Abstract
ATP generation by both glycolysis and glycerol catabolism is autocatalytic, because the first kinases of these pathways are fuelled by ATP produced downstream. Previous modeling studies predicted that either feedback inhibition or compartmentation of glycolysis can protect cells from accumulation of intermediates. The deadly parasite Trypanosoma brucei lacks feedback regulation of early steps in glycolysis yet sequesters the relevant enzymes within organelles called glycosomes, leading to the proposal that compartmentation prevents toxic accumulation of intermediates. Here, we show that glucose 6-phosphate indeed accumulates upon glucose addition to PEX14 deficient trypanosomes, which are impaired in glycosomal protein import. With glycerol catabolism, both in silico and in vivo, loss of glycosomal compartmentation led to dramatic increases of glycerol 3-phosphate upon addition of glycerol. As predicted by the model, depletion of glycerol kinase rescued PEX14-deficient cells of glycerol toxicity. This provides the first experimental support for our hypothesis that pathway compartmentation is an alternative to allosteric regulation.
Keywords: glycosome, PEX14, regulation of glycolysis, systems biology, Trypanosoma brucei
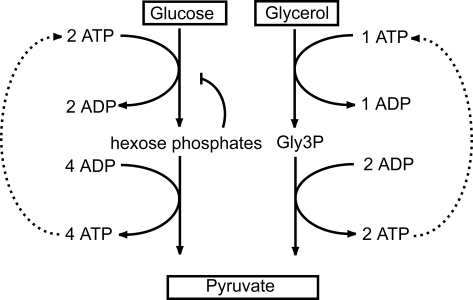
Cellular pathways that require investment of an end product before net production takes place necessitate tight regulation to prevent the accumulation of intermediate metabolites. Both glucose and glycerol catabolism are examples of such autocatalytic pathways (1) [Fig. 1 and supporting information (SI)Fig. S1]. In glycolysis, ATP is first invested in 2 phosphorylation reactions, catalyzed by hexokinase (HXK) and phosphofructokinase (PFK). Only further downstream in the pathway the ATP is regained and then a surplus of ATP is generated. In glycerol metabolism, ATP is invested in the reaction catalized by glycerol kinase (GK) (Fig. 1).
Fig. 1.
The autocatalytic reaction scheme of glucose and glycerol breakdown. In both glucose and glycerol breakdown, initially ATP is invested to phosphorylate the substrates, while further downstream ATP is produced. The ATP produced downstream can fuel the upstream reactions (dashed lines), which may result in accumulation of phosphorylated intermediates. Most organisms prevent this accumulation by negative feedback of the hexose phosphates on HXK. This regulation is absent from trypanosomes (see Introduction). For a more detailed scheme, see SI Text.
In the absence of specific regulation of HXK and PFK, the ATP produced by glycolysis could boost the flux through these enzymes above the capacity of the enzymes downstream, and hexose phosphates [glucose 6-phosphate (Glc6P), fructose 6-phosphate (Fru6P) and fructose 1,6-phosphate (Fru1,6BP)] would accumulate to extreme levels. By analogy to the turbo engine (which uses engine exhaust to boost performance), this property was called the “turbo-design”of glycolysis (2). Many organisms avoid the negative side effects of the autocatalytic design of glycolysis by a tight feedback regulation of HXK and PFK, e.g., inhibition of HXK by Glc6P (3) or trehalose 6-phosphate (5). In yeast, deletion of trehalose-6-phosphate synthase leads to glucose toxicity, accumulation of hexose phosphates and rapid consumption of ATP (4). This “turbo-explosion” phenotype is rescued by reducing the expression of HXK (5) or glucose transporters (6).
Trypanosoma brucei, the tropical parasite that causes the deadly African sleeping sickness, lacks feedback regulation of HXK and PFK (7–9). The parasite has a complex life cycle that alternates between insect and mammalian hosts; in the latter it lives in the bloodstream, supplied with a ready source of glucose. How then are trypanosomes protected against a possible turbo-explosion of glycolysis?
A key feature of trypanosome glycolysis is the compartmentation of the first 7 enzymes of glycolysis and 2 involved in glycerol metabolism inside peroxisome-like organelles called glycosomes (10). In the glycosome, ATP and redox levels are balanced (11–13). Net ATP synthesis coupled to glycolysis is deferred to the final cytosolic portion of the pathway. This ATP would not be available to HXK or PFK, because the glycosomal membrane is not permeable to small molecules. Based on a detailed kinetic model of glycolysis of bloodstream-form trypanosomes (14), we predicted that the compartmentation of glycolysis provides an alternative mechanism to protect trypanosomes against the turbo-explosion (15). We showed, in silico, that the glycosomal compartmentation prevents the accumulation of presumably toxic levels of Glc6P, Fru6P, and Fru1,6BP. We further postulated that protection against turbo-explosion is a present-day biological function of the compartmentation of much of trypanosome glycolysis. Although we have presented circumstantial experimental evidence (16) to support this hypothesis, the model predictions have not been tested experimentally in a direct and quantitative way in vivo. Hence, the postulated role of the glycosomal compartmentation has remained a hypothesis.
In this study, we tested the hypothesis that compartmentation is an alternative mechanism to avoid toxic accumulation of intermediates in autocatalytic pathways. We used procyclic (insect-stage) PEX14-RNAi mutants in which protein import into the glycosomes is impaired. We measured enzyme localization, growth, and metabolite concentrations upon addition of glucose or glycerol. We find that indeed glycosomal compartmentation can serve the function of protecting against the metabolic explosion associated with the autocatalytic nature of catabolism.
Results
Addition of Glucose Leads to Accumulation of Glc6P if Protein Import into Glycosomes Is Impaired.
We first tested how an updated version of the computer model of trypanosome glycolysis (17) responded to removal of the glycosomal membrane, dispersing all metabolites and enzymes throughout the cytosol. With a glycosome present, the concentrations of phosphorylated intermediates of glycolysis remained low. When the glycosomal membrane was removed, the model predicted accumulation of Glc6P,Fru6P and Fru1,6BP to very high levels (Fig. 2A and Fig. S2). These results were in qualitative agreement with the earlier study (15).
Fig. 2.
Glc6P profiles upon addition of glucose. Model versus experiment. Glucose (25 mM) was added at t = 0 in the model and in experiments. (A) Calculated concentrations of Glc6P in the model with (solid line) or without (dashed line) the glycosome. (B) Intracellular concentrations of Glc6P were measured in time in PEX14-RNAi cultures. Filled symbols, uninduced cultures; open symbols, Tet-induced cultures. Squares and circles show biological replicates. Error bars represent standard deviations of 2 measurements in the same sample.
The PEX14 protein is required for protein import into glycosomes. To test the model predictions, we measured the concentrations of hexose phosphates in a tetracycline (Tet)-inducible PEX14-RNAi mutant (18). All of the mutants used in this study were generated in the procyclic form of the parasite, because, unlike the bloodstream forms, the procyclic stage does not require glucose for survival and can grow on proline (Fig. S1). In essence, glucose and glycerol catabolism are similar in bloodstream-form and procyclic trypanosomes. Procyclic cells (unlike bloodstream-form cells) generate extra ATP in the respiratory pathway, which should only aggravate the turbo problem in procyclic cells, because it strengthens the dangerous feedback loop. Other key differences between the procyclic form and the bloodstream form are (i) the abundance of the glycolytic enzymes (lower in procyclic forms) and (ii) the localization of phosphoglycerate kinase (PGK), which is cytosolic in procyclic forms and glycosomal in bloodstream forms (19). We anticipate that the differences between the 2 differentiation stages yield quantitative differences, but do not effect the dangerous design qualitatively.
The procyclic PEX14-RNAi cell line was pregrown in the absence of glucose on 10 mM proline as the main source of free energy. Tet was added 5–7 generations before the addition of 25 mM glucose to ensure depletion of PEX14 protein (18). In line with the computer model, within 1 h after addition of glucose Glc6P accumulated to 50–60 mM in the PEX14-depleted cells and cells started dying, whereas uninduced cells maintained a Glc6P concentration of ≈10 mM (Fig. 2B) and remained viable. However, unlike in the glycosome-deficient model, the concentrations of Fru6P (Fig. S2B) and Fru1,6BP (Fig. S2D) did not rise after the glucose pulse in the PEX14 knockdown cells.
Subsequently, we measured the localization of the glycolytic enzymes. Previous studies on the role of PEX14 in protein import reported on the localization of only a few representative glycosomal enzymes (18, 20). They showed a shift toward cytosolic localization in the PEX14 mutant, but also suggested that a fraction of some enzymes may still be glycosomal. For the present study, however, the localization of all glycolytic enzymes is relevant. The accumulation of hexose phosphates should occur when the activities of all glycolytic enzymes reside in the cytosol. It should also occur when a fraction of every glycolytic enzyme would be relocalized to the cytosol. The persistence of a parallel glycosomal route that functions properly should not disturb this. Enzyme-activity and Western blot analyses of digitonin-treated Western blot (SI Text and Fig. S3) showed that of the glycolytic proteins normally present in the glycosome only glucose-6-phosphate isomerase (PGI) was severely mislocalized to the cytosol. HXK, PFK, and aldolase (ALD) were only slightly shifted to the cytosol (<20% released at a digitonin concentration of 0.1 mg·mL−1).
We concluded that the PEX14 mutant on glucose does not provide a stringent test of the model, because we could not prove that there is a complete parallel glycolytic pathway in the cytosol. An alternative explanation for the accumulation of Glc6P would be the truncation of the glycosomal pathway at PGI.
Addition of Glycerol Leads to Accumulation of Gly3P if Protein Import into Glycosomes Is Impaired.
Because the turbo design and its implications are based on the autocatalytic nature of the pathway, glycerol catabolism, which requires ATP investment via GK, also provides an opportunity to test our hypothesis (Fig. 1). Production of ATP results from the reactions catalized by PGK and pyruvate kinase (PYK) (Fig. S1). Like HXK, the GK-catalyzed reaction has a highly negative Gibbs-free energy, which may lead to product accumulation if the activity is not tightly regulated. We wondered whether the glycosomal localization of GK might prevent such product accumulation by shielding the enzyme from the ATP produced by cytosolic PGK and PYK. In agreement with this hypothesis, we showed in ref. 16 that even low concentrations of glycerol were toxic to the PEX14-RNAi mutant but not to wild-type trypanosomes.
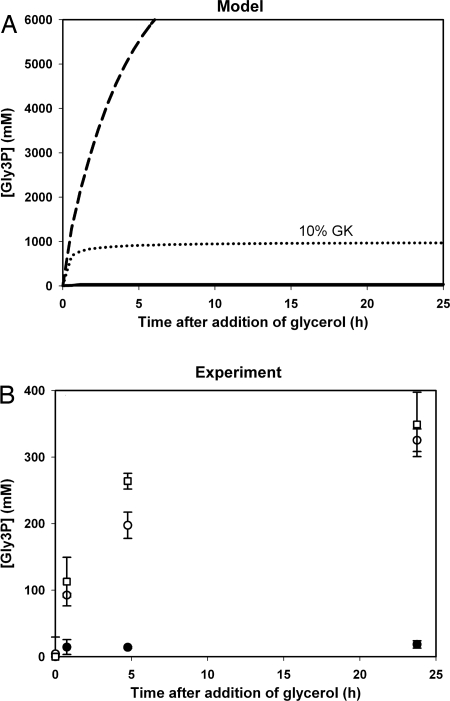
Indeed, in the model with a glycosome, glycerol 3-phosphate (Gly3P) concentrations remained low after administration of 25 mM glycerol. In contrast, in the model without the glycosome, Gly3P accumulated rapidly to several-hundred-millimolar concentrations (Fig. 3A). Modeling a decreased GK activity (10% remaining) in the absence of a glycosome showed significantly less accumulation of Gly3P after addition of glycerol (Fig. 3A).
Fig. 3.
Gly3P profiles upon addition of glycerol. Model versus experiment. Glycerol (25 mM) was added at t = 0 in the model and in experiments. (A) The concentration of Gly3P was calculated in time in a model with (solid line) and without (dashed line) a glycosome. The dotted lines show results from a time simulation in the model without the glycosome and with only 10% of the usual cellular GK activity. (B) The measured intracellular Gly3P concentration in cells induced or not induced for PEX14 RNAi. Open symbols, Tet-induced cultures; filled symbols, uninduced cultures. Squares and circles show independent biological replicates. Error bars represent standard deviations of 2 measurements of the same sample.
To test the effect of glycerol on the concentration of Gly3P experimentally, PEX14-RNAi cultures were again pregrown on proline and induced with Tet. After induction for 5–7 generations with Tet in proline-containing medium without glucose or glycerol, the cultures were incubated in medium with 25 mM glycerol (time 0, Fig. 3B). The intracellular concentration of Gly3P rose in 1 h to 100 mM and increased even further in the next 24 h. Gly3P levels in uninduced cultures remained at 15 mM. After 24 h, 75% of the induced cells became immotile but did not lyse. We used total cell counts to calculate the internal Gly3P concentration. Clearly, the estimated intracellular concentration would have been even higher had we based our calculation on motile cells.
As a control, we checked that at least a fraction of all the glycosomal enzymes of glycerol metabolism were relocalized to the cytosol. The glycosomal part of the pathway consists of GK, glycerol-3-phosphate dehydrogenase (G3PDH), triosephosphate isomerase (TIM) and the glycosomal isoenzyme of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In uninduced cells, the former 3 enzymes behaved as glycosomal proteins in a digitonin titration (Fig. 4), being released at 0.5 mg·mL−1 digitonin. In contrast, in cells induced for PEX14 RNAi, 20–40% of all 3 activities was already released at 0.1 mg·mL−1 digitonin, consistent with partial mislocalization (Fig. 4). This proves that the PEX14-RNAi mutant has a complete parallel glycerol pathway in the cytosol rather than a truncation in the glycosome, which makes PEX14 RNAi cells a valid experimental tool to test the model predictions for glycerol catabolism.
Fig. 4.
Subcellular localization of the enzymes in the pathway from glycerol to pyruvate. PEX14-RNAi cells were permeabilized by increasing concentrations of digitonin and the enzyme activities were measured in the supernatant. (Left) Results for uninduced cells, the Right for Tet-induced cells. PGK was used as a cytosolic control. In each culture, the enzyme activities at 1.5 milligrams of digitonin per milliliter were taken as 100%. The error bars represent standard deviations of 2 independent biological replicates. open squares, GK; open circles, G3PDH; inverted filled triangles, TIM; filled circles, PGK.
Depleting GK Rescues the PEX14-RNAi Mutant from Glycerol Toxicity.
Because the model predicted that the accumulation of Gly3P would be attenuated by down-regulation of GK, we isolated stable transfectants bearing inducible single and double RNAi constructs of GK and PEX14. Northern blot analysis confirmed the down-regulation of the corresponding mRNAs (Fig. S4). Western blot analyses showed that after 4 days induction with Tet (in medium lacking glycerol), the abundance of the corresponding proteins was strongly reduced (Fig. 5A). In medium lacking glucose but containing glycerol, cells induced for GK RNAi alone showed no morphological or growth phenotype, whereas the growth defect of the PEX14 RNAi mutant appeared after 3 days. Concomitant reduction of GK rescued the PEX14-RNAi cells from the lethal effects of glycerol, as measured by population growth (Fig. 5B). Images of cells from day 4 revealed the stark difference between the induced and uninduced cultures of the PEX14 mutant—only 12% of the cells from the induced culture appeared normal (Fig. 5C). Most cells were rounded and dying. In contrast, the PEX14-GK double knockdown cells showed normal morphology.
Fig. 5.
Knockdown of GK rescues the PEX14-depletion phenotype on glycerol. (A) A Western blot of samples of PEX14-RNAi, GK-RNAi, and PEX14-GK RNAi cells taken just before (day 0) and 4 days after Tet-induction in standard medium. NOG1, a protein involved in ribosome biogenesis, was used as the loading control. (B) Cumulative cell densities of uninduced (solid line) and Tet-induced (dashed line) cultures of PEX14-RNAi, GK-RNAi, and PEX14-GK RNAi strains in glucose-free medium supplemented with 10 mM glycerol. (C) Light microscopy images of uninduced and Tet-induced PEX14-RNAi and PEX14-GK RNAi mutants grown in glucose free-medium with 10 mM glycerol.
We attempted to investigate whether mislocalization of GK alone was toxic, by expressing cytosolically localized GFP- or myc-fused GK. Unfortunately, we were not able to detect enzymatic activity of these fusion proteins (data not shown). Remarkably, we observed that depletion of GK also partly rescued the PEX14-RNAi mutant from glucose toxicity (Fig. S5).
Discussion
In this article, we give experimental support for our hypothesis that compartmentation of an autocatalytic (“turbo”) pathway is an alternative to allosteric enzyme regulation in preventing the metabolic explosion otherwise predicted from the turbo design. This conclusion was primarily reached by analysis of glycerol catabolism, which is fuelled by ATP (via the action of GK) before net ATP production takes place. Because GK is highly active in trypanosomes and its reaction has a highly negative free-energy difference, the product Gly3P tends to accumulate. Our computer modeling predicted that this was prevented by the localization of the first enzymes of the pathway in glycosomes such that GK could not access the ATP produced downstream by PYK. In a PEX14 knockdown mutant, the entire glycerol pathway was localized in the cytosol and in agreement with the computer model this led to extreme accumulation of intracellular Gly3P. This phenotype was rescued in the model and experimentally by decreasing the GK activity.
No regulation of activity by any metabolites has been found for T. brucei GK (21). Organisms with cytosolic glycerol metabolism regulate GK differently: In Escherichia coli and Haemophilus influenzae, GK is inhibited by Fru1,6BP (22, 23), and, in E. coli and in Salmonella typhimurium, the enzyme is regulated by glucose-dependent binding to protein IIAGlc of the phosphotransferase system (24, 25).
Autocatalytic metabolic pathways are widespread in living cells. Examples are the activation of sugars, fatty acids, ketone bodies, and some amino acids in catabolism by high-energy compounds, such as ATP and/or acetylCoA. This may lead to special properties, including bistability and oscillations, and requires a tight regulation of the so-called “sparking reactions” in the beginning of the pathways (1, 2). That this tight regulation may not only be achieved by (allosteric) effectors or products of the reaction but also by compartmentation, is a relatively new concept (15) that had not yet been explicitly tested experimentally. This study illuminates another critical function of organelles, in addition to the established functions of partitioning reactions likely to generate toxic compounds, preventing interference with other pathways, and exploiting transmembrane electrochemical gradients for energy transduction.
After the discovery of glycosomes in T. brucei (10), the compartmentation of a large part of glycolysis in these organelles was perceived to facilitate a high glycolytic flux (11). However, quantitative analysis suggested that trypanosome glycolysis would not be diffusion-limited, even if the enzymes were dispersed in the cytosol (15), which eliminated the underlying argument for the high-flux hypothesis. In this study, the experiments on glucose toxicity with the PEX14 knockdown mutant were consistent with our hypothesis: Strong accumulation of Glc6P accompanied by growth inhibition (growth data not shown, see also ref. 18) in the mutant, but not in the wild-type, when cells were confronted with extracellular glucose. However, the results presented here could also support a competing explanation of the same results, namely truncation of the glycosomal pathway at the level of PGl, which would also lead to accumulation of intraglycosomal Glc6P. A more stringent test of the model predictions for glucose requires a viable mutant in which there is either no glycosomal glycolysis left or which has complete pathways in the glycosome and in the cytosol. We were able to validate our hypothesis for glycerol metabolism, indirectly supporting the possibility that compartmentation fulfills a similar regulatory function for glucose metabolism in trypanosomes.
We repeated the above experiments with another cell line (PEX14-RNAi cell line PF449), which is also derived from strain 427 (20, 26) (Fig. S6–S8). The key results corresponded between the 2 cell lines, including different degrees of mislocalization of the enzymes with PGI being most severely mislocalized and the accumulation of Gly3P after a glycerol pulse. There were quantitative differences although. The PEX14 RNAi mutant made from cell line 449 was more sensitive to glycerol and less sensitive to glucose. In the 449-derived mutant, we could not detect any significant accumulation of Glc6P after addition of glucose (Fig. S7A). This correlated with a 60-fold lower activity of HXK in the latter cell line (PGI and PFK activities were more similar) (see Table S1). Although these data do not bear on the central hypothesis being tested in this work, they point out the need for analysis of multiple strains when evaluating metabolic activities of the parasites.
Although there were quantitative differences between the model and experiments, the qualitative agreement was sufficient to provide proof of principle in the case of glycerol catabolism. In the model, the accumulation of Gly3P was more severe than in the experiment. This may be explained in part by the fact that the model was generated for bloodstream forms while the experiments were done on procyclic cultures, which have different enzyme activities (Table S1), different connecting pathways (solid versus dashed lines in Fig. S1) and different kinetic parameters for glycerol uptake. Incorporating the glycerol uptake rate measured in procyclic forms (27) would considerably diminish the predicted Gly3P accumulation (data not shown).
The most enigmatic result was that GK knockdown both rescued the PEX14 phenotype on glycerol and partially rescued growth on glucose. Because we verified that no trace of glycerol was present in the starting medium, our most plausible explanation for this observation is that glycerol is produced from glucose through the glycosomal route, as far as it is functional in the mutant, and consumed via the pathological cytosolic route. Knockdown of GK would reduce the consumption of this glycerol and partially rescue the cells. It is known that even under aerobic conditions some glycerol is produced in the wild type trypanosomes (28, 29), and, if our cells happened to be oxygen-limited, more glycerol may have been made.
The study presented in this article is a convincing example of the value of quantitative models of metabolic pathways. The model produced a testable hypothesis that was subsequently verified experimentally. This shows that the systems biology approach is successful in predicting a function of a unique cellular organization. The present study is, to our knowledge, one of the rare cases in biology in which a regulatory mechanism found by kinetic computer modeling was published before experimental verification was possible. We consider this as an important proof-of-principle of the bottom-up systems-biology approach (30) that deserves to be followed by many more examples as the field matures.
Finally, the results further strengthen the status of the glycosomal protein import as a target for the design of drugs (31, 32). Even an incomplete knockdown of PEX14 expression, leading to a partial mislocalization of glycosomal enzymes, caused metabolic defects that killed the parasites. In this respect, the results with glucose are as relevant as those with glycerol, because both are present in the blood.
Experimental Procedures
Mathematical Modeling.
Details on the modeling are described in SI Appendix.
Parasite Cultivation and Induction of RNAi.
Procyclic T. brucei cell line 29–13, stably transfected with a PEX14-RNAi construct (18) and/or a GK-RNAi construct (see below) was cultivated in SDM79 medium (JRH Biosciences) or in the same medium lacking glucose and glucosamine as described in ref. 18. To adapt cells to growth on proline, cells were pregrown for at least 20 generations on medium lacking glucose. RNAi was induced with 1–4 μg·mL−1 Tet dissolved in DMSO. Control cultures were given the same amount of solvent alone.
Construction of RNAi Knockdown Plasmids.
RNAi constructs were based on the plasmid pZJM (33), which allows for Tet-regulated expression of the introduced fragments from opposing T7 promoters. HindIII restriction sites were added to the 5′ (5′-AAGCTTCGTCGGATCCATTGACCAGG-3′) and 3′ (5′-AAGCTTATGCGTCAGCAACAGCTGG-3′) primers, which were designed to amplify nucleotides 9–484 of GK. Cloned fragments were ligated into the HindIII site of the vector pZJM-PEX14 (18) with GK in either orientation relative to PEX14. From the tail to tail orientation, XhoI restriction dropped out PEX14 and 25 bp of the GK sequence, leaving a 442-bp fragment of GK in pZJM-GK. The tail to head orientation of PEX14 and GK (pZJM-PEX14/GK) was used for RNAi studies and used to transfect procyclic 29–13 cells (34).
Metabolite Measurements.
For metabolite measurements, cultures were grown for 4–5 generations in the presence or absence of Tet. At time point zero, 25 mM glucose or 25 mM glycerol was added to the cultures. Samples of 1 mL were taken just before addition of these substrates and at several time points after the addition. Cells were pelleted by centrifugation (7.5 min at 6,000 × g) and pellets were resuspended in 0.1 mL of the medium and a sample was reserved for determining cell number. Metabolism was quenched immediately by the addition of 10 μL of ice-cold 35% (vol/vol) perchloric acid, and, after 10 min on ice, the sample was neutralized with 11 μL of 5 M KOH/0.2 M Mops (35). After a further incubation on ice for 10 min, the precipitated protein was removed by centrifugation for 10 min at 11,000 × g.
Glc6P, Fru6P, Fru16P, and Gly3P were measured via NAD(P)-linked reactions in 96-wells plates in a Novostar spectrophotometer (BMG Labtech) in fluorescence mode (excitation wavelength 340 nm; emission wavelength 460 nm). Reaction mixtures for Glc6P,Fru6P and Fru1,6BP were essentially as described in ref. 36. Minor modifications are described in SI Text. Gly3P was measured according to ref. 37.
Intracellular concentrations were calculated based on 175 mg of total cell protein corresponding to 1 mL of total cellular volume (38) and 1.94 × 108 cells contain 1 mg of protein (39). Cell counts included both motile and nonmotile intact cells. Unless mentioned, >90% of the cells were motile.
Fractionation by Digitonin Titration for Enzyme Activity Assays.
Cells were washed in STE-buffer [25 mM Tris·HCl, 250 mM sucrose, 1 mM EDTA (pH 7.8)] and resuspended in STE supplemented with 150 mM NaCl. After addition of digitonin (Sigma), cells were incubated for 4 min at 25 °C and immediately centrifuged for 2 min at 12,000 × g (40). Supernatants were collected rapidly for enzyme-activity assays and kept on ice before and in between measurements. Enzyme activities in samples were measured with a Cobas FARA or Cobas BIO analyzer (Roche Diagnostics), using assays as described in ref. 41, except for ALD, which was carried out as described in ref. 42.
Western Blot Analysis on PEX14-GK Double RNAi Mutant.
Approximately 5 × 106 cell equivalents were loaded per lane onto 10% polyacrylamide gels (Cambrex; PAGEr). After electrophoresis, proteins were transferred to nitrocelluose membranes (Protran). For immunoblots the following conditions were implemented. Anti-TbGK (1:1,000), anti-TbPEX14 (1:100), and anti-TbNOG1 (1:5,000) were used. Bound antibodies were detected with horseradish peroxidase-coupled protein A (1:3,000) (Bio-Rad) and chemiluminescent substrates (Perkin–Elmer; Western Lightening). These were imaged on Kodak BioMax MS film.
Immunofluorescence Analysis.
Parasites (2 × 106) were washed in PBS, and ≈2 × 104 parasites were placed on glass slides. These were fixed with 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 8% nonfat milk in PBS. Samples were incubated for 1 h with rabbit anti-glycosome antiserum (1:100) in the blocking solution. For detection, FITC-conjugated goat anti-rabbit IgG (H+L) (1:50) was used. Slides were mounted in Antifade (Molecular Probes) for fluorescence microscopy on a Nikon Eclipse E300 equipped with Metamorph software.
Supplementary Material
Acknowledgments.
We thank Alexander Lindenbergh and Jildau Bouwman for technical assistance and Jaap van Hellemond and Lodewijk Tielens for insightful discussions. This work was supported by a Nederlandse Organisatie voor Wetenschappelijk Onderzoek-vernieuwingsimpuls grant (to B.M.B.); National Institutes of Health Grant R01 AI22635 (M.P.); Fonds de la Recherche Scientifique médicale 2.4652.06 (to P.A.M.M.); and grants from the Nederlandse Organisatie voor Wetenschappelijk Onderzoek, Biotechnology and Biological Sciences Research Council, and European Union-Framework Programme 7-Biosym, Nucsys (to H.V.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806664105/DCSupplemental.
References
- 1.Reich JG, Sel'kov EE. Energy Metabolism of the Cell: A Theoretical Treatise. London: Academic; 1981. [Google Scholar]
- 2.Teusink B, Walsh MC, van Dam K, Westerhoff HV. The danger of metabolic pathways with turbo design. Trends Biochem Sci. 1998;23:162–169. doi: 10.1016/s0968-0004(98)01205-5. [DOI] [PubMed] [Google Scholar]
- 3.Colowick SP. In: The Enzymes. Boyer PD, editor. New York: Academic; 1973. pp. 1–48. [Google Scholar]
- 4.Thevelein JM, Hohmann S. Trehalose synthase: Guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 5.Hohmann S, et al. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr Genet. 1993;23:281–289. doi: 10.1007/BF00310888. [DOI] [PubMed] [Google Scholar]
- 6.Luyten K. Disruption of the Kluyveromyces lactis GGS1 gene causes inability to grow on glucose and fructose and is suppressed by mutations that reduce sugar uptake. Eur J Biochem. 1993;217:701–713. doi: 10.1111/j.1432-1033.1993.tb18296.x. [DOI] [PubMed] [Google Scholar]
- 7.Nwagwu M, Opperdoes FR. Regulation of glycolysis in Trypanosoma brucei: Hexokinase and phosphofructokinase activity. Acta Trop. 1982;39:61–72. [PubMed] [Google Scholar]
- 8.Cronin CN, Tipton KF. Purification and regulatory properties of phosphofructokinase from Trypanosoma (Trypanozoon) brucei brucei. Biochem J. 1985;227:113–124. doi: 10.1042/bj2270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronin CN, Tipton KF. Kinetic studies on the reaction catalysed by phosphofructokinase from Trypanosoma brucei. Biochem J. 1987;245:13–18. doi: 10.1042/bj2450013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opperdoes FR, Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: The glycosome. FEBS Lett. 1977;80:360–364. doi: 10.1016/0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- 11.Opperdoes FR. Compartmentation of carbohydrate metabolism in trypanosomes. Annu Rev Microbiol. 1987;41:127–151. doi: 10.1146/annurev.mi.41.100187.001015. [DOI] [PubMed] [Google Scholar]
- 12.Hammond DJ, Aman RA, Wang CC. The role of compartmentation and glycerol kinase in the synthesis of ATP within the glycosome of Trypanosoma brucei. J Biol Chem. 1985;260:15646–15654. [PubMed] [Google Scholar]
- 13.Hammond DJ, Bowman IB. Studies on glycerol kinase and its role in ATP synthesis in Trypanosoma brucei. Mol Biochem Parasitol. 1980;2:77–91. doi: 10.1016/0166-6851(80)90033-x. [DOI] [PubMed] [Google Scholar]
- 14.Bakker BM, Michels PA, Opperdoes FR, Westerhoff HV. Glycolysis in bloodstream form Trypanosoma brucei can be understood in terms of the kinetics of the glycolytic enzymes. J Biol Chem. 1997;272:3207–3215. doi: 10.1074/jbc.272.6.3207. [DOI] [PubMed] [Google Scholar]
- 15.Bakker BM, et al. Compartmentation protects trypanosomes from the dangerous design of glycolysis. Proc Natl Acad Sci USA. 2000;97:2087–2092. doi: 10.1073/pnas.030539197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler PS, Parsons M. Probing the role of compartmentation of glycolysis in procyclic form Trypanosoma brucei: RNA interference studies of PEX14, hexokinase, and phosphofructokinase. J Biol Chem. 2005;280:9030–9036. doi: 10.1074/jbc.M412033200. [DOI] [PubMed] [Google Scholar]
- 17.Albert M-A, et al. Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei. J Biol Chem. 2005;280:28306–28315. doi: 10.1074/jbc.M502403200. [DOI] [PubMed] [Google Scholar]
- 18.Furuya T, et al. Glucose is toxic to glycosome-deficient trypanosomes. Proc Natl Acad Sci USA. 2002;99:14177–14182. doi: 10.1073/pnas.222454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blattner J, Clayton CE. The 3′-untranslated regions from the Trypanosoma brucei phosphoglycerate kinase genes mediate developmental regulation. Gene. 1995;162:153–156. doi: 10.1016/0378-1119(95)00366-e. [DOI] [PubMed] [Google Scholar]
- 20.Moyersoen J, et al. Characterization of Trypanosoma brucei PEX14 and its role in the import of glycosomal matrix proteins. Eur J Biochem. 2003;270:2059–2067. doi: 10.1046/j.1432-1033.2003.03582.x. [DOI] [PubMed] [Google Scholar]
- 21.Kralova I, Rigden DJ, Opperdoes FR, Michels PA. Glycerol kinase of Trypanosoma brucei. Cloning, molecular characterization and mutagenesis. Eur J Biochem. 2000;267:2323–2333. doi: 10.1046/j.1432-1327.2000.01238.x. [DOI] [PubMed] [Google Scholar]
- 22.Zwaig N, Lin EC. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1996;153:755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]
- 23.Pawlyk AC, Pettigrew DW. Subcloning, expression, purification, and characterization of Haemophilus influenzae glycerol kinase. Protein Expr Purif. 2001;22:52–59. doi: 10.1006/prep.2001.1408. [DOI] [PubMed] [Google Scholar]
- 24.Postma PW, Epstein W, Schuitema AR, Nelson SO. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J Bacteriol. 1984;158:351–353. doi: 10.1128/jb.158.1.351-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novotny MJ, Frederickson WL, Waygood EB, Saier MH., Jr Allosteric regulation of glycerol kinase by enzyme IIIglc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1985;162:810–816. doi: 10.1128/jb.162.2.810-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biebinger S, Wirtz LE, Clayton CE. Vectors for inducible over-expression of potentially toxic gene products in bloodstream and procyclic Trypanosoma brucei. Mol Biochem Parasitol. 1997;85:99–112. doi: 10.1016/s0166-6851(96)02815-0. [DOI] [PubMed] [Google Scholar]
- 27.Wille U, Schade B, Duszenko M. Characterization of glycerol uptake in bloodstream and procyclic forms of Trypanosoma brucei. Eur J Biochem. 1998;256:245–250. doi: 10.1046/j.1432-1327.1998.2560245.x. [DOI] [PubMed] [Google Scholar]
- 28.Eisenthal R, Panes A. The aerobic/anaerobic transition of glucose metabolism in Trypanosoma brucei. FEBS Lett. 1985;181:23–27. doi: 10.1016/0014-5793(85)81106-6. [DOI] [PubMed] [Google Scholar]
- 29.Darling TN, Balber AE, Blum JJ. A comparative study of D-lactate, L-lactate and glycerol formation by four species of Leishmania and by Trypanosoma lewisi and Trypanosoma brucei gambiense. Mol Biochem Parasitol. 1988;30:253–257. doi: 10.1016/0166-6851(88)90094-1. [DOI] [PubMed] [Google Scholar]
- 30.Bruggeman F, Westerhoff H. The nature of systems biology. Trends Microbiol. 2007;15:45–50. doi: 10.1016/j.tim.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Parsons M, Furuya T, Pal S, Kessler P. Biogenesis and function of peroxisomes and glycosomes. Mol Biochem Parasitol. 2001;115:19–28. doi: 10.1016/s0166-6851(01)00261-4. [DOI] [PubMed] [Google Scholar]
- 32.Moyersoen J, Choe J, Fan E, Hol WG, Michels PA. Biogenesis of peroxisomes and glycosomes: Trypanosomatid glycosome assembly is a promising new drug target. FEMS Microbiol Rev. 2004;28:603–643. doi: 10.1016/j.femsre.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Morris JC, Drew ME, Englund PT. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J Biol Chem. 2000;275:40174–40179. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- 34.Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 35.Rossell S, van der Weijden C, Kruckeberg A, Bakker B, Westerhoff H. Hierarchical and metabolic regulation of glucose influx in starved Saccharomyces cerevisiae. FEMS Yeast Res. 2005;5:611–619. doi: 10.1016/j.femsyr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 36.de Koning W, van Dam K. A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Anal Biochem. 1992;204:118–123. doi: 10.1016/0003-2697(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 37.Bergmeyer HU. Methods of enzymatic analysis. New York: Academic; 1974. [Google Scholar]
- 38.Opperdoes FR, et al. Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei. J Cell Biol. 1984;98:1178–1184. doi: 10.1083/jcb.98.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakker BM, Michels PA, Opperdoes FR, Westerhoff HV. What controls glycolysis in bloodstream form Trypanosoma brucei? J Biol Chem. 1999;274:14551–14559. doi: 10.1074/jbc.274.21.14551. [DOI] [PubMed] [Google Scholar]
- 40.Heise N, Opperdoes FR. Purification, localisation and characterisation of glucose-6-phosphate dehydrogenase of Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:21–32. doi: 10.1016/s0166-6851(98)00176-5. [DOI] [PubMed] [Google Scholar]
- 41.Misset O, Opperdoes FR. Simultaneous purification of hexokinase, class-I fructose-bisphosphate aldolase, triosephosphate isomerase and phosphoglycerate kinase from Trypanosoma brucei. Eur J Biochem. 1984;144:475–483. doi: 10.1111/j.1432-1033.1984.tb08490.x. [DOI] [PubMed] [Google Scholar]
- 42.Misset O, Bos OJM, Opperdoes FR. Glycolytic enzymes of Trypanosoma brucei: Simultaneous purification, intraglycosomal concentrations and physical properties. Eur J Biochem. 1986;157:441–453. doi: 10.1111/j.1432-1033.1986.tb09687.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.