Abstract
Skeletal muscle mitochondrial dysfunction occurs in many conditions including aging and insulin resistance, but the molecular pathways of the mitochondrial dysfunction remain unclear. Presently, no methodologies are available to measure synthesis rates of individual mitochondrial proteins, which limits our ability to fully understand the translational regulation of gene transcripts. Here, we report a methodology to measure synthesis rates of multiple muscle mitochondrial proteins, which, along with large-scale measurements of mitochondrial gene transcripts and protein concentrations, will enable us to determine whether mitochondrial alteration is due to transcriptional or translational changes. The methodology involves in vivo labeling of muscle proteins with l-[ring-13C6]phenylalanine, protein purification by two-dimensional gel electrophoresis of muscle mitochondrial fraction, and protein identification and stable isotope abundance measurements by tandem mass spectrometry. Synthesis rates of 68 mitochondrial and 23 nonmitochondrial proteins from skeletal muscle mitochondrial fraction showed a 10-fold range, with the lowest rate for a structural protein such as myosin heavy chain (0.16 ± 0.04%/h) and the highest for a mitochondrial protein such as dihydrolipoamide branched chain transacylase E2 (1.5 ± 0.42%/h). This method offers an opportunity to better define the translational regulation of proteins in skeletal muscle or other tissues.
Keywords: protein synthesis, two-dimensional gel electrophoresis
muscle mitochondrial dysfunction has been reported to occur in a variety of conditions, including type 2 diabetes (1, 26, 48, 51), insulin resistance (37), obesity (27, 39), aging (36, 46), and sepsis (7, 18). A reduction in muscle mitochondrial protein concentrations and mitochondrial DNA copy number has been reported to occur with aging, indicating that reduction in muscle ATP production capacity in aging is related to reduction in mitochondrial DNA and protein abundance (4, 46). A reduction in mitochondrial content has also been reported in severely insulin-resistant offspring of type 2 diabetic patients, in association with a reduction in muscle mitochondrial ATP production (32). In contrast, despite a failure to enhance insulin-stimulated muscle mitochondrial ATP production, insulin-resistant type 2 diabetic patients have mitochondrial DNA copy numbers similar to those of nondiabetics (1). To understand the cause of altered mitochondrial oxidative phosphorylation in these different conditions, it is important to determine the regulation of mitochondrial biogenesis at multiple molecular levels. Measurement of steady-state levels of protein abundance and transcript levels of nuclear regulatory factors and mitochondrial proteins is presently possible (1, 46, 50, 51), but these approaches are not sufficient to fully define the molecular pathways involved in the regulation of mitochondrial biogenesis.
Large-scale approaches for identification and relative quantification of proteins are a rapidly expanding field in biological research (11, 35), but the present quantitative proteomic approaches lack sufficient precision to measure changes in protein concentrations during a short experimental period, especially for proteins such as myosin heavy chain (MHC) with a very low fractional synthesis rate of <1.5% per day in human skeletal muscle. These approaches also have limitations in measuring the concentration changes in low-abundance proteins with a rapid turnover rate (34). Moreover, protein concentration is determined by the net effect of synthesis and degradation and does not offer any understanding on the regulatory pathways. To understand the regulation of translation of transcripts into proteins, it is important to measure the rate at which individual proteins are synthesized.
Proteomic strategies using in vivo isotope labeling have been developed for the relative measurement of protein turnover in cultures of single-cell organisms such as yeast (38) and bacteria (9) as well as in chickens (13). However, these approaches require a high isotopic enrichment of proteins that can only be achieved in single-cell organisms over a multiple number of generations or by feeding animals with highly enriched isotopes for a longer period. None of the above approaches is a direct measurement of the synthesis rate of proteins or practical in human studies. Most recently, a method has been reported to measure synthesis and degradation rate of β-amyloid in human cerebrospinal fluid by stable isotope-labeled amino acid incorporation and tandem mass spectrometry (MS) quantitation (SILT) (5). This method requires quantification of at least one specific peptide with an isotope-labeled amino acid. Although very promising, turnover measurement of multiple proteins by this method is yet to be reported.
Relative protein concentrations of several skeletal muscle mitochondrial proteins have been shown to decline with age (46). The reduction in the concentrations of these individual mitochondrial proteins, in turn, may occur because of a reduction in the synthesis rate of these muscle mitochondrial proteins as reported with aging (20, 40) or because of an increase in degradation of these proteins. The previously reported measurement of mitochondrial protein synthesis rate (13) is an estimation of average synthesis rate of all proteins in the muscle mitochondrial fraction, which may also contain proteins from myofibrillar and sarcoplasmic fractions. As it has been shown in type 2 diabetes, changes in gene transcripts in insulin-resistant type 2 diabetic patients do not involve all genes encoding mitochondrial proteins (1). It is also possible that the changes in translational rate are not global to all gene transcripts in specific physiological and pathological conditions. Measurement of synthesis rates of individual mitochondrial proteins is the only logical approach to assess translational rates of gene transcripts, but presently no methodology is available to measure the synthesis rates of individual mitochondrial proteins. We therefore sought to measure the synthesis rate of specific muscle mitochondrial proteins, and here we report a methodology to simultaneously measure the in vivo synthesis rates of multiple skeletal muscle mitochondrial proteins.
MATERIALS AND METHODS
Animals.
Six male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) ∼8 wk old and weighing 260–300 g were used for the study. Study protocol and procedures were approved by the Institutional Animal Care and Use Committee and followed the guidelines of the National Research Council's Guide for the Care and Use of Laboratory Animals. Rats were housed individually in plastic boxes under a 12:12-h light-dark cycle with water and standard commercial rat chow.
Study protocol.
On the day of the study, rats were transferred to a smaller holding cage with light restraint to facilitate access to the tail. Each rat received an intravenous injection of [ring-13C6]phenylalanine (Cambridge Isotope Laboratories, Cambridge, MA; 99 atom percent excess, 10 mg/ml solution) at a dose of 15 mg/kg body wt (90.9 μmol/kg). An overdose of pentobarbital sodium (Nembutal, 50 mg/kg, intraperitoneal injection) was given 15 min after the tracer injection. At 20 min, individual hindlimb quadriceps muscles were rapidly removed under anesthesia. Muscle samples were blotted of blood, and any visible fat or connective tissue was removed. Samples were kept on ice in saline-soaked gauze for mitochondrial isolation. A small portion of the muscle sample was quickly frozen in liquid nitrogen and stored at −80°C.
Purification of mitochondrial proteins.
Skeletal muscle mitochondria were initially separated using a differential centrifugation method (47), using ∼1 g of muscle tissue from each rat separately, and all individual samples were separately processed for protein purification. The mitochondrial pellets obtained were dissolved in 500 μl of lysis buffer (35 mM Tris, 9 M urea, 4% CHAPS, 65 mM DTT) and trace of bromophenol blue. The dissolved protein mixture was centrifuged at 14,000 rpm for 5 min to remove any undissolved material. Protein concentration was estimated by the Bradford method (Bio-Rad Laboratories, Hercules, CA). Individual proteins were isolated from the mixture by performing large, high-resolution, two-dimensional gel electrophoresis (2DGE). Approximately 400 μg of each protein sample were dissolved in lysis buffer to a final volume of 900 μl. These samples were used to rehydrate 24-cm, pH 4–7 and 6–9, immobilized pH gradient (IPG) strips (Bio-Rad Laboratories, Hercules, CA) in a rehydration tray overnight. The rehydrated IPG strips were subjected to isoelectric focusing in a Protean IEF Cell (Bio-Rad) using a three-step protocol: 1) the focusing was achieved with an initial step of 250 V for 15 min, 2) continued with a maximum of 10,000 V increased linearly from 250 V over 6 h, and 3) continued at 10,000 V for 60,000 volt hours. The cell temperature was kept at 20°C with a maximum current of 50 μA/strip. The IPG strips were then equilibrated for the SDS-PAGE in a two-step equilibration using 5 ml of equilibration buffer per strip (6 M urea, 2% SDS, 0.375 M Tris·HCl, pH 8.8, and 20% glycerol) with 130 mM DTT in the first step and 135 mM iodoacetamide in the second step. The equilibration steps were done in an equilibration tray for 10 min each on a rotary shaker at room temperature. The second-dimension separation by subunit molecular weight was performed by vertical 12%, 24 × 20-cm dimension SDS-PAGE (Ettan DALT system; GE Healthcare Bio-Sciences, Piscataway, NJ). The IPG strips were mounted into the IPG well with molten agarose and then run at 75 V for 24 h or until the dye front reached the bottom of the gel. The protein gel spots were visualized by staining with Coomassie blue (GelCode Blue Stain Reagent; Pierce, Rockford, IL). Duplicate 2DGE was performed for both pH ranges of each sample to get enough protein from gel spots for further analysis. Pairs of 190 gel spots that were the most resolved and intense from both gels were excised and analyzed for protein identification by liquid chromatography-tandem MS (LC/MS/MS) and for isotopic enrichment by gas chromatography-quadrupole MS (GC/MS/MS). Although we used 1 g of muscle sample for the methodology development, for analysis, only 200 μg of protein were required for each gel and were obtained from a 100-mg muscle sample.
Protein identification by tandem MS.
Gel spots were subjected to in-gel reduction and alkylation, followed by trypsin digestion, as previously described (24, 42). Peptides were extracted three times, first by adding 2% trifluoroacetyl acid (TFA) in water to the digest mixture and then by an equal volume of 60% acetonitrile-0.1% TFA-40% water followed by a final extraction with 100% acetonitrile. Extracts were dried in a SpeedVac to remove organic solvent. Samples were dissolved in 0.1% formic acid-2% acetonitrile solution just before injection into the mass spectrometer.
MS analyses were done on a quadrupole linear trap mass spectrometer (LTQ, Thermo Electron). The digested peptides were introduced into the LTQ through an automated nanoscale LC system (LC Packings, Dionex). The chromatographic separation was performed on a 100-μm inside diameter (ID) by 15-cm C18 column (Zorbax, Agilent Technologies), with a linear gradient elution from 100% buffer A (0.1% formic acid-acetonitrile, 98:2 vol/vol) to 60% buffer B (0.1% formic acid-acetonitrile, 2:98 vol/vol) in 60 min. The MS/MS raw data were converted to DTA files using ThermoElectron Bioworks 3.2 and correlated to theoretical fragmentation patterns of tryptic peptide sequences from a rat subset of the National Center for Biotechnology Information (NCBI) nonredundant (December 2003) database using TurboSEQUEST (14) (version 27, revision 12; ThermoElectron, San Jose, CA) in Bioworks 3.2. The rat database contained 27,365 entries. All searches were conducted with cysteine modifications of +57 for carboxamidomethyl-cysteines and modifications allowing +16 with methionines for methionine sulfoxide. The search was restricted to trypsin-generated peptides, allowing for two missed cleavages from rat protein entries. Peptide mass search tolerance was set to 1.5 Da, and fragment mass tolerance was set to ±1.0 Da. Protein identifications were considered when at least two unique consensus peptides with individual cross correlations (×Corr) exceeded a set threshold depending on the precursor charge state (+1 ×Corr >1.8, +2 ×Corr >2.3, +3 ×Corr >2.8), thus ranking as the hit for that MS/MS spectra. These thresholds correctly identified 50 fmol of the myoglobin standard (in similar matrix as the samples) as the only protein.
Isotopic enrichment of individual muscle proteins derived from gel spots.
For GC/MS/MS analysis, duplicate gels were run for each of six rat samples. Gel spots were washed several times with deionized water and then hydrolyzed overnight at 110°C in 6 M HCl. The amino acids from the hydrolyzed gel pieces were purified over an AG-50W cation exchange column. They were then eluted with 4 M NH4OH and dried down in the SpeedVac. The amino acid residues were derivatized to their N-heptafluorobutyryl methyl esters (15) and analyzed by tandem MS using a ThermoFinnigan TSQ 7000 GC/MS/MS under negative ion chemical ionization conditions, using isobutane as the reactant gas. The derivatives were dissolved in ethyl acetate and separated on a 30 m × 0.25 mm ID × 0.25 μm RTX-1701 fused silica column (Restek, Bellefonte, PA), using splitless injection (2 μl). The 13C enrichment of [ring-13C6]phenylalanine was measured by MS/MS by monitoring transition fragments of the [M-HF]− parent ions corresponding to the [m+2] and [m+6] species using transitions m/z [357 → 306] and m/z [361 → 290], respectively. The technique of measuring the ratio of [m+6] to [m+2] instead of [m+6] to [m+0] for determination of low levels of enrichment (<0.5%) on a conventional organic mass spectrometer has been described by Calder et al. (8) and Slater et al. (49).
Isotopic enrichment of free phenylalanine in muscle tissue fluid.
Tissue fluid amino acids were extracted from muscle tissue of each rat using perchloric acid as described (6) and analyzed as their t-butyldimethylsilyl ester derivative (45). The molar percent excess of [13C6]phenylalanine was calculated above background as previously described (22).
Fractional synthesis rate of skeletal muscle proteins.
The fractional synthesis rate (FSR) for individual proteins for all six rats was calculated from the isotopic enrichment of [13C6]phenylalanine (Ie) in the proteins and using the tissue fluid free phenylalanine enrichment (Pe) as the precursor pool. The equation used is as previously described (55): FSR (%/h) = [Ie/(Pe * t)] * 100, where t represents time (0.33 h).
Pathway analysis and grouping of proteins.
Eighty-two identified proteins were used as “focus proteins” in Ingenuity Pathway Analysis (IPA; Ingenuity Systems, Redwood City, CA). All proteins in the Ingenuity Protein Knowledge Base (PKB) database were used as reference. Eighty-two proteins were identified using GenBank Identification (GI) numbers. The corresponding gene symbols for each GI number were obtained using IPA's GI-gene symbol matching tool. The functional annotations for each gene symbol, “biological process” and “cellular component” of Gene Ontology, protein name, and pathways, were obtained using NetAffx (http://www.affymetrix.com/analysis/index.affx; Affymetrix, Santa Clara, CA).
Statistical analyses.
All values are expressed as means ± SE. Unpaired t-test was used for detecting differences in fractional synthesis rates between individual proteins or between protein groups.
RESULTS
Separation and identification of individual skeletal muscle proteins.
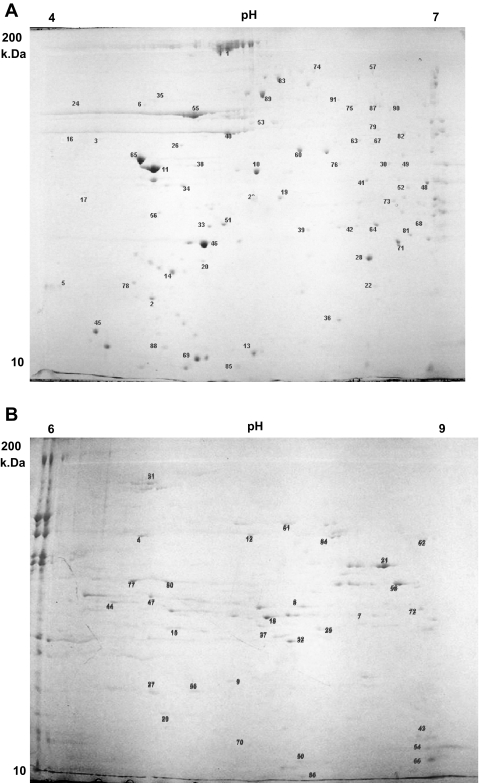
Individual proteins separated from mitochondrial pellets by 2DGE are shown in Fig. 1. The majority of proteins were isolated in the pH range of 4–7 (Fig. 1A) and the remaining proteins in the pH range of 6–9 (Fig. 1B). We collected a total of 190 gel spots from the two pH ranges. Of these 190 gel spots, 91 individual muscle proteins were identified by LC/MS/MS, and 91 protein gel spots representing these proteins were selected for isotopic enrichment measurements. We based our choice of protein gel spots on their reproducibility of appearance in 2DGE, intensity and resolution of the spot, and protein identification with high confidence. The selected proteins are shown in Table 1 with their corresponding Sequest score, accession number, percent amino acid coverage, and number of peptides (14, 52). Of 91 proteins, 5 proteins were identified only with single peptides, and the details are given in Table 2. The majorities (68) of the identified proteins were mitochondrial proteins, and all of them were encoded by nuclear genes. We also noted that the mitochondrial protein fraction contained contractile and sarcoplasmic proteins as well, demonstrating that the muscle mitochondrial protein fraction was not entirely pure.
Fig. 1.
Separation of proteins in mitochondrial isolate by 2-dimensional gel electrophoresis (2DGE). A: 2DGE using pH 4–7 immobilized pH gradient (IPG) strip in the 1st dimension. B: 2DGE using pH 6–9 IPG strip in the 1st dimension. Protein gel spot nos. correspond to list of proteins in Tables 1 and 2. Gel pictures are representative of an individual sample from a single rat.
Table 1.
Skeletal muscle proteins separated and purified by 2DGE and identified by tandem mass spectrometry
| No. | Protein Name | Accession No. | %AA Coverage | Sequest Score | Unique Peptides | Subcellular Location | Protein Function |
|---|---|---|---|---|---|---|---|
| 1 | myosin heavy chain, skeletal muscle, adult 1 | 34870884 | 13.55 | 234.32 | 23 | cytoskeleton | muscle contraction |
| 2 | myosin, light polypeptide 2 | 6981238 | 45.72 | 90.25 | 9 | cytoskeleton | actin-dependent ATPase activity, calcium ion binding, motor activity |
| 3 | tropomodulin 4 | 34858039 | 15.94 | 60.25 | 6 | cytoskeleton | muscle contraction |
| 4 | creatine kinase, muscle form | 6978661 | 28.28 | 138.25 | 13 | cytoplasm | reversibly catalyzes the transfer of phosphate between ATP and various phosphogens |
| 5 | troponin C, skeletal muscle | 34860691 | 30.90 | 40.26 | 4 | cytoskeleton | regulation of muscle contraction |
| 6 | leucine zipper-EF-hand containing transmembrane protein 1 | 34878524 | 10.28 | 70.28 | 7 | mitochondrion, plasma membrane | signal transduction, development |
| 7 | 3-hydroxyacyl-CoA dehydrogenase type II | 7387724 | 30.33 | 70.30 | 6 | cytoplasm, mitochondrion, plasma membrane | lipid metabolism |
| 8 | coiled-coil-helix-coiled-coil-helixdomaincontaining3 | 34855114 | 21.54 | 80.27 | 8 | mitochondrion | protein binding |
| 9 | NAD+-isocitrate dehydrogenase, gamma subunit | 414185 | 9.52 | 48.20 | 4 | mitochondrion | carbohydrate metabolism, tricarboxylic acid cycle |
| 10 | pyruvate dehydrogenase (lipoamide) beta | 34869518 | 27.21 | 90.29 | 9 | mitochondrion | glucose metabolism, glycolysis, tricarboxylic acid cycle |
| 11 | striated muscle alpha tropomyosin | 57406 | 35.26 | 70.25 | 7 | cytoskeleton | cell motility, regulation of muscle contraction, regulation of heart contraction, fatty acid biosynthesis |
| 12 | KCRS_RAT creatine kinase, sarcomeric mitochondrial precursor | 125313 | 11.10 | 80.22 | 7 | mitochondrion | generation of precursor metabolites and energy, muscle contraction |
| 13 | cytochrome c oxidase subunit Vb | 16758362 | 21.86 | 30.18 | 3 | mitochondrial envelope | electron transport, respiratory gaseous exchange |
| 14 | troponin T, fast skeletal muscle isoforms beta/alpha | 136385 | 9.99 | 40.25 | 7 | cytoskeleton | regulation of striated muscle contraction |
| 15 | ubiquinol-cytochrome c reductase, Rieske iron-sulfur polypeptide 1 | 34875396 | 9.25 | 30.26 | 3 | mitochondrion, membrane | electron transport, transcription, regulation of transcription-DNA-dependent, ATP synthesis coupled proton transport, transcription from RNA polymerase II promoter |
| 16 | hypothetical protein MGC36907 | 34868312 | 4.08 | 10.22 | 1 | ||
| 17 | complement component 1 Q subcomponent-binding protein | 34870494 | 5.38 | 10.24 | 1 | mitochondrial matrix | immune response |
| 18 | DNA segment, Chr 10, Johns Hopkins University 81 expressed | 27705010 | 24.22 | 60.24 | 6 | cell wall, mitochondrion | protein binding |
| 19 | prohibitin | 34851184 | 47.23 | 110.28 | 11 | nucleus, mitochondrial inner membrane, | regulation of progression through cell cycle, DNA replication, signal transduction, histone deacetylation, regulation of apoptosis |
| 20 | acyl-CoA dehydrogenase, very long chain | 6978435 | 6.66 | 50.28 | 5 | mitochondrion | electron transport, lipid metabolism, fatty acid metabolism, fatty acid beta-oxidation, energy derivation by oxidation of organic compounds |
| 21 | malate dehydrogenase 2 | 13592145 | 37.39 | 120.27 | 11 | mitochondrion, mitochondrial matrix | glycolysis, tricarboxylic acid cycle, malate metabolism, tricarboxylic acid cycle intermediate metabolism |
| 22 | LRP16 protein | 21326475 | 4.2 | 10.19 | 2 | mitochondrion | may play a role in cell proliferation |
| 23 | pyruvate dehydrogenase (lipoamide) alpha 1 | 34880551 | 21.09 | 80.22 | 8 | extracellular space, mitochondrion | acetyl-CoA metabolism, glycolysis |
| 24 | calreticulin | 11693172 | 15.5 | 60.31 | 6 | endoplasmic reticulum, cytosol, nucleus | regulation of transcription, protein folding, protein export from nucleus, calcium ion homeostasis, regulation of apoptosis |
| 25 | NADP+-specific isocitrate dehydrogenase | 34857317 | 39.01 | 70.26 | 6 | mitochondrion | role in intermediary metabolism and energy production, carbohydrate metabolic process |
| 26 | heat shock cognate 71-kDa protein | 34876476 | 9.72 | 50.24 | 5 | cytoplasm | chaperone, response to unfolded protein, protein binding |
| 27 | NAD+-specific isocitrate dehydrogenase b subunit | 34858691 | 19.73 | 80.23 | 8 | mitochondrion | tricarboxylic acid cycle, isocitrate metabolism |
| 28 | ATP synthase subunit d | 9506411 | 29.06 | 50.24 | 5 | mitochondrion | ion transport, ATP synthesis coupled proton transport |
| 29 | peroxiredoxin 5 precursor | 16758404 | 14.35 | 40.21 | 4 | mitochondrion, peroxisome | inflammatory response, response to oxidative stress |
| 30 | hydroxyacyl-CoA dehydrogenase | 18677763 | 12.50 | 90.27 | 9 | mitochondrion | lipid metabolism, fatty acid metabolism, fatty acid beta-oxidation |
| 31 | mitochondrial aconitase | 38541404 | 22.43 | 160.27 | 15 | mitochondrion | generation of precursor metabolites and energy, tricarboxylic acid cycle, citrate metabolism |
| 32 | unnamed protein product | 56691 | 27.60 | 70.26 | 7 | mitochondrion | age-dependent response to reactive oxygen species, regulation of transcription from RNA polymerase II promoter, superoxide metabolism, cellular defense response |
| 33 | myosin, light polypeptide 3 | 6981240 | 38.38 | 60.26 | 6 | cytoskeleton | muscle contraction |
| 34 | succinyl-CoA ligase beta-chain, mitochondrial | 34874487 | 11.02 | 40.29 | 4 | mitochondrion | carbohydrate metabolism; tricarboxylic acid cycle |
| 35 | prolyl 4-hydroxylase, beta polypeptide | 6981324 | 29.29 | 160.29 | 16 | extracellular region, endoplasmic reticulum | protein folding, peptidyl-proline hydroxylation to 4-hydroxy-L-proline |
| 36 | electron-transfer-flavoprotein, beta polypeptide | 27731305 | 12.58 | 30.18 | 3 | mitochondrial matrix | electron transport |
| 37 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 10, 22 kDa | 27667934 | 34.68 | 70.20 | 7 | mitochondrial inner membrane | transfer of electrons from NADH to the respiratory chain. |
| 38 | polymerase (RNA) II (DNA directed) polypeptide C | 34851240 | 10.94 | 30.26 | 3 | nucleus | transcription, transcription from RNA polymerase II promoter |
| 39 | dihydrolipoamide succinyltransferase | 266684 | 3.07 | 10.24 | 3 | mitochondrion | generation of precursor metabolites and energy, tricarboxylic acid cycle, metabolism |
| 40 | alpha 1 actin precursor | 4501881 | 12.9 | 40.32 | 4 | cytoskeleton | muscle thin filament assembly, skeletal muscle fiber development |
| 41 | 3-mercaptopyruvate sulfurtransferase | 20304123 | 4.33 | 10.23 | 1 | mitochondrial matrix | sulfate transport, cyanate catabolism, response to toxin |
| 42 | acetyl-CoA dehydrogenase, long-chain | 6978431 | 14.56 | 50.31 | 5 | mitochondrion | electron transport, lipid metabolism, fatty acid metabolism |
| 43 | ATPase, Ca2+ transporting, cardiac muscle, fast twitch 1 | 17157987 | 20.90 | 90.22 | 9 | smooth endoplasmic reticulum | cation transport, calcium ion transport, regulation of striated muscle contraction, proton transport |
| 44 | enoyl CoA hydratase, short chain 1 | 17530977 | 21.97 | 70.25 | 7 | mitochondrion | generation of precursor metabolites and energy, lipid metabolism, fatty acid metabolism, fatty acid beta-oxidation |
| 45 | ATP synthase, H+ transporting, mitochondrial F1 complex, delta subunit | 20806153 | 13.32 | 20.24 | 2 | mitochondrion, membrane fraction | ion transport, ATP synthesis coupled proton transport, proton transport, ATP biosynthesis |
| 46 | fast myosin alkali light chain | 13487933 | 37.56 | 50.23 | 7 | cytoskeleton | muscle development |
| 47 | H+-transporting ATP synthase | 57029 | 22.78 | 78.22 | 8 | mitochondrion, membrane fraction | ion transport, ATP synthesis coupled proton transport, proton transport, ATP biosynthesis |
| 48 | dihydrolipoamide dehydrogenase | 27717159 | 11.47 | 50.33 | 5 | cytoplasm, mitochondrion | electron transport |
| 49 | Tu translation elongation factor, mitochondrial | 34859187 | 24.73 | 80.26 | 8 | mitochondrion, membrane | protein biosynthesis, translational elongation, transport, |
| 50 | NADH dehydrogenase (ubiquinone) Fe-S protein 6 | 9506913 | 20.11 | 30.22 | 3 | mitochondrion | mitochondrial electron transport- NADH to ubiquinone |
| 51 | 24-kDa subunit of mitochondrial NADH dehydrogenase | 34877948 | 25.29 | 60.26 | 6 | mitochondrion, membrane fraction | mitochondrial electron transport, NADH to ubiquinone, nervous system development |
| 52 | 3-hydroxyisobutyrate dehydrogenase | 34855881 | 23.86 | 100.30 | 10 | mitochondrion | pentose-phosphate shunt, valine metabolism |
| 53 | gamma actin-like protein | 34856512 | 14.94 | 50.25 | 5 | cytoskeleton | structural constituent of cytoskeleton, sarcomere organization |
| 54 | Atp5c1 protein | 37747914 | 22.07 | 88.21 | 7 | membrane fraction, mitochondrion, | generation of precursor metabolites and energy, ion transport, ATP synthesis coupled proton transport, ATP biosynthesis |
| 55 | ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypept | 34862189 | 45.73 | 180.28 | 18 | mitochondrion, integral to membrane, | generation of precursor metabolites and energy, ion transport, ATP synthesis coupled proton transport |
| 56 | creatine kinase, sarcomeric mitochondrial precursor | 34853112 | 6.11 | 30.24 | 3 | mitochondrion inner membrane | reversibly catalyzes the transfer of phosphate between ATP and various phosphogens (e.g., creatine phosphate). |
| 57 | propionyl-CoA carboxylase, alpha polypeptide | 34876107 | 18.82 | 90.26 | 9 | mitochondrion; mitochondrial matrix | carbohydrate metabolism; propanoate metabolism |
| 58 | voltage-dependent anion-selective channel protein 1 | 10720216 | 22.20 | 70.25 | 7 | mitochondrial outer membrane | anion transport, apoptotic program, ion transport, apoptosis |
| 59 | cytochrome c-1 | 34866853 | 12.45 | 30.23 | 3 | mitochondrion, membrane | electron transport |
| 60 | isocitrate dehydrogenase 3 (NAD+) alpha | 16758446 | 24.44 | 90.22 | 9 | mitochondrion | carbohydrate metabolism, tricarboxylic acid cycle |
| 61 | fumarate hydratase 1 | 8393358 | 23.69 | 130.28 | 12 | cytoplasm, mitochondrion | tricarboxylic acid cycle, fumarate metabolism, cell cycle, negative regulation of progression through cell cycle, fumarate metabolism |
| 62 | glutamate oxaloacetate transaminase 2 | 6980972 | 29.23 | 170.27 | 14 | mitochondrion | amino acid metabolism, aspartate catabolism |
| 63 | NADH dehydrogenase (ubiquinone) flavoprotein 1 | 34861382 | 19.82 | 100.26 | 10 | mitochondrial inner membrane | electron transport, mitochondrial electron transport-NADH to ubiquinone, generation of precursor metabolites and energy |
| 64 | lysophospholipase 1 | 6981362 | 22.27 | 40.21 | 44 | membrane fraction | Lipid metabolism, fatty acid metabolism, |
| 65 | tropomyosin 4 | 14134107 | 4.92 | 40.25 | 4 | cytoskeleton | cell motility, structural constituent of muscle, calcium dependent regulation of muscle contraction, Binds to actin filaments in muscle and nonmuscle cells |
| 66 | ubiquinol-cytochrome c reductase binding protein | 27677468 | 41.59 | 70.20 | 7 | mitochondrial inner membrane | ubiquinol-cytochrome-c reductase activity, electron transport |
| 67 | NADH dehydrogenase 1 alpha subcomplex 10-like protein | 34877665 | 14.95 | 50.25 | 5 | membrane fraction, mitochondrion | generation of precursor metabolites and energy, nucleobase, nucleoside, nucleotide and nucleic acid metabolism |
| 68 | methylmalonate semialdehyde dehydrogenase gene | 13591997 | 10.99 | 60.20 | 6 | mitochondrion | pyrimidine nucleotide metabolism, valine metabolism, thymine metabolism |
| 69 | cytochrome c oxidase, subunit Va | 24233541 | 17.93 | 38.23 | 4 | mitochondrion | electron transport |
| 70 | NADH dehydrogenase (ubiquinone) Fe-S protein 2 | 34880923 | 12.76 | 40.24 | 4 | membrane fraction, mitochondrion | electron transport, mitochondrial electron transport-NADH to ubiquinone |
| 71 | mitochondrial H+-ATP synthase alpha subunit | 38512279 | 21.99 | 110.27 | 11 | membrane fraction, mitochondrion | ion transport, ATP synthesis coupled proton transport, proton transport,ATP biosynthesis |
| 72 | hydroxyacyl dehydrogenase, subunit B | 19424338 | 13.18 | 70.20 | 6 | mitochondrial envelope | lipid metabolism, fatty acid metabolism, fatty acid beta-oxidation |
| 73 | citrate synthase | 18543177 | 9.28 | 30.25 | 3 | cytoplasm, mitochondrion | tricarboxylic acid cycle, main pathways of carbohydrate metabolism |
| 74 | inner membrane protein | 34855983 | 20.94 | 128.27 | 13 | mitochondrial inner membrane | critical organizer of the mitochondrial cristae morphology and thus indispensable for normal mitochondrial function |
| 75 | ubiquinol-cytochrome c reductase core protein 1 | 34866355 | 18.08 | 80.23 | 8 | mitochondrion, membrane | oxidative phosphorylation, aerobic respiration, electron transport, transport |
| 76 | enolase 3, beta; muscle-specific enolase | 6978811 | 8.28 | 40.23 | 4 | phosphopyruvate hydratase complex | Glycolysis |
| 77 | electron transfer flavoprotein alpha-subunit | 27720225 | 30.22 | 80.27 | 8 | mitochondrial matrix | electron transport |
| 78 | MD-1 [Rattus norvegicus] | 34875250 | 3.43 | 10.14 | 1 | plasma membrane | apoptosis, inflammatory response, humoral immune response, signal transduction, cell proliferation, immune response |
| 79 | isovaleryl CoA dehydrogenase | 6981112 | 24.31 | 110.23 | 11 | mitochondrial matrix | electron transport, metabolism |
| 80 | voltage-dependent anion channel 2 | 13786202 | 20.26 | 50.31 | 5 | mitochondrial outer membrane | anion transport, ion transport |
| 81 | dihydrolipoamide acetyltransferase | 220838 | 12.72 | 60.25 | 6 | mitochondrion | acetyl-CoA biosynthesis, glycolysis |
| 82 | short chain acyl-CoA dehydrogenase | 11968090 | 16.91 | 60.24 | 6 | mitochondrion | electron transport, lipid metabolism, fatty acid metabolism, fatty acid beta-oxidation, generation of precursor metabolites and energy |
| 83 | dnaK-type molecular chaperone grp75 precursor | 2119726 | 18.86 | 118.24 | 12 | mitochondrion | control of cell proliferation and cellular aging; it may also act as a chaperone |
| 84 | acetyl-coenzyme A acetyltransferase 1 | 8392836 | 21.73 | 80.29 | 9 | mitochondrion | catalyzes the conversion of 2 acetyl-CoA molecules to acetoacetyl-CoA plus CoA |
| 85 | ubiquinol-cytochrome C reductase complex core protein 2 | 27679580 | 3.16 | 10.27 | 2 | mitochondrial inner membrane | electron transport, oxidative phosphorylation, proteolysis, aerobic respiration |
| 86 | cytochrome c oxidase, subunit VIa, polypeptide 2 | 6978691 | 12.48 | 10.17 | 1 | mitochondrial envelope | electron transport, generation of precursor metabolites and energy |
| 87 | mitochondrial aldehyde dehydrogenase | 25990263 | 29.36 | 120.28 | 12 | mitochondrion | carbohydrate metabolism, alcohol metabolism, metabolism |
| 88 | parvalbumin (calcium binding protein) | 11968064 | 30.41 | 50.23 | 5 | cytoplasm | calcium-binding protein is involved in muscle relaxation |
| 89 | heat shock protein, mitochondrial precursor (Hsp60) | 34876438 | 11.13 | 128.20 | 7 | mitochondrion matrix | mitochondrial protein import and macromolecular assembly, facilitates correct folding of imported proteins. |
| 90 | dihydrolipoamide branched chain transacylase E2 | 34859889 | 14.67 | 60.22 | 6 | mitochondrion matrix | amino acid metabolism |
| 91 | hypothetical protein FLJ10759 | 34860175 | 21.99 | 80.29 | 8 | intracellular | transcription regulator activity |
Mitochondrial fractions of rat quadriceps muscles were prepared by differential centrifugation as described in materials and methods. The mitochondrial pellets were used to perform 2-dimensional gel electrophoresis (2DGE) for protein purification. The protein serial no. sequence is according to Figs. 1 and 4. Proteins were identified by tandem mass spectrometry followed by Sequest database search. Proteins are listed with their identification parameters such as accession no., %amino acid (%AA) coverage, Sequest score, and no. of specific peptides obtained along with their subcellular location and function.
Table 2.
Proteins identified with single peptide, with their corresponding sequence, precursor m/z, charge, and score (XC)
| Protein No. | Protein Name | Peptide | Precursor m/z | Charge | XC |
|---|---|---|---|---|---|
| 16 | hypothetical protein MGC36907 | FLEQQNKVLETK | 738.26 | 2 | 4.49 |
| 17 | complement component 1 Q subcomponent-binding protein | MSGDWELEVNGTEAK | 841.72 | 2 | 4.75 |
| 41 | 3-mercaptopyruvate sulfurtransferase | THEDILENLDAR | 713.58 | 2 | 4.51 |
| 78 | MD-1 [Rattus norvegicus] | KLFLDIAFVAK | 632.98 | 2 | 2.77 |
| 86 | cytochrome c oxidase, subunit VIa, polypeptide 2 | GDHGGAGANTWR | 600.12 | 2 | 3.40 |
Isotopic enrichment of individual muscle proteins.
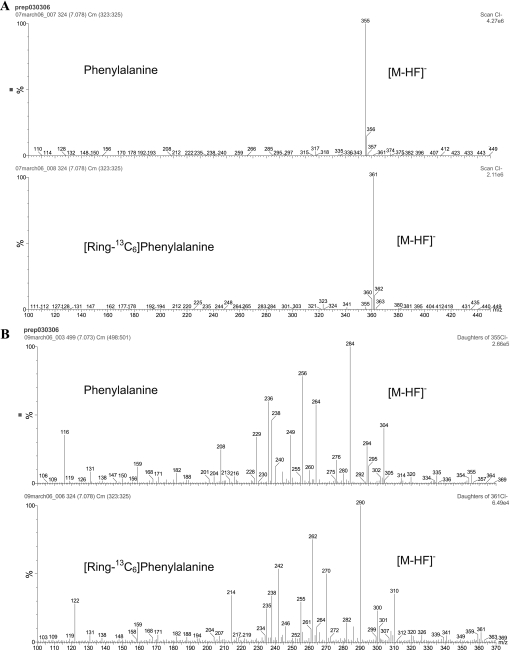
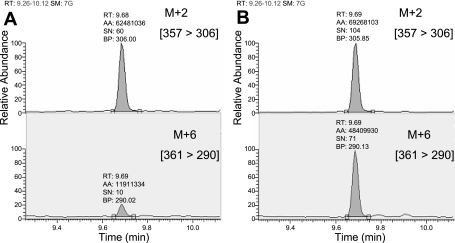
Isotopic enrichment in protein gel spots was measured by a previous methodology we used to measure very low levels of isotopic enrichment of amino acids in similarly low amounts of purified individual plasma proteins (25). Unlike the chromatographic approach we utilized for plasma protein purification, we have used a 2DGE approach in the present study to purify muscle mitochondrial proteins because of the limited amount of mitochondrial proteins available from muscle samples. The MS analysis was also modified by utilizing an MS/MS approach to reduce the interference of co-eluting compounds such as acrylamide monomers arising from the gel preparation. Since co-eluting compounds do not normally have the same ion transitions, the MS/MS approach offered greater sensitivity and specificity than GC/MS for the measurement of isotopic enrichment. The MS spectrum of the N-heptafluorobutyryl methyl ester derivative of the parent ions (m+0 and m+6) and MS/MS spectra of their daughter (product) ions are shown in Fig. 2. The MS/MS spectrum of the labeled phenylalanine clearly shows a shift of 6 mass units from the unlabeled species for many of the fragments. Fragment ions at m/z 290 and 306 were chosen to monitor the labeled and unlabeled species, respectively, which relates to the [m+6] to [m+2] fragments as described in materials and methods. Since the same fragment in the labeled and unlabeled components gave disproportionate signal intensities in the enriched samples, different m/z fragments were chosen for each analog to achieve a 1:1 relationship between the labeled and unlabeled signals, for optimum precision of measurement. Samples were analyzed against an enrichment curve prepared by plotting the area ratio of [m+6]/[m+2] vs. the theoretical molar percent excess of [13C6]phenylalanine. In the present study, we have successfully used this approach to measure tracer enrichment in gel spots of individual muscle proteins. The natural abundance measured in rats that did not receive isotopic infusion was subtracted from the samples of animals that were infused with [13C6]phenylalanine. Figure 3 shows chromatograms of phenylalanine measured from protein gel spots of very low isotopic enrichment (Fig. 3A) and high enrichment (Fig. 3B). Interassay precision was measured over a 6-mo period using a sample derived from a pool of protein gel spots. The interassay precision was 0.218%(SD0.016), percent coefficient of variation = 7.34%.
Fig. 2.
Mass spectra of unlabeled and labeled phenylalanine showing MS spectra (A) and MS/MS spectra of the [M-HF]− species (B). MS/MS ions containing 13C6 are mass shifted by 6 Da. These mass spectrums are products from a standard containing both labeled and unlabeled phenylalanine.
Fig. 3.
Chromatograms of phenylalanine from protein gel spots representing very low isotopic enrichment (A) and high enrichment (B). The chromatographic peaks shown relate to transitions of the m+2 fragment [357 → 306] and m+6 fragment [361 → 290].
Fractional synthesis rate of skeletal muscle proteins.
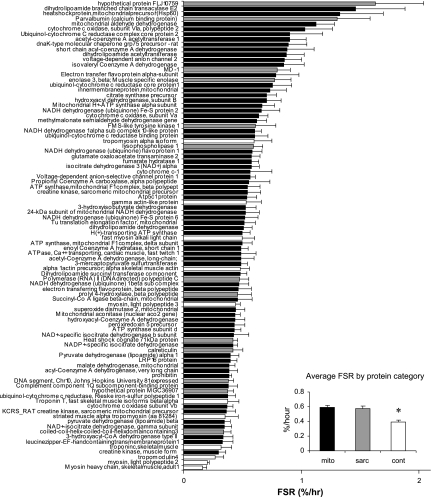
FSR values for all 91 muscle proteins (68 mitochondrial proteins and 23 nonmitochondrial proteins) identified are shown in Fig. 4. An almost 10-fold range in FSR was observed, with the lowest values observed for structural proteins such as MHCs (0.16 ± 0.04%/h) and the highest values for mitochondrial proteins such as dihydrolipoamide branched chain transacylase E2 (1.5 ± 0.42%/h). The FSR values of mitochondrial proteins were distributed between 0.30%/h ± 0.07 for the lowest value and 1.5 ± 0.42%/h for the highest, showing a fivefold difference. The average FSR of contractile proteins (0.39 ± 0.02%/h) was significantly lower (P < 0.002) than the average FSR value for both mitochondrial (0.59 ± 0.03%/h) and sarcoplasmic (0.67 ± 0.04%/h) proteins. There was no difference in the average FSR values for mitochondrial and sarcoplasmic proteins.
Fig. 4.
Fractional synthesis rate (FSR) of rat skeletal muscle proteins. Mitochondria were prepared from skeletal muscle of 6 male rats infused with [13C6]phenylalanine. Individual proteins were separated by 2DGE. Sixty-eight mitochondrial (mito), 11 contractile (cont), and 13 sarcoplasmic (sarc) proteins were identified by LC/MS/MS. Isotopic enrichment of each protein was measured by GC/MS/MS, and FSR was calculated using tissue fluid isotopic enrichment. Data are expressed as means ± SE. Inset: average synthesis rate of mitochondrial, sarcoplasmic, and contractile proteins. FSR of contractile proteins is significantly lower than for the other 2 fractions.
Functional/pathway analyses.
We performed IPA for the proteins identified to determine whether protein clusters demonstrate differences in their FSR based on their cellular location and biological functions. All mitochondrial proteins were identified using the Gene Ontology cellular component information, and some mitochondrial proteins were classified as membrane and nonmembrane proteins. There were no differences between the average FSR of membrane (0.595 ± 0.046%/h) and nonmembrane proteins (0.594 ± 0.048%/h). We also explored the common mitochondrial functions including TCA cycle, electron transport chain, fatty acid metabolism, glycolysis, and amino acid metabolism. There were no differences in the average FSR values among these five functional groups (data not shown). However, the results indicate that several individual proteins belonging to the same functional pathways have a wide range in their synthesis rates, as shown in Table 1.
DISCUSSION
We report a methodology to measure the synthesis rates of 68 individual mitochondrial proteins in skeletal muscle by measuring the incorporation of isotopically labeled phenylalanine into these proteins. Proteins were labeled in vivo by intravenous administration of [ring-13C6]phenylalanine in rats. Individual mitochondrial proteins were purified from the mitochondrial fraction of quadriceps muscle by 2DGE, and identification of individual proteins and measurement of isotopic enrichment of phenylalanine in these proteins and muscle tissue fluid were performed by tandem MS to calculate the FSR of these proteins. The present methodology is based on an animal study following a bolus injection of a tracer. This methodology could be easily adapted to humans and other species, using either the bolus or continuous infusion of labeled amino acids. Similar measurements can also be performed in tissues other than skeletal muscle.
The approach described in the present paper offers many advantages. Most of the recent developments in quantitative proteomics have focused on improving the technology for measuring protein abundance, which is the net result of protein synthesis and breakdown. Protein abundance data, in the absence of protein turnover information, can overlook several proteins that are affected by a particular biological condition, because the turnover rate of a protein can be altered by a condition without any change in protein concentration. For example, a simultaneous increase in synthesis rate and breakdown of a protein results in no changes in concentration, although substantial changes in biological functions may occur. Moreover, as discussed in the introduction, the presently available quantitative proteomic approaches do not have sufficient precision to detect changes in low-abundance proteins, including many enzymes that have major biological functions (34), and short-term studies are unlikely to detect changes in concentrations of proteins that turn over slowly. The present approach circumvents these problems by measuring the in vivo synthesis rate of proteins, although no information on protein degradation is available. In some cases, simultaneous measurements of synthesis rate and concentration of a protein at baseline and following intervention offer qualitative measurement of degradation rate.
There are many logistical reasons to recommend the present approach. One of the main limitations of performing proteomic studies in humans is the limitation of the size of tissue samples. The MS/MS approach we have used relies on purifying proteins from a fraction or compartment of interest and requires as little as 1 μg or less of each individual protein for the measurement. There exists well-established stable isotope tracer methods for measuring the synthesis of total muscle proteins (33) and muscle protein fractions including sarcoplasmic (3) and mitochondrial proteins (3, 40, 41). The average synthesis rates of mitochondrial, sarcoplasmic, and myofibrillar proteins in the present study (Fig. 4) agree with the previously reported average synthesis rates. The main advantage of the present method is that it measures synthesis rates of highly purified individual mitochondrial proteins unlike the average FSR of mixed muscle proteins or different subcellular fractions that were previously reported. Moreover, the proteins of mitochondrial fractions prepared by differential centrifugation have in the present study been shown to be contaminated with proteins from the other compartments such as myofibrillar and sarcoplasmic protein fractions. If the contributions of myofibrillar and sarcoplasmic components to mitochondrial fractions isolated by differential centrifugation are stable under different study conditions, it is potentially feasible to estimate fractional synthesis rates of mixed mitochondrial proteins.
In general, the previous methods measure the abundance of isotopic enrichment in muscle proteins by using GC-combustion-isotope ratio MS (GC/C/IRMS) of the derivatized amino acids from the hydrolysis of proteins. Such approaches are sensitive and accurate but also limited, since they require relatively large amounts of protein. However, the GC/C/IRMS approach is not sensitive for isotopic enrichment measurement of individual proteins obtained from a 2DGE, as in the present study, because of the limitation of very small sample size. It is possible to purify sufficient quantities of the individual muscle contractile proteins such as MHC by preparative continuous elution gel electrophoresis (2), or MHC and actin by SDS-PAGE (23). However, these are relatively high-abundance proteins in muscle, and ∼0.3–1.0 mg of purified protein is required to be able to measure isotopic enrichment by GC/C/IRMS. Moreover, it is not realistic to use these methods to purify multiple individual muscle proteins for isotopic enrichment measurement. By combining 2DGE for protein purification and GC/MS/MS to measure isotopic enrichment in the present study, we were able to calculate the FSR of multiple individual muscle proteins simultaneously.
We chose to focus on the mitochondrial cellular fraction from rat skeletal muscle for this study, mainly because of substantial interest in mitochondrial changes as a potential mechanism in aging (46) and many other metabolic conditions (31, 50, 51). The exact number of mitochondrial proteins is not known but is believed to be ∼1,000 proteins (43). Most of the proteomic analyses performed to date have identified mitochondrial proteins using high-throughput experiments. For example, Gaucher et al. (19) mapped 680 proteins associated with human heart mitochondria (19) from three separate studies using high-throughput proteomics (19, 53, 54). A report of proteomic analyses in combination with RNA expression data produced a list of 591 mitochondrial proteins using mouse brain, heart, kidney, and liver (30), while another study identified 689 mitochondrial proteins using rat liver, heart, and skeletal muscle (16). Although the above studies have attempted only to identify or profile mitochondrial proteins, there have also been reports to purify individual mitochondrial proteins from various organs, but not skeletal muscle. A study using the human neuroblastoma cell line SH-SY5Y isolated and identified 60 mitochondrial proteins by 2DGE (44), and another two studies using rat liver produced 80 (17) and 78 (29) mitochondrial proteins by 2DGE. The present study, to the best of our knowledge, is the first study reporting large-scale purification of skeletal muscle mitochondrial proteins by 2DGE. Of the 91 individual muscle proteins, only 68 (73.9%) were mitochondrial proteins, indicating that the muscle mitochondrial fraction obtained through differential centrifugation does not entirely consist of mitochondrial proteins. These proteins are synthesized in cytoplasmic compartments and transported into mitochondria. There are about 13 mitochondrial proteins encoded by mitochondrial genes that were not purified in the present approach. Although we used two different pH ranges and a very-large-size (24 × 24 cm) 2DGE to achieve superior resolving ability for purification, the present approach was not ideal to purify many membrane proteins. The present approach also did not isolate many hydrophobic proteins in mitochondria that can be purified by using an alternative electrophoretic approach.
The present study reports synthesis rates of only nuclear-encoded proteins in mitochondria. 2DGE has its own limitations, such as its inability to detect very-low-abundance or extremely small proteins (21), a lower yield in the isolation of number of proteins, and its inability to separate hydrophobic proteins (19). However, we chose to use 2DGE-based protein purification in the present study because it offered the ability to visually select a large number of proteins that are purified and resolved on a single platform. Another potential limitation in this study is that the application of tracer infusion is delivered as a single-bolus intravenous injection instead of a continuous steady-state infusion. Nevertheless, our technique could be easily applied to measure the synthesis rate of multiple proteins in steady-state tracer infusion studies as well. For fractional protein synthesis rate calculations, we used isotopic enrichment in muscle tissue fluid, which consists of ∼85% intracellular and 15% extracellular fluid and offers the best approximation to amino acyl tRNA (28). Ideally, amino acyl tRNA enrichment representing the obligatory precursor of protein synthesis should be used for calculations of fractional protein synthesis rate. Although calculations using amino acid enrichment in tissue fluid as the precursor underestimate the fractional protein synthesis rates, intervention studies have shown that calculations based on both tissue fluid and plasma enrichment demonstrate similar directional changes as those measured using amino acyl tRNA (10, 12). Measurement of amino acyl tRNA is not practical in most studies because of the sample size required (∼300- to 500-mg muscle sample). Because, in the present study, we used the same precursor pool for fractional protein synthesis rate measurements of all individual proteins, the relationship between synthesis rates of different proteins would not be altered even if we used amino acyl tRNA as precursor.
Of interest is the finding from the present study that the synthesis rates of skeletal muscle proteins vary substantially. Our results demonstrate a fivefold range between the lowest and the highest FSR values among the mitochondrial proteins. However, the FSR among all the proteins analyzed spans a 10-fold range, demonstrating the lowest rates for MHC, which is a structural protein, and the highest for dihydrolipoamide branched chain transacylase E2, which is a mitochondrial protein. As indicated in the results, we could not find any differences in average synthesis rates of proteins clustered based on different functions or based on anatomic locations within mitochondria. For example, we did not observe any difference in the FSR between membrane vs. nonmembrane proteins or for proteins classified within the five functional groups of the citric acid cycle, electron transport chain, fatty acid metabolism, glycolysis and gluconeogenesis, and amino acid metabolism. A potential explanation for this is that the study was conducted in normal healthy animals under baseline conditions and also because of the lack of coverage of all the known muscle proteins. However, a key finding was that individual proteins within a functional group have vastly different synthesis rates. For example, 3-hydroxyacyl-CoA dehydrogenase type II (FSR = 0.34 ± 0.08%/h) and short chain acetyl CoA (FSR = 0.88 ± 0.23%/h) are involved in mitochondrial fatty acid metabolism but have significantly (P = 0.04) different synthesis rates. Even with the single respiratory enzyme complex cytochrome c oxidase, we found that the FSR of individual subunits VIa (FSR = 1.13 ± 0.42%/h) and Vb (FSR = 0.37 ± 0.05%/h) varied threefold (P = 0.04). There are many other examples to show the wide range in synthesis rates of proteins with similar functions (Table 1 and Supplemental Table; supplemental data are available at the online version of this article). No clear explanation for this novel observation is evident from the present study. For mitochondrial function, it is important to have a complement of all proteins in each protein complex, and the differences in the synthesis rates of proteins with similar function may be an unappreciated step in functional regulation. It remains to be determined whether these vastly different protein synthesis rates remain similar under conditions of changing fuel oxidation.
To the best of our knowledge, this is the first study demonstrating the synthesis rate of multiple muscle proteins from animals or humans. Although we did not isolate and analyze all of the known muscle proteins, our results demonstrate a significant difference in the FSR between the contractile proteins and the mitochondrial and sarcoplasmic proteins. This is very important, because, although we prepared purified mitochondrial fractions through centrifugation, the mitochondrial pellet still contained numerous proteins from the sarcoplasmic and structural compartments of muscle. This fact should not be overlooked while measuring the synthesis rate of total proteins from various fractions of skeletal muscle, as happens in most studies. During stable isotope labeling, all proteins being synthesized are labeled simultaneously, which offers the possibility of measuring synthesis rates of multiple proteins from the sarcoplasmic and myofibrillar fractions from the same muscle sample, which contain more sarcoplasmic proteins and structural proteins, respectively. In addition, the present methodology could be easily adapted to measure synthesis rates of multiple proteins from any other organ tissue or biological fluid.
The FSR measurement of individual proteins represents the best measurement of translational rate of gene transcripts and thus offers a potential opportunity to understand in vivo regulation of specific genes. The present approach measures the dynamics of gene expression at the translational level and offers an opportunity to understand the regulation of gene expression in a new dimension. Quantification of the abundance of gene transcripts and proteins provides a valuable measure of steady-state net changes in transcript and protein turnover rates. However, when used in combination with protein translational rates, substantial opportunities are available to understand the pathophysiology of many clinical conditions involving muscle mitochondrial dysfunction.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-AG-09531, R01-DK-41973, and UL1-RR-024150-01 from the National Center for Research Resources, a component of the NIH, and NIH Roadmap for Medical Research. K. S. Nair is also supported by a David Murdock Dole Endowed Professorship.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55: 3309–3319, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal P, Ljungqvist O, Nair KS. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans. Am J Physiol Endocrinol Metab 272: E45–E50, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab 273: E790–E800, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 275: 3343–3347, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bateman RJ, Munsell LY, Chen X, Holtzman DM, Yarasheski KE. Stable isotope labeling tandem mass spectrometry (SILT) to quantify protein production and clearance rates. J Am Soc Mass Spectrom 18: 997–1006, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann PQ, Stirewalt WS, O'Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol Endocrinol Metab 267: E203–E209, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 6: 421–424, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Cargile BJ, Bundy JL, Grunden AM, Stephenson JL Jr. Synthesis/degradation ratio mass spectrometry for measuring relative dynamic protein turnover. Anal Chem 76: 86–97, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Caso G, Ford GC, Nair KS, Garlick PJ, McNurlan MA. Aminoacyl-tRNA enrichment after a flood of labeled phenylalanine: insulin effect on muscle protein synthesis. Am J Physiol Endocrinol Metab 282: E1029–E1038, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Sun L, Yu Y, Xue Y, Yang P. Amino acid-coded tagging approaches in quantitative proteomics. Exp Rev Proteomics 4: 25–37, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin's anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab 291: E729–E736, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Doherty MK, Whitehead C, McCormack H, Gaskell SJ, Beynon RJ. Proteome dynamics in complex organisms: using stable isotopes to monitor individual protein turnover rates. Proteomics 5: 522–533, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5: 976–989, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Ford GC, Cheng KN, Halliday D. Analysis of (1-13C)leucine and (13C)KIC in plasma by capillary gas chromatography/mass spectrometry in protein turnover studies. Biomed Mass Spectrom 12: 432–436, 1985. [DOI] [PubMed] [Google Scholar]
- 16.Forner F, Foster LJ, Campanaro S, Valle G, Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol Cell Proteomics 5: 608–619, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Fountoulakis M, Berndt P, Langen H, Suter L. The rat liver mitochondrial proteins. Electrophoresis 23: 311–328, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Fredriksson K, Hammarqvist F, Strigard K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab 291: E1044–E1050, 2006. [Erratum. Am J Physiol Endocrinol Metab 292(4): E1240, 2007.] [DOI] [PubMed] [Google Scholar]
- 19.Gaucher SP, Taylor SW, Fahy E, Zhang B, Warnock DE, Ghosh SS, Gibson BW. Expanded coverage of the human heart mitochondrial proteome using multidimensional liquid chromatography coupled with tandem mass spectrometry. J Proteome Res 3: 495–505, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab 31: 5S20–25S26, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Gygi SP, Corthals GL, Zhang Y, Rochon Y, Aebersold R. Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proc Natl Acad Sci USA 97: 9390–9395, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliday D, Ford GC. Stable isotopes in clinical investigations. In: Clinical Biochemistry: Principles, Methods, Applications, edited by Lawson AM. New York: Walter deGruyter, 1989, p. 684–726.
- 23.Hasten DL, Morris GS, Ramanadham S, Yarasheski KE. Isolation of human skeletal muscle myosin heavy chain and actin for measurement of fractional synthesis rates. Am J Physiol Endocrinol Metab 275: E1092–E1099, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellman U, Wernstedt C, Gonez J, Heldin CH. Improvement of an “In-Gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem 224: 451–455, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Jaleel A, Nehra V, Persson XM, Boirie Y, Bigelow M, Nair KS. In vivo measurement of synthesis rate of multiple plasma proteins in humans. Am J Physiol Endocrinol Metab 291: E190–E197, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE, Mintun MA, Watkins SC, Simoneau JA, Jadali F, Fredrickson A, Beattie J, Theriault R. The effect of non-insulin-dependent diabetes mellitus and obesity on glucose transport and phosphorylation in skeletal muscle. J Clin Invest 97: 2705–2713, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljungqvist OH, Persson M, Ford GC, Nair KS. Functional heterogeneity of leucine pools in human skeletal muscle. Am J Physiol Endocrinol Metab 273: E564–E570, 1997. [DOI] [PubMed] [Google Scholar]
- 29.McDonald T, Sheng S, Stanley B, Chen D, Ko Y, Cole RN, Pedersen P, Van Eyk JE. Expanding the subproteome of the inner mitochondria using protein separation technologies: one- and two-dimensional liquid chromatography and two-dimensional gel electrophoresis. Mol Cell Proteomics 5: 2392–2411, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115: 629–640, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115: 3587–3593, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol Endocrinol Metab 254: E208–E213, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Nair KS, Jaleel A, Asmann YW, Short KR, Raghavakaimal S. Proteomic research: potential opportunities for clinical and physiological investigators. Am J Physiol Endocrinol Metab 286: E863–E874, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Ong SE, Mann M. Mass spectrometry-based proteomics turns quantitative. Nat Chem Biol 1: 252–262, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pratt JM, Petty J, Riba-Garcia I, Robertson DH, Gaskell SJ, Oliver SG, Beynon RJ. Dynamics of protein turnover, a missing dimension in proteomics. Mol Cell Proteomics 1: 579–591, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93: 15364–15369, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooyackers OE, Balagopal P, Nair KS. Measurement of synthesis rates of specific muscle proteins using needle biopsy samples. Muscle Nerve Suppl 5: S93–S96, 1997. [PubMed] [Google Scholar]
- 42.Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem 203: 173–179, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Scharfe C, Zaccaria P, Hoertnagel K, Jaksch M, Klopstock T, Dembowski M, Lill R, Prokisch H, Gerbitz KD, Neupert W, Mewes HW, Meitinger T. MITOP, the mitochondrial proteome database: 2000 update. Nucleic Acids Res 28: 155–158, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheffler NK, Miller SW, Carroll AK, Anderson C, Davis RE, Ghosh SS, Gibson BW. Two-dimensional electrophoresis and mass spectrometric identification of mitochondrial proteins from an SH-SY5Y neuroblastoma cell line. Mitochondrion 1: 161–179, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Schwenk WF, Berg PJ, Beaufrere B, Miles JM, Haymond MW. Use of t-butyldimethylsilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem 141: 101–109, 1984. [DOI] [PubMed] [Google Scholar]
- 46.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102: 5618–5623, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Short KR, Nygren J, Barazzoni R, Levine J, Nair KS. T(3) increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and -3. Am J Physiol Endocrinol Metab 280: E761–E769, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83: 166–171, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Slater C, Preston T, McMillan DC, Stuart Falconer J, Fearon CH. GC/MS analysis of [2H5]phenylalanine at very low enrichment: measurement of protein synthesis in health and disease. J Mass Spectrom 30: 1325–1332, 1995. [Google Scholar]
- 50.Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes 51: 1913–1920, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100: 7996–8001, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabb DL, McDonald WH, Yates JR 3rd. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res 1: 21–26, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor SW, Fahy E, Zhang B, Glenn GM, Warnock DE, Wiley S, Murphy AN, Gaucher SP, Capaldi RA, Gibson BW, Ghosh SS. Characterization of the human heart mitochondrial proteome. Nat Biotechnol 21: 281–286, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Taylor SW, Warnock DE, Glenn GM, Zhang B, Fahy E, Gaucher SP, Capaldi RA, Gibson BW, Ghosh SS. An alternative strategy to determine the mitochondrial proteome using sucrose gradient fractionation and 1D PAGE on highly purified human heart mitochondria. J Proteome Res 1: 451–458, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Waterlow JC, Golden J, Picou D. The measurements of rates of protein turnover, synthesis, and breakdown in man and the effects of nutritional status and surgical injury. Am J Clin Nutr 30: 1333–1339, 1977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.