3):170–174. doi: 10.1016/1043-4666(90)90012-i. [DOI] [PubMed] [Google Scholar]
- 119.Meldrum DR, Partrick DA, Cleveland JC, Jr., Shenkar R, Meldrum KK, Raiesdana A, Ayala A, Brown JW, Harken AH. On-pump coronary artery bypass surgery activates human myocardial NF-kappaB and increases TNF-alpha in the heart. J Surg Res. 2003;112(2):175–179. doi: 10.1016/s0022-4804(03)00122-7. [DOI] [PubMed] [Google Scholar]
- 120.Song Y, Shi Y, Ao LH, Harken AH, Meng XZ. TLR4 mediates LPS-induced HO-1 expression in mouse liver: role of TNF-alpha and IL-1beta. World J Gastroenterol. 2003;9(8):1799–1803. doi: 10.3748/wjg.v9.i8.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100(4):581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 122.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42(4):792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50(Suppl 1):S24–33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- 124.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S. Topical transplantation of mesenchymal stem cells accelerates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G778–786. doi: 10.1152/ajpgi.00468.2007. [DOI] [PubMed] [Google Scholar]
- 125.Jaquet K, Krause KT, Denschel J, Faessler P, Nauerz M, Geidel S, Boczor S, Lange C, Stute N, Zander A, Kuck KH. Reduction of myocardial scar size after implantation of mesenchymal stem cells in rats: what is the mechanism? Stem Cells Dev. 2005;14(3):299–309. doi: 10.1089/scd.2005.14.299. [DOI] [PubMed] [Google Scholar]
- 126.Xu X, Xu Z, Xu Y, Cui G. Selective down-regulation of extracellular matrix gene expression by bone marrow derived stem cell transplantation into infarcted myocardium. Circ J. 2005;69(10):1275–1283. doi: 10.1253/circj.69.1275. [DOI] [PubMed] [Google Scholar]
- 127.Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, Meldrum DR. Sex dimorphisms in activated mesenchymal stem cell function. Shock. 2006;26(6):571–574. doi: 10.1097/01.shk.0000233195.63859.ef. [DOI] [PubMed] [Google Scholar]
- 128.Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex Steroids and Stem Cell Function. Mol Med. 2008 doi: 10.2119/2008-00004.Ray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Markel TA, Crisostomo PR, Wang M, Herring CM, Meldrum DR. Activation of individual tumor necrosis factor receptors differentially affects stem cell growth factor and cytokine production. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G657–662. doi: 10.1152/ajpgi.00230.2007. [DOI] [PubMed] [Google Scholar]
- 130.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42(6):1009–1015. doi: 10.1016/j.yjmcc.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hsieh YC, Frink M, Kawasaki T, Thobe BM, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Downregulation of TLR4-dependent ATP production is critical for estrogen-mediated immunoprotection in Kupffer cells following trauma-hemorrhage. J Cell Physiol. 2007;211(2):364–370. doi: 10.1002/jcp.20943. [DOI] [PubMed] [Google Scholar]
- 132.Kuebler JFJD, Toth B, Bland KI, Rue L, 3rd, Wang P, Chaudry IH. Estradiol administration improves splanchnic perfusion following trauma-hemorrhage and sepsis. Archives of Surgery. 2002;137(1):74–79. doi: 10.1001/archsurg.137.1.74. [DOI] [PubMed] [Google Scholar]
- 133.Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on mods and cytokines after multiple injuries. Shock. 2007;27(2):151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 134.Meldrum KK, Meldrum DR, Meng X, Ao L, Harken AH. TNF-alpha-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2002;282(2):H540–546. doi: 10.1152/ajpheart.00072.2001. [DOI] [PubMed] [Google Scholar]
- 135.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274(3 Pt 2):R577–595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 136.Meldrum KK, Metcalfe P, Leslie JA, Misseri R, Hile KL, Meldrum DR. TNF-alpha neutralization decreases nuclear factor-kappaB activation and apoptosis during renal obstruction. J Surg Res. 2006;131(2):182–188. doi: 10.1016/j.jss.2005.11.581. [DOI] [PubMed] [Google Scholar]
- 137.Panes J, Gomollon F, Taxonera C, Hinojosa J, Clofent J, Nos P. Crohn's disease: a review of current treatment with a focus on biologics. Drugs. 2007;67(17):2511–2537. doi: 10.2165/00003495-200767170-00005. [DOI] [PubMed] [Google Scholar]
- 138.Meng X, Ao L, Meldrum DR, Cain BS, Shames BD, Selzman CH, Banerjee A, Harken AH. TNF-alpha and myocardial depression in endotoxemic rats: temporal discordance of an obligatory relationship. Am J Physiol. 1998;275(2 Pt 2):R502–508. doi: 10.1152/ajpregu.1998.275.2.R502. [DOI] [PubMed] [Google Scholar]
- 139.Meng X, Harken AH. The interaction between Hsp70 and TNF-alpha expression: a novel mechanism for protection of the myocardium against post-injury depression. Shock. 2002;17(5):345–353. doi: 10.1097/00024382-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 140.Pillekamp F, Reppel M, Brockmeier K, Hescheler J. Stem cells and their potential relevance to paediatric cardiology. Cardiol Young. 2006;16(2):117–124. doi: 10.1017/S1047951106000023. [DOI] [PubMed] [Google Scholar]
- 141.Gardner SL. Application of stem cell transplant for brain tumors. Pediatr Transplant. 2004;8(Suppl 5):28–32. doi: 10.1111/j.1398-2265.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 142.Santner-Nanan B, Peek MJ, McCullagh P, Nanan R. Therapeutic potential of stem cells in perinatal medicine. Aust N Z J Obstet Gynaecol. 2005;45(2):102–107. doi: 10.1111/j.1479-828X.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 143.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1073–1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 144.van Haaften T, Thebaud B. Adult bone marrow-derived stem cells for the lung: implications for pediatric lung diseases. Pediatr Res. 2006;59(4 Pt 2):94R–99R. doi: 10.1203/01.pdr.0000203550.50258.5a. [DOI] [PubMed] [Google Scholar]
- 145.Cuffari C. Inflammatory bowel disease in children: a pediatrician's perspective. Minerva Pediatr. 2006;58(2):139–157. [PubMed] [Google Scholar]
- 146.Latella G, Fiocchi C, Caprilli R. Late-breaking news from the “4th International Meeting on Inflammatory Bowel Diseases” Capri, 2006. Inflamm Bowel Dis. 2007;13(8):1031–1050. doi: 10.1002/ibd.20127. [DOI] [PubMed] [Google Scholar]
- 147.Ditschkowski M, Einsele H, Schwerdtfeger R, Bunjes D, Trenschel R, Beelen DW, Elmaagacli AH. Improvement of inflammatory bowel disease after allogeneic stem-cell transplantation. Transplantation. 2003;75(10):1745–1747. doi: 10.1097/01.TP.0000062540.29757.E9. [DOI] [PubMed] [Google Scholar]
- 148.Cassinotti A, Annaloro C, Ardizzone S, Onida F, Della Volpe A, Clerici M, Usardi P, Greco S, Maconi G, Porro GB, Deliliers GL. Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn's disease. Gut. 2008;57(2):211–217. doi: 10.1136/gut.2007.128694. [DOI] [PubMed] [Google Scholar]
- 149.Srivastava AS, Feng Z, Mishra R, Malhotra R, Kim HS, Carrier E. Embryonic stem cells ameliorate piroxicam-induced colitis in IL10−/− KO mice. Biochem Biophys Res Commun. 2007;361(4):953–959. doi: 10.1016/j.bbrc.2007.07.139. [DOI] [PubMed] [Google Scholar]
- 150.Masuda J, Mitsuyama K, Yamasaki H, Takedatsu H, Okamura T, Andoh A, Murohara T, Asahara T, Sata M. Depletion of endothelial progenitor cells in the peripheral blood of patients with ulcerative colitis. Int J Mol Med. 2007;19(2):221–228. [PubMed] [Google Scholar]
- 151.Gratwohl A, Passweg J, Bocelli-Tyndall C, Fassas A, van Laar JM, Farge D, Andolina M, Arnold R, Carreras E, Finke J, Kotter I, Kozak T, Lisukov I, Lowenberg B, Marmont A, Moore J, Saccardi R, Snowden JA, van den Hoogen F, Wulffraat NM, Zhao XW, Tyndall A. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 2005;35(9):869–879. doi: 10.1038/sj.bmt.1704892. [DOI] [PubMed] [Google Scholar]
- 152.Ahmed T, Ein S, Moore A. The role of peritoneal drains in treatment of perforated necrotizing enterocolitis: recommendations from recent experience. J Pediatr Surg. 1998;33(10):1468–1470. doi: 10.1016/s0022-3468(98)90476-6. [DOI] [PubMed] [Google Scholar]
- 153.Horwitz JR, Lally KP, Cheu HW, Vazquez WD, Grosfeld JL, Ziegler MM. Complications after surgical intervention for necrotizing enterocolitis: a multicenter review. J Pediatr Surg. 1995;30(7):994–998. doi: 10.1016/0022-3468(95)90328-3. discussion 998−999. [DOI] [PubMed] [Google Scholar]
- 154.Schimpl G, Hollwarth ME, Fotter R, Becker H. Late intestinal strictures following successful treatment of necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:80–83. doi: 10.1111/j.1651-2227.1994.tb13251.x. [DOI] [PubMed] [Google Scholar]
- 155.Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161(6):583–590. doi: 10.1001/archpedi.161.6.583. [DOI] [PubMed] [Google Scholar]
- 156.Ringden O, Uzunel M, Sundberg B, Lonnies L, Nava S, Gustafsson J, Henningsohn L, Le Blanc K. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21(11):2271–2276. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 157.Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S. Topical Transplantation of Mesenchymal Stem Cells Accelerates Gastric Ulcer Healing in Rats. Am J Physiol Gastrointest Liver Physiol. 2008 doi: 10.1152/ajpgi.00468.2007. [DOI] [PubMed] [Google Scholar]
- 158.Hackam DJ, Reblock KK, Redlinger RE, Barksdale EM., Jr. Diagnosis and outcome of Hirschsprung's disease: does age really matter? Pediatr Surg Int. 2004;20(5):319–322. doi: 10.1007/s00383-004-1188-5. [DOI] [PubMed] [Google Scholar]
- 159.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130(2 Suppl 1):S16–28. doi: 10.1053/j.gastro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 160.Lloyd DA, Gabe SM. Managing liver dysfunction in parenteral nutrition. Proc Nutr Soc. 2007;66(4):530–538. doi: 10.1017/S002966510700585X. [DOI] [PubMed] [Google Scholar]
- 161.Chungfat N, Dixler I, Cohran V, Buchman A, Abecassis M, Fryer J. Impact of parenteral nutrition-associated liver disease on intestinal transplant waitlist dynamics. J Am Coll Surg. 2007;205(6):755–761. doi: 10.1016/j.jamcollsurg.2007.06.299. [DOI] [PubMed] [Google Scholar]
- 162.Ladd AP, Grosfeld JL, Pescovitz OH, Johnson NB. The effect of growth hormone supplementation on late nutritional independence in pediatric patients with short bowel syndrome. J Pediatr Surg. 2005;40(2):442–445. doi: 10.1016/j.jpedsurg.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 163.Pereira PM, Bines JE. New growth factor therapies aimed at improving intestinal adaptation in short bowel syndrome. J Gastroenterol Hepatol. 2006;21(6):932–940. doi: 10.1111/j.1440-1746.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 164.Modi BP, Javid PJ, Jaksic T, Piper H, Langer M, Duggan C, Kamin D, Kim HB. First report of the international serial transverse enteroplasty data registry: indications, efficacy, and complications. J Am Coll Surg. 2007;204(3):365–371. doi: 10.1016/j.jamcollsurg.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


