Abstract
All eukaryotic cells present at the cell surface a specific set of plasma membrane proteins that modulate responses to internal and external cues and whose activity is also regulated by protein degradation. We characterized the lytic vacuole-dependent degradation of membrane proteins in Arabidopsis thaliana by means of in vivo visualization of vacuolar targeting combined with quantitative protein analysis. We show that the vacuolar targeting pathway is used by multiple cargos including PIN-FORMED (PIN) efflux carriers for the phytohormone auxin. In vivo visualization of PIN2 vacuolar targeting revealed its differential degradation in response to environmental signals, such as gravity. In contrast to polar PIN delivery to the basal plasma membrane, which depends on the vesicle trafficking regulator ARF-GEF GNOM, PIN sorting to the lytic vacuolar pathway requires additional brefeldin A-sensitive ARF-GEF activity. Furthermore, we identified putative retromer components SORTING NEXIN1 (SNX1) and VACUOLAR PROTEIN SORTING29 (VPS29) as important factors in this pathway and propose that the retromer complex acts to retrieve PIN proteins from a late/pre-vacuolar compartment back to the recycling pathways. Our data suggest that ARF GEF- and retromer-dependent processes regulate PIN sorting to the vacuole in an antagonistic manner and illustrate instrumentalization of this mechanism for fine-tuning the auxin fluxes during gravitropic response.
Keywords: gravitropism, polar auxin transport, posttranslational regulation, vesicle trafficking
Plants have evolved a remarkable developmental plasticity to shape their individual growth according to environmental stresses. The signal molecule auxin has been ultimately linked to the flexible plant expansion. The distribution of the phytohormone auxin depends largely on its directional transport from cell to cell (1). Auxin efflux carriers of the PIN-FORMED (PIN) family show a polar localization that correlates with and determines the direction of auxin flow (2, 3). By this delivery system, auxin accumulates in spatial and temporal patterns, which are read out to direct plant growth and development (4, 5). Throughout the plant's lifespan, PIN-dependent auxin distribution contributes to developmental adaptation, such as postembryonic organ formation, apical dominance, tissue regeneration, and tropisms (6–9).
PIN auxin efflux carrier targeting is a highly dynamic process with constitutive cycling of the PIN proteins between the plasma membrane and (an) endosomal compartment(s). This process depends on ADP-ribosylation factor GTP-exchange factors (ARF-GEFs), such as GNOM (also called EMB40), and is sensitive to vesicle trafficking inhibitors, such as brefeldin A (BFA) (10–12). GNOM appears to regulate PIN recycling strictly to the basal plasma membrane (13), whereas the putative retromer complex component SORTING NEXIN1 (SNX1) resides in intracellular structures distinct from GNOM and has been suggested to be involved in recycling of apical PIN2 but not basal PIN1 (14). SNX1 genetically interacts with other putative retromer complex members, such as VACUOLAR PROTEIN SORTING29 (VPS29) (15). In contrast to SNX1, the VPS29 has been proposed to be involved preferentially in basal PIN1 targeting and has been suggested to function downstream of GNOM (15). Nevertheless, the contribution of the retromer complex to the PIN recycling remained controversial (16).
However, the overall function of the PIN-constitutive cycling is still unclear, although it has been proposed to enable the rapid changes in PIN polarity in response to environmental stimuli (12, 13) or to control the levels of PIN proteins at the plasma membrane and thus the auxin transport rate (17). The activity of the PIN-dependent auxin distribution network can be regulated at multiple levels, including transcription (18–20), polar subcellular localization (7–9, 12), endocytosis (17), and, not least, protein degradation (21–24). In particular, the mechanisms that regulate PIN degradation are still widely elusive. However, after gravistimulation, proteasome-dependent variations in PIN2 localization and degradation at the upper and lower sides of the root result in asymmetric distribution of PIN2, suggesting that it is functionally important for posttranslational regulation of the PIN stability in plant development (22).
To date, the mechanisms underlying the degradation of plasma membrane proteins are not well understood in plants; hence, we examined the cellular and molecular requirements of this process with a special focus on PIN efflux carriers. We used a drug-free assay to visualize the PIN degradation in lytic vacuoles and to illustrate the importance of posttranslational PIN regulation for fine-tuning auxin transport during plant development. Our data suggest that a partially BFA-sensitive ARF-GEF promotes the PIN protein transition from endosomes to the prevacuolar compartment (PVC), whereas SNX1 can counteract this process by retrieving PIN proteins from the PVC, which appears to be functionally important for temporal PIN degradation during gravitropic responses.
Results and Discussion
Auxin Efflux Carrier PIN2 Displays Dynamic Turnover in the Lytic Vacuole.
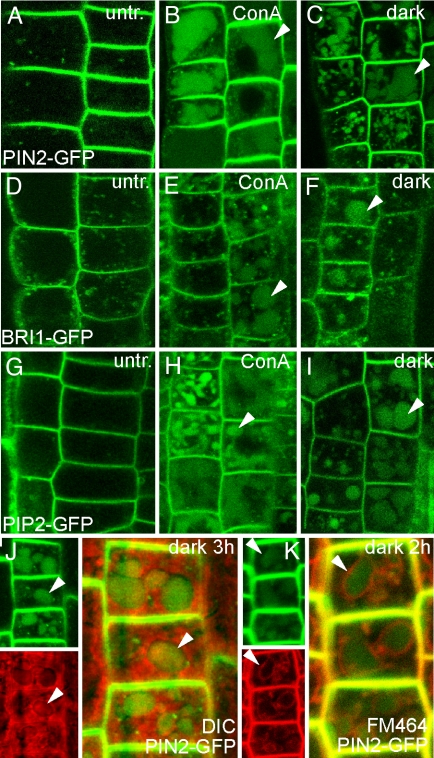
To visualize the targeting to the lytic vacuole for degradation, we initially used concanamycin A, a well-established and specific inhibitor of vacuolar H-ATPases, to reduce acidification of lytic compartments and, thus, protein degradation (25). Under our experimental conditions, concanamycin A treatments enabled us to observe accumulation and, hence, trafficking of fluorescently tagged plasma membraneproteins to the lytic vacuole for degradation, namely of the auxin efflux carrier PIN2 (Fig. 1 A and B), the brassinosteroid receptor BRI1, (Fig. 1 D and E), and the aquaporin PIP2 (Fig. 1 G and H).
Fig. 1.
Visualization of trafficking of plasma membrane proteins to the lytic vacuole. (A) PIN2-GFP localization at the plasma membrane and endocytic intracellular compartments in untreated epidermal root cells. (B and C) Appearance of GFP signal in lytic vacuoles in PIN2-GFP-expressing seedlings after concanamycin A (1 μM for 6 h) treatment (B) or incubation (6 h) in the dark (C). (D) BRI1-GFP-expressing transgenic lines showing BRI1 localization at the plasma membrane and in intracellular structures. (E and F) BRI1-GFP degradation in lytic vacuoles after concanamycin A (E) and dark (F). (G–I) PIP2-GFP distribution in epidermis cells (G) after concanamycin A (H) and dark (I) treatments. (J and K) Appearance of diffuse vacuolar GFP signal (in green) in PIN2-GFP-expressing cells after dark treatment (2 h) identified morphologically in transmission light image (in red) (J) or by endocytic uptake of FM4-64 (K) dye (in red), labeling the tonoplast around the diffuse GFP signal. Arrowheads indicate vacuolar occurrence of GFP signals.
Because of the possible unwanted side effects of concanamycin A on protein trafficking (26), we made use of the fact that GFP and related proteins are more stable in lytic vacuoles under dark conditions than in the light because of conformational changes (27) to study plasma membrane protein degradation in a drug-independent manner. Indeed, dark treatment led to vacuole-like accumulation of the GFP signal in various transgenic lines, including PIN2-GFP, BRI1-GFP, and PIP2-GFP (Fig. 1 C, F, and I).
GFP localization in dark-treated PIN2-GFP coincided with vacuolar occurrence in transmission light images (Fig. 1J). Moreover, the endocytic dye FM4-64, which labels the tonoplast within 2 h of incubation (26), surrounded the diffuse GFP signal in PIN2-GFP-expressing seedlings after 2 h in the dark (Fig. 1K). These findings confirm that GFP resides in the lumen of the tonoplast after dark treatment, thus illustrating the degradation of PIN2 in lytic vacuoles.
To roughly estimate the turnover of PIN2, we analyzed the earliest GFP accumulation in lytic vacuoles. A diffuse GFP occurrence in lytic vacuoles was already detectable after 1–2 h in the dark in two independent PIN2-GFP (22, 28) lines (Fig. 1K and data not shown). In contrast, BRI1-GFP fusions (29, 30) displayed their earliest vacuolar signals within 4–6 h (Fig. 1F and data not shown), in agreement with previous half-life estimations of BRI1-GFP (30). To investigate the PIN2 protein stability independent of the light-to-dark transition, dexamethasone-inducible TA:PIN2-GFP lines were used (22). After induction of the transgene and subsequent removal of dexamethasone, PIN2-GFP was rapidly depleted, further indicating a fast, proteolytic turnover of this protein [supporting information (SI) Fig. S1].
These data show that integral plasma membrane proteins, such as PIN2, BRI1, and PIP2, are targeted to the vacuole for degradation. Moreover, the rapid turnover of the PIN2 protein suggests a tight posttranslational regulation during plant development.
ARF-GEF-Dependent Trafficking of PIN2 to the Lytic Vacuole.
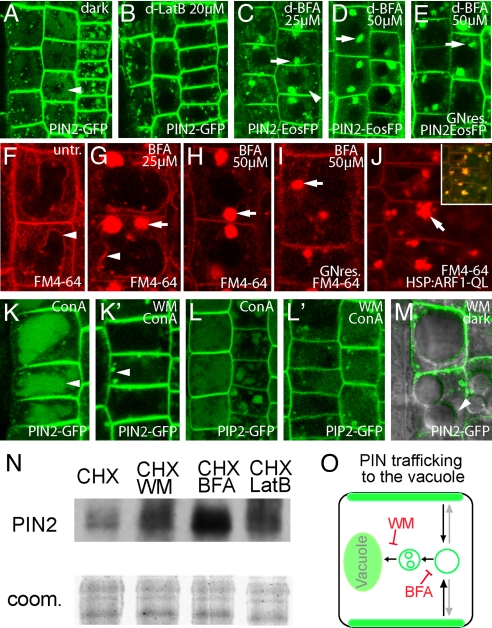
To gain insights into the biological function of the PIN2 targeting to the lytic vacuole, we examined the cellular requirements for endocytic trafficking of PIN2. The PIN2-GFP targeting to the vacuole for degradation depends on the actin cytoskeleton, because latrunculin B-induced depolymerization of actin inhibited GFP accumulation in lytic vacuoles and stabilized PIN2 as manifested by increased total levels of the PIN2 protein (Fig. 2 B and N), implying an actin-dependent vesicle transport for vacuolar PIN2 trafficking. BFA, an inhibitor of ARF-GEF-type vesicle transport regulators, restrains recycling to the plasma membrane and leads to PIN accumulation in aggregated endosomes (10) and also elevates PIN2 protein levels (Fig. 2N) (22). Notably, BFA reduced the PIN2 targeting to the lytic vacuole (Fig. 2D), suggesting the involvement of an ARF-GEF. Moreover, BFA also inhibited the uptake of FM4-64 to the tonoplast membrane (Fig. 2H) and stabilized BRI1 in endosomes (30), hinting at a general requirement for BFA-sensitive ARF-GEF activity in the control of membrane trafficking from endosomes to the vacuole. Reduced BFA concentrations sufficient to inhibit ARF GEF GNOM-dependent PIN recycling to the basal cell side (31) did not fully abolish endocytic PIN2-GFP or FM4-64 trafficking to the tonoplast (Fig. 2 C and G), which might hint at a GNOM-independent mode of action for BFA. To determine whether BFA affects the PIN2 trafficking to the vacuole independent of GNOM, we used an engineered BFA-resistant version of GNOM (11). The BFA-resistant GNOMML lines still showed BFA sensitivity for endocytic uptake of PIN2 and FM4-64 to the vacuole (Fig. 2 E and I), indicating a GNOM-independent vacuolar trafficking and involvement of an additional ARF-GEF. This involvement of ARF and ARF-GEF activity in the PIN2 vacuolar targeting has been further substantiated by conditional overexpression of a constitutively active version of an ARF-GEF substrate ARF1 (28) that strongly interfered with the endocytic FM4-64 trafficking to the tonoplast (Fig. 2J).
Fig. 2.
Cellular and molecular requirements of the PIN trafficking to lytic vacuoles. (A and B) Latrunculin B (LatB; 20 μM) treatment (B) compared with control (A) revealing actin-dependent trafficking of the PIN2-GFP to the vacuole as visualized by dark treatment (2 h). (C and D) Dark treatments (2 h) showing the reduction of PIN2-EosFP in vacuolar targeting at 25 μM BFA (C) and a complete block at 50 μM BFA (D). (E) In the transgene carrying the engineered BFA-resistant version of GNOM ARF GEF (GNOMML), the vacuolar trafficking of PIN2-EosFP is still BFA-sensitive. (F–J) ARF GEF-dependent FM4-64 uptake (2 h) to the tonoplast (indicated by arrowheads) of untreated cells (F) and cells treated with 25 μM BFA (G), 50 μM BFA (H), and 50 μM BFA in the BFA-resistant version of GNOMML (I) and after heat-shock induction (37 °C for 2 h) of the constitutively active ARF1QL (J). (Inset) FM4-64 and ARF1QL-YFP colocalization. (K and L) Concanamycin A (1 μM, 6 h)-visualized trafficking of PIN2-GFP (K) and PIP2-GFP (L) to vacuoles inhibited by wortmannin (15 μM, 6 h) (K′ and L′) treatment. (M) Occasional PIN2 localization at the tonoplast after wortmannin (15 μM for 3 h in the dark) treatment. (N) PIN2 protein-stabilizing effect of wortmannin (WM) brefeldin A (BFA), and latrunculin B (LatB) by Western blot analysis. Concomitant drug treatment with protein biosynthesis inhibitor cycloheximide (CHX) was done for inhibition of the PIN2 secretion. (O) Schematic representation of the ARF-GEF (BFA) and PI3K (WM)-sensitive sorting events of PIN2 to the lytic vacuole for degradation. Arrows mark BFA-induced accumulation, and arrowheads mark the PIN2 occurrence in vacuoles or endocytic mistargeting.
Thus, whereas polar recycling of PIN proteins to the basal plasma membrane depends on the GNOM function, endocytic translocation of plasma membrane components, such as PIN2, to the vacuole is GNOM-independent and most probably utilizes another BFA-sensitive ARF-GEF activity. Importantly, these findings indicate that, after internalization, the PIN recycling to the plasma membrane and the alternative posting to the vacuole for degradation are molecularly distinct by ARF-GEF utilization.
Vacuolar Trafficking of PIN2 Depends on Phosphatidylinositol-Dependent Pathways.
Progress has been made in the elucidation of the cellular machinery, by which cargo is delivered to the lysosome/vacuole in animal, yeast, and plant cells (32). In plants, wortmannin, an inhibitor of phosphatidylinositol-3-kinase (PI3K) and at 10-fold higher concentration of PI4K, affects recycling of vacuolar sorting receptors between the prevacuolar compartment (PVC) and the trans-Golgi network (TGN) and thus alters the PVC identity (33, 34). In accordance, wortmannin severely affects the PVC morphology of treated plant cells (35).
Notably, wortmannin has been shown to interfere with apical PIN2 localization in the epidermis, but not with basal PIN1 targeting in stele cells, indicating that wortmannin interferes with apical but not basal PIN trafficking (14). However, ectopic expression of the basal PIN1 cargo in root epidermis cells resulted in comparable wortmannin sensitivity (data not shown). Similarly, we observed wortmannin-sensitive PIN1 targeting in steles after prolonged wortmannin treatments (data not shown). Hence, differential sensitivity to wortmannin could be explained by cell type dependency or drug uptake. This finding shows that wortmannin treatment does not discriminate between apical and basal polar cargos in plant cells.
Although wortmannin has been suggested to interfere with PIN2 recycling events to the plasma membrane (14), the underlying mechanisms remain unclear. We show that wortmannin abolishes vacuolar trafficking of PIN2-GFP (Fig. 2 K and K′) as well as that of additional plasma membrane proteins, such as PIP2-GFP (Fig. 2 L and L′ and data not shown). Consistent with the observed effects of wortmannin on vacuolar targeting, the drug also increased the total PIN2 protein levels in membrane protein preparations (Fig. 2N). From these findings, we concluded that posttranslational regulation of plasma membrane proteins, such as PIN2, involves PI3K signaling as a regulator of protein translocation to the lytic vacuole. However, we cannot rule out that, in addition to PI3K, PI4K-dependent processes could contribute to the observed effects to some extent.
Under our experimental conditions, wortmannin affects the endocytic targeting of plasma membrane proteins to the vacuole by inhibiting intracellular sorting events rather than endocytosis (36) at the plasma membrane (Fig. S2). In accordance, in some cases we observed mistargeting of PIN2-GFP to the tonoplast membrane after wortmannin treatment (Fig. 2M), suggesting sorting/invagination defects at the multivesicular body (MVB)/PVC (35).
Taken together, the effect of wortmannin on the intracellular compartments disrupting vacuolar sorting of plasma membrane proteins, such as PIN2, strongly indicates that PI3K activity is required for the regulation of vacuolar degradation of PIN2.
SNX1 Localization and Conditional Mutant Phenotypes Suggest Their Involvement in Vacuolar Targeting.
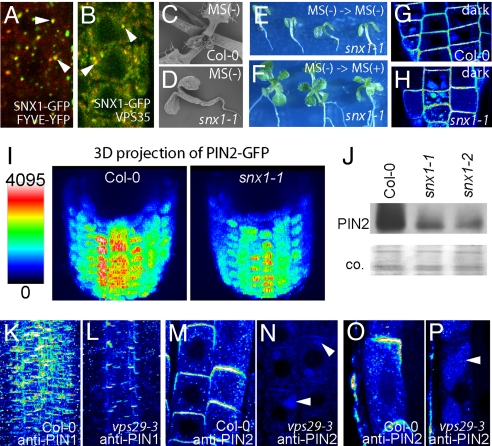
SNX1 is a potential downstream effector of PI3K because it bears a phosphatidylinositol binding PX domain (37) and colocalizes with a marker for phosphatidylinositol-3-phosphate (PI3P)-enriched membrane subdomains (Fig. 3A). Yeast orthologs of SNX1 are retromer complex components required for vacuolar receptor retrieval from the PVC to the TGN in a clathrin- and COP-independent manner (38). In plants, putative retromer components localize to the PVC and might interact with vacuolar sorting receptors (39). Moreover, SNX1 has been shown to colocalize and genetically interact with the putative plant retromer component VPS29 (15). In agreement with these findings, we found another putative plant retromer component (39), VPS35, that colocalizes with SNX1 at the PVC (Fig. 3B).
Fig. 3.
Regulation of PIN degradation by plant retromer components. (A and B) Merged images of colocalization (arrowheads) of SNX1-GFP (green) with the PI3P-binding domain FYVE-YFP (red) (A) and retromer component VPS35 (red) (B). (C and D) Two-week-old wild-type seedlings with normal growth on media without sucrose (C) and snx1 mutants arrested in growth (D). (E and F) Rescue of growth defects (E) by transfer on sucrose-containing media (F). (G and H) Substantial vacuolar targeting of the PIN2-GFP in the wild type (G) and even more pronounced in snx1 mutant (H) after dark treatments for 2 h. (I) Reduced PIN2-GFP levels at the plasma membrane in snx1 mutant. Three-dimensional animation of z-stacks (80 μM with 2-μM steps) was obtained. Roots were digitally tilted for outlook at the apical cell surface. False color code was used for PIN2-GFP intensity visualization. (J) Reduced total PIN2 protein levels in snx1 mutants by Western blot analysis. (K and L) Reduced PIN1 protein levels in vps29 (L) compared with wild-type seedlings (K) by z-stack analysis and maximum projection of the PIN1 immunolocalization (≈80 μM, 2-μM steps). (M–P) PIN2 immunolocalization in wild type (M and O) and vps29-3 mutants (N and P). PIN2 accumulation in vps29 was ectopically localized in vacuolar-like structures in meristematic (N) and in elongated root cells (P), suggesting enhanced degradation in vacuoles. Arrowheads depict PIN occurrence in vacuole-like structures. False color code depicts relative fluorescent intensity (I and K–P).
Mutants in the VPS29 gene, which has been shown to be crucial for protein storage vacuole formation in Arabidopsis embryos (40), show conditional growth arrest phenotypes on sucrose-depleted medium (Fig. S3). Similarly, snx1 seedlings exhibited a pronounced growth arrest on sucrose-depleted medium (Fig. 3D). In the most severe cases, snx1 mutant seedlings arrested growth after cotyledon formation (Fig. 3D). The growth arrest of sucrose-depleted snx1 seedlings was conditional and could be fully rescued by sucrose application (Fig. 3F). Remarkably, similar growth phenotypes have been described for mutants in GRAVITROPISM DEFECTIVE 2 (GRV2), an Arabidopsis homolog of RECEPTOR MEDIATED ENDOCYTOSIS 8 from Caenorhabditis elegans (41). Grv2 mutants are characterized by deficiencies in shoot gravitropism and exhibit a conditional growth arrest phenotype when grown in the absence of sucrose (41). These phenotypes have been attributed primarily to defects in the late steps of the endocytic pathway that interfere with the proper cargo delivery to storage as well as to lytic vacuoles (41, 42).
Hence, we assume that interference with storage vacuole formation affects the energy-consuming postembryonic leaf organ formation, leading to growth-arrested phenotypes that can be rescued by exogenous sucrose. The analogy to the grv2 growth phenotype could indicate that the SNX1/VPS29-dependent retromer complex is involved in protein storage vacuole formation and in late steps of the endocytic pathway for lytic transmembrane protein degradation.
The Putative Retromer Components SNX1 and VPS29 Mediate Vacuolar Targeting and Steady-State Levels of PIN Proteins.
The similarity of the phenotypes observed in either vps29 or snx1 mutants might indicate overlapping, interdependent functions of the corresponding gene products in the control of vacuolar targeting. Therefore, we analyzed abundance and intracellular distribution of the PIN proteins in these mutants. Mutants in snx1 interfere with steady-state PIN2 levels, reflected in reduced PIN2-GFP abundance at the epidermal plasma membrane and in diminished PIN2 protein levels in snx1-1 and snx1-2 membrane protein preparations (Fig. 3 I and J). Changes in PIN protein abundance in snx1 mutants were largely independent of transcriptional regulation and hint at a posttranslational regulation (data not shown). Similar observations were made when analyzing endogenous PIN1 and PIN2 in a vps29 mutant, demonstrating reduced amounts of both proteins at the plasma membrane and elevated amounts of PIN2 signals in vacuole-like structures, being suggestive for enhanced turnover of the protein (Fig. 3 K–P). Moreover, our data indicate that retromer-dependent sorting events at the PVC affect both apical and basal PIN2 or PIN1 targeting.
PIN proteins have been shown to constantly cycle between plasma membrane and an endosomal compartment whose identity is not fully determined yet (10, 11, 13). It has been suggested that the putative retromer complex is required for polar recycling of PIN proteins by regulating PIN exocytosis to the plasma membrane downstream of GNOM (15). In this scenario, disturbance of the retromer-dependent exocytotic branch of PIN cycling could also lead to the observed changes in PIN abundance at the plasma membrane in snx1 or vps29 by default targeting to the vacuole. However, even prolonged pharmacological interference with the SNX1-labeled PVCs did not inhibit PIN targeting to the plasma membrane (data not shown and Fig. S4 D and H). In contrast, BFA inhibition of GNOM leads to reversible accumulation of basal PIN cargos in agglomerating endosomes and has been extensively used to study recycling of PIN proteins (10, 13). To investigate especially polar recycling from endosomal compartments to the basal plasma membrane in snx1, we used BFA washout experiments in the presence and absence of the protein biosynthesis inhibitor cycloheximide (Fig. S4 and data not shown). These experiments demonstrated that polar PIN localization, BFA response, and recycling/exocytosis from endosomes to the plasma membrane after BFA removal remain unaffected in snx1 mutants (Fig. S4 A–H and data not shown). This finding contradicts previous models in which the retromer complex at the PVC is involved in polar PIN exocytosis downstream of GNOM and illustrates that PIN2 and PIN1 recycling does not directly require the function of the retromer complex. Thus, although SNX1 activity appears crucial for PIN2 homeostasis, it appears not to be directly involved in polar localization or in an exocytic recycling step of PIN proteins to the plasma membrane, illustrating again the interdependent, but distinct, regulation of PIN recycling and vacuolar degradation.
Remarkably, deficiencies in putative plant retromer components result in defects in plasma membrane protein localization and turnover that differ from those observed upon wortmannin treatments. Specifically, whereas the pharmacological inhibition of the SNX1-labeled PVC by wortmannin reduced PIN2 accumulation in the vacuole, loss of SNX1 function did not, but preferentially enhanced vacuolar translocation (Fig. 3 G and H). Moreover, unlike wortmannin treatments that lead to increased PIN2 protein levels, PIN2-GFP abundance at the epidermal plasma membrane and total PIN2 protein levels were reduced in snx1 mutants (compare Figs. 2N and 3J). Moreover, in contrast to wortmannin treatments (Fig. S2G), endocytic FM4-64 uptake was not significantly altered in snx1 mutants (data not shown). Thus, although the effects of wortmannin on the PIN localization and turnover might arise from a more general interference with the sorting of plasma membrane proteins at the PVC, such as MVB formation, the mutant analysis was consistent with a more specific function of SNX1 and VPS29 in the control of vacuolar targeting. In agreement, PIN2 targeting remained sensitive to wortmannin treatment in snx1 mutants (data not shown). The snx1 mutant phenotypes, as well as SNX1 subcellular localization, suggest SNX1 involvement in vacuolar sorting events at the PVC. In such a scenario, SNX1/VPS29 seems to have a gating function for endocytic translocation of PIN2 to the lytic vacuole for degradation.
Differential PIN2 Degradation in Lytic Vacuoles in Response to External Stimuli.
Posttranslational mechanisms that determine protein abundance of the PIN auxin efflux carriers and their contribution to the plant development are still ill-defined. External signals, such as light or gravitropic stimulation, have been reported to trigger intracellular redistribution to the vacuole and/or increased degradation of PIN2, respectively (22, 24). Quantitative Western blot analysis revealed that total PIN2 protein levels are reduced after both prolonged dark treatment (Fig. S5) and gravity stimulation (Fig. S6). In particular, PIN2 is down-regulated in response to gravity stimulus only in a transient manner and recovers after prolonged stimulation, suggesting a defined and complex regulation of the PIN2 turnover in response to external stimuli.
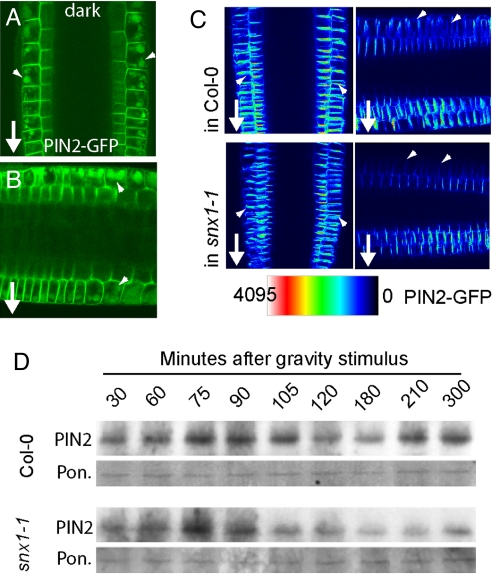
To investigate whether the gravity stimulus induces PIN2 degradation via this pathway, we used dark treatments to monitor PIN2 targeting to the lytic vacuole. Notably, in PIN2-GFP seedlings, higher GFP accumulation was detectable invacuoles of epidermis cells at the upper side of gravity-stimulated roots (Fig. 4 A and B and Fig. S7), suggesting a spatially differential regulation of the vacuolar PIN2 trafficking during gravitropism. Our previous findings suggested that the PIN2 degradation requires proteasome activity (22). Notably, pharmacological inhibition of the proteasome function interfered with internalization and vacuolar translocation of PIN2 (ref. 22 and data not shown), indicating an involvement of the proteasome in the mechanism of PIN2 vacuolar targeting. Thus, the previously reported effect of proteasome-targeting drugs, such as MG132 on PIN2 stability (22), could be explained by their effect on additional molecular determinants that regulate PIN2 turnover and are themselves under control of the proteasome. Thus, it needs to be seen what is the mechanism underlying the involvement of proteasome in PIN2 turnover.
Fig. 4.
Differential PIN targeting to the lytic vacuole during plant development. (A and B) Increased PIN2 targeting to the vacuole in epidermal cells at the upper root side in contrast to the symmetric vacuolar signal in vertically grown roots (A) after dark treatment of gravistimulated (for 3 h) PIN2-GFP roots (B). (C) Differential down-regulation of PIN2-GFP in upper epidermal cells after gravitropic stimulus for 3 h in snx1 mutant seedlings. (D) Enhanced gravity-induced degradation of PIN2 in snx1 mutants revealed by quantitative time course of total PIN2 protein abundance after gravity stimulation. Arrowheads mark differential PIN degradation in lytic vacuoles.
Our findings substantiate previous reports on asymmetric PIN2 stability (22) and indicate involvement of differential vesicle trafficking of PIN2 to the lytic vacuoles during the gravitropic response. In this scenario, asymmetric vesicle transport for vacuolar degradation of PIN2 with an increased activity at the upper side of gravistimulated roots limits PIN2 abundance and, hence, auxin flux into the elongation zone, eventually inducing differential cell elongation that results in root bending toward the gravity vector.
SNX1 Is Required for the Temporal PIN2 Degradation During the Gravitropic Response.
Interestingly, PIN2 preferentially resides in SNX1-labeled PVC after gravity stimulations (14), and, moreover, snx1 mutants are defective in gravitropic response (14). Because differential degradation of PIN2 in lytic vacuoles regulates gravitropic responses (Fig. 4 A and B) (22), we analyzed the potential SNX1 involvement in this process. PIN2 showed gravity-induced differential down-regulation of upper epidermal cell files in the wild type and snx1 mutants (Fig. 4C). To address potential quantitative differences, we monitored PIN2 protein levels after gravity stimulation. Western analysis revealed that regulation of PIN2 degradation and subsequent replenishment were altered in snx1 mutants (Fig. 4D), suggesting that the SNX1 function is required for temporally defined vacuolar targeting of PIN2 after gravity stimulation and might account for the gravitropic defects observed in snx1 mutants (14). In this scenario, SNX1-dependent protein retrieval from the PVC prevents PIN2 degradation after prolonged gravitropic responses.
Our finding substantiates previous findings on gravity-induced targeting of PIN2 (14, 22), which depends on the SNX1 function. Also, it further illustrates that PIN2 is translocated to the vacuole for degradation via an SNX1-dependent pathway. Moreover, the SNX1-dependent feedback mechanism for PIN2 retrieval and subsequent recovery for recycling appears to be functionally important for the gravitropic response. In this scenario, the retromer complex prevents transition of PIN proteins through the PVC and shuffles the proteins to the recycling endosomes, thus revealing its interdependency but also enabling independent regulation of polar targeting to the plasma membrane and posting to the vacuole for degradation.
Conclusions
Our data provide insights into the turnover mechanism of plant plasma membrane proteins, including the PIN auxin efflux carriers. The PIN proteins regulate important decisions during plant development by limiting rate and direction of the polar auxin transport (2, 3). The PIN-dependent auxin transport can be controlled at the level of PIN transcription (18, 19), polar targeting (7, 8, 43, 44), endocytosis (17), or protein stability (22).
We show that degradation of PIN proteins is mediated by their targeting to the vacuole that depends on the actin cytoskeleton and most likely on PI3K activity. PIN2 vacuolar trafficking and its polar recycling to the plasma membrane are interdependent, yet molecularly distinct, thus enabling the independent modulation of PIN2 protein abundance and polar localization. The ARF GEF GNOM regulates the PIN recycling rate to the basal plasma membrane (11, 13), whereas (an) additional partially BFA-sensitive ARF GEF(s) appear(s) to regulate the vacuolar degradation of PIN2, most probably upon trafficking from endosomes to the PVC. On the other hand, activities of the putative retromer components SNX1 and VPS29 appear to regulate the defined rate of PIN2 translocation from the PVC to the vacuole. Notably, during Drosophila development a similar retromer-dependent mechanism regulates late endosome-to-Golgi retrieval of Wntless/Evi/Sprinter that is instrumental for Wingless (Wnt) transport to the plasma membrane. In the absence of the retromer, Wntless is degraded in lysosomes and Wnt secretion is impaired (45). In plants, the retromer complex similarly appears to promote PIN2 retrieval from the PVC most likely to the TGN but is not directly involved in polar PIN targeting to the plasma membrane (Fig. S8).
PIN vacuolar trafficking and, henceforth, PIN levels can be controlled by external signals, such as light and gravity, providing an additional mechanism, besides transcytosis-like PIN polarity changes (12, 13), for translating environmental signals into modulation of auxin fluxes and, consequently, auxin-dependent development.
Materials and Methods
Lines.
PIN1:PIN1-GFP(8); PIN2:PIN2-GFP(1) (28); PIN2:PIN2-GFP(2), and TA:PIN2-GFP (22); BRI1:BRI1-GFP(1) (29, 46); BRI1:BRI1-GFP(2) (30); 35S:PIP2-GFP (47); snx1-1, snx1-2, and SNX1-GFP, SNX1-RFP (14); vps29-3 (obtained from The Salk Institute) (15); and YFP-2xFYVE (48) have been described previously. Data presented in the figures were all obtained with PIN2:PIN2-GFP(1) and BRI1:BRI1-GFP(1). Control experiments with PIN2:PIN2-GFP(2) and BRI1:BRI1-GFP(2) led to similar results.
Growth Conditions.
Plants were grown on soil or Murashige and Shoog (MS) plates (with or without sucrose) as described (6) under a 16-h light/8-h dark photoperiod at 21/18 °C. Dark experiments were carried out in the light period. All experiments were performed as previously described (13, 22); for detailed information see SI Text.
Supplementary Material
Acknowledgments.
We gratefully acknowledge T. Gaude, T. Munnik, D. Robinson, E. Rojo, B. Scheres, C. R. Somerville, and S. C. de Vries (all from the Ecole Normale Superieure de Lyon, Lyon, France) for making available the materials used in this study; the Arabidopsis Stock Centre for providing seed stocks; S. Vanneste and J. Ding for technical help with the quantitative transcription analysis; S. Robert for critical reading of the manuscript; and M. De Cock for help in preparing it. This work was supported by grants to J.F. from the Research Foundation Flanders (Odysseus) and the European Molecular Biology Organization Young Investigator Program, Fonds zur Förderung der Wissenschaftlichen Forschung Grant P19585 (to C.L.), and the Vienna Science and Technology Fund Grant LS0535 (to C.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808073105/DCSupplemental.
References
- 1.Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell Mol Life Sci. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrášek J, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 3.Wišniewska J, et al. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 4.Leyser O. Dynamic integration of auxin transport and signalling. Curr Biol. 2006;16:R424–R433. doi: 10.1016/j.cub.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- 6.Friml J, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 7.Friml J, et al. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 8.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 9.Sauer M, et al. Canalization of auxin flow by Aux/IAA-ARF-dependent feed-back regulation of PIN polarity. Genes Dev. 2006;20:2902–2911. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 11.Geldner N, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 12.Friml J, Wišniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 13.Kleine-Vehn J, et al. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol. 2008;18:526–531. doi: 10.1016/j.cub.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Jaillais Y, Fobis-Loisy I, Miège C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–109. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- 15.Jaillais Y, et al. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Jürgens G, Geldner N. The high road and the low road: Trafficking choices in plants. Cell. 2007;130:977–979. doi: 10.1016/j.cell.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Paciorek T, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 18.Peer WA, et al. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell. 2004;16:1898–1911. doi: 10.1105/tpc.021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieten A, et al. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;132:4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- 20.Izhaki A, Bowman JL. KANADI and class III HD-Zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis. Plant Cell. 2007;19:495–508. doi: 10.1105/tpc.106.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieberer T, et al. Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Curr Biol. 2000;10:1595–1598. doi: 10.1016/s0960-9822(00)00861-7. [DOI] [PubMed] [Google Scholar]
- 22.Abas L, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 23.Malenica N, et al. MODULATOR OF PIN genes control steady-state levels of Arabidopsis PIN proteins. Plant J. 2007;51:537–550. doi: 10.1111/j.1365-313X.2007.03158.x. [DOI] [PubMed] [Google Scholar]
- 24.Laxmi A, Pan J, Morsy M, Chen R. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS ONE. 2008;3:e1510. doi: 10.1371/journal.pone.0001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Páli T, Dixon N, Kee TP, Marsh D. Incorporation of the V-ATPase inhibitors concanamycin and indole pentadiene in lipid membranes. Spin-label EPR studies. Biochim Biophys Acta. 2004;1663:14–18. doi: 10.1016/j.bbamem.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura K, et al. Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J. 2003;35:545–555. doi: 10.1046/j.1365-313x.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Scheres B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell. 2005;17:525–536. doi: 10.1105/tpc.104.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedrichsen DM, Joazeiro CAP, Li J, Hunter T, Chory J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 2000;123:1247–1255. doi: 10.1104/pp.123.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleine-Vehn J, et al. Cellular and Molecular Requirements for Polar PIN Targeting and Transcytosis in Plants. Mol Plant. 2008 doi: 10.1093/mp/ssn062. [DOI] [PubMed] [Google Scholar]
- 32.Bassham DC, Raikhel NV. Unique features of the plant vacuolar sorting machinery. Curr Opin Cell Biol. 2000;12:491–495. doi: 10.1016/s0955-0674(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka K, Bassham DC, Raikhel NV, Nakamura K. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol. 1995;130:1307–1318. doi: 10.1083/jcb.130.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.daSilva LLP, et al. Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell. 2005;17:132–148. doi: 10.1105/tpc.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tse YC, et al. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16:672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emans N, Zimmermann S, Fischer R. Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell. 2002;14:71–86. doi: 10.1105/tpc.010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanoosthuyse V, Tichtinsky G, Dumas C, Gaude T, Cock JM. Interaction of calmodulin, a sorting nexin and kinase-associated protein phosphatase with the Brassica oleracea S locus receptor kinase. Plant Physiol. 2003;133:919–929. doi: 10.1104/pp.103.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seaman MNJ. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Oliviusson P, et al. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell. 2006;18:1239–1252. doi: 10.1105/tpc.105.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada T, et al. AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 2006;47:1187–1194. doi: 10.1093/pcp/pcj103. [DOI] [PubMed] [Google Scholar]
- 41.Silady RA, et al. The GRV2/RME-8 protein of Arabidopsis functions in the late endocytic pathway and is required for vacuolar membrane flow. Plant J. 2008;53:29–41. doi: 10.1111/j.1365-313X.2007.03314.x. [DOI] [PubMed] [Google Scholar]
- 42.Tamura K, et al. Arabidopsis KAM2/GRV2 is required for proper endosome formation and functions in vacuolar sorting and determination of the embryo growth axis. Plant Cell. 2007;19:320–332. doi: 10.1105/tpc.106.046631. and erratum (2007) 19:3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhardt D, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 44.Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franch-Marro X, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russinova E, et al. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR. Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA. 2000;97:3718–3723. doi: 10.1073/pnas.97.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vermeer JEM, et al. Visualization of Ptdlns3P dynamics in living plant cells. Plant J. 2006;47:687–700. doi: 10.1111/j.1365-313X.2006.02830.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.