Abstract
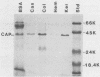
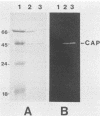
Six Candida spp. that were previously characterized for cutaneous pathogenicity were assessed for Candida acid proteinase (CAP) production in albumin-supplemented, nitrogen-restricted media. C. albicans CAP production was compared in media supplemented with albumin, casein, collagen, hemoglobin, or keratin and in TC medium 199. C. albicans, C. stellatoidea, and C. tropicalis, which are cutaneous pathogens in murine infections, produced 3.3 to 4.7 times more CAP than did the nonpathogens C. parapsilosis and C. guilliermondii. C. krusei, a nonpathogen, produced negligible amounts of enzyme. C. albicans CAP production was similar in each protein-supplemented medium, and only a single acid proteinase was recovered from each one. Rapid CAP purification from culture supernatants was achieved by hollow fiber and stirred cell ultrafiltration followed by Affi-Gel blue and Sephacryl column chromatographies. The highest yield, purity, and specific activity of CAP were obtained from keratin-supplemented medium supernatants, producing 2.86 mg of purified CAP from a 7-liter culture. CAP was characterized as a 41,500-dalton glycoprotein, with a pI of 4.5; a pH range of 2.5 to 5.5; and broad substrate specificity, including that for keratin, denatured collagen, hemoglobin, casein, and albumin. Isolation of CAP also isolated the keratinolytic proteinase of Candida spp. CAP was inhibited by pepstatin A, but not by EDTA or phenylmethylsulfonyl fluoride. Monospecific antibody to CAP was produced in mice and reacted only to the 41,500-dalton protein, as determined by immunoblot analysis. High CAP production by cutaneous pathogenic Candida spp. supports the fact that CAP is a potential virulence factor that may facilitate Candida colonization and invasion of skin. CAP production from keratin-supplemented medium was superior to that from the other media that were tested and yielded sufficient and suitable enzyme for use in immunoassays of CAP antigen and antibody.
Full text
PDF

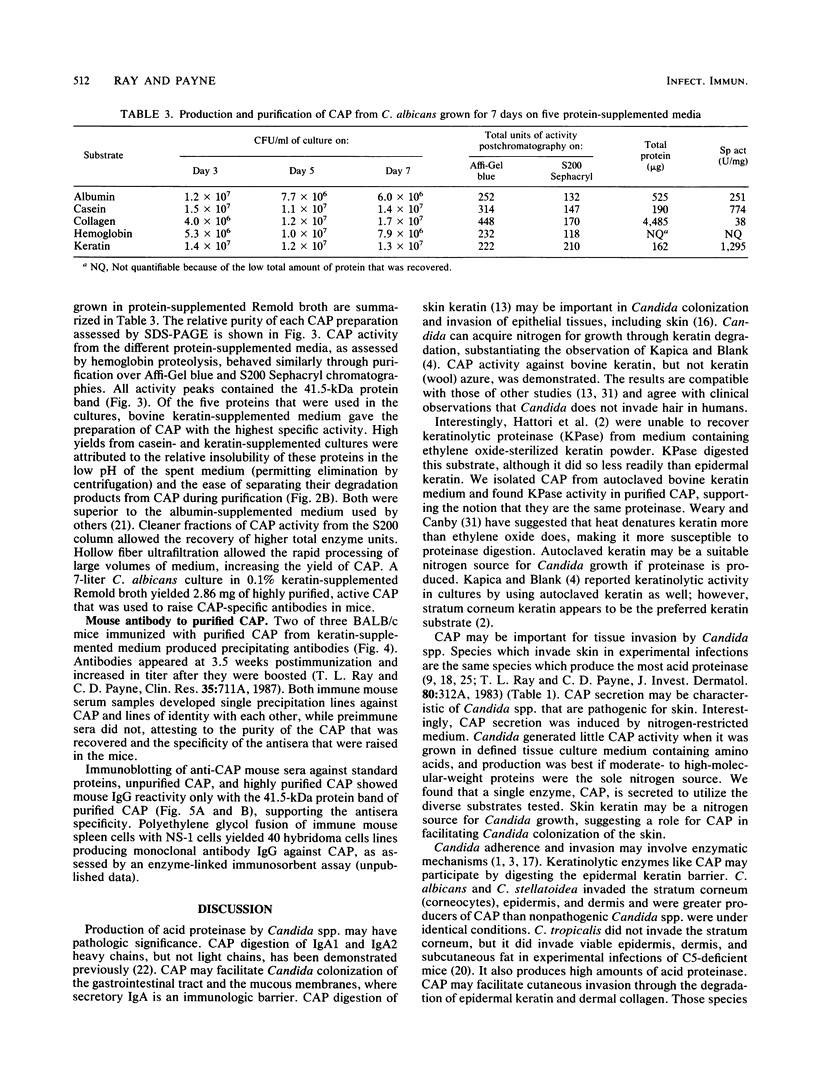
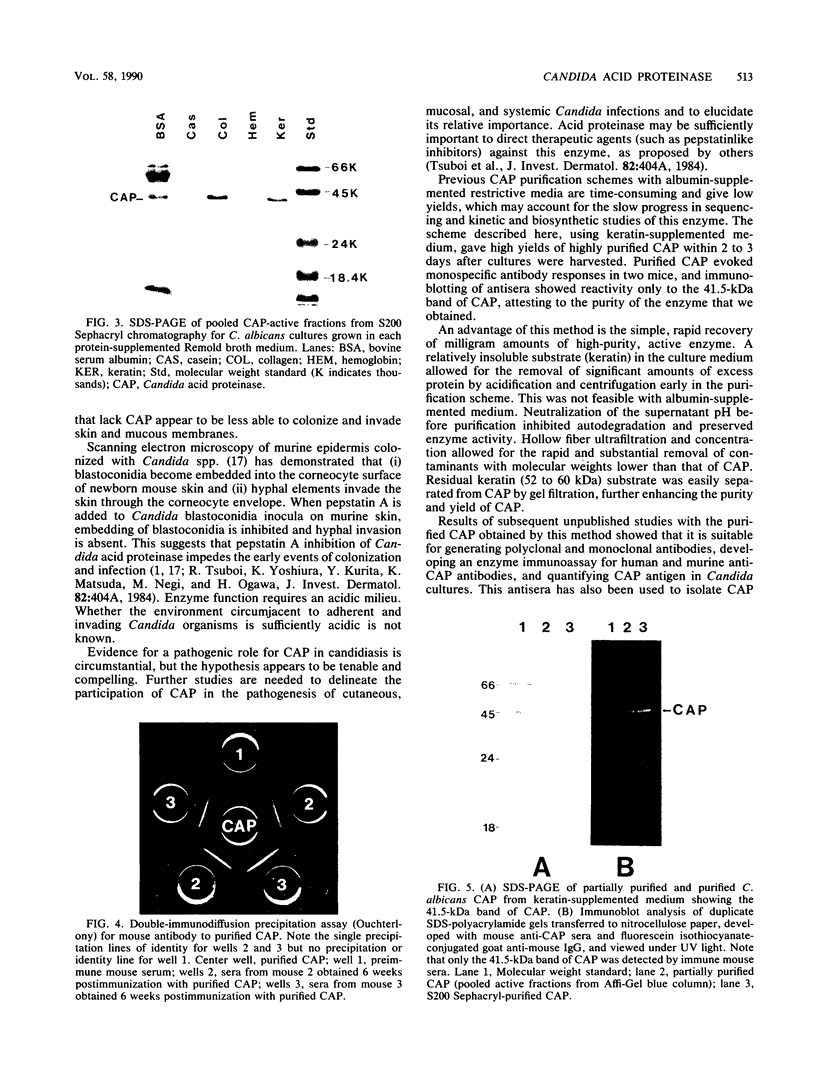

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg M., Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988 Mar;56(3):626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Yoshiura K., Negi M., Ogawa H. Keratinolytic proteinase produced by Candida albicans. Sabouraudia. 1984;22(3):175–183. [PubMed] [Google Scholar]
- Howlett J. A., Squier C. A. Candida albicans ultrastructure: colonization and invasion of oral epithelium. Infect Immun. 1980 Jul;29(1):252–260. doi: 10.1128/iai.29.1.252-260.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPICA L., BLANK F. Growth of Candida albicans on keratin as sole source of nitrogen. Dermatologica. 1957 Aug;115(2):81–105. doi: 10.1159/000255990. [DOI] [PubMed] [Google Scholar]
- Kondoh Y., Shimizu K., Tanaka K. Proteinase production and pathogenicity of Candida albicans. II. Virulence for mice of C. albicans strains of different proteinase activity. Microbiol Immunol. 1987;31(11):1061–1069. doi: 10.1111/j.1348-0421.1987.tb01338.x. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980 Aug;13(3):423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Purified Candida albicans proteinase in the serological diagnosis of systemic candidosis. JAMA. 1980 Jun 20;243(23):2409–2411. [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- Macdonald F. Secretion of inducible proteinase by pathogenic Candida species. Sabouraudia. 1984;22(1):79–82. [PubMed] [Google Scholar]
- Moore G. L. Use of azo-dye-bound collagen to measure reaction velocities of proteolytic enzymes. Anal Biochem. 1969 Oct 15;32(1):122–127. doi: 10.1016/0003-2697(69)90111-0. [DOI] [PubMed] [Google Scholar]
- Negi M., Tsuboi R., Matsui T., Ogawa H. Isolation and characterization of proteinase from Candida albicans: substrate specificity. J Invest Dermatol. 1984 Jul;83(1):32–36. doi: 10.1111/1523-1747.ep12261656. [DOI] [PubMed] [Google Scholar]
- Odds F. C. Candida albicans proteinase as a virulence factor in the pathogenesis of Candida infections. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Dec;260(4):539–542. doi: 10.1016/s0176-6724(85)80069-9. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Digre K. B., Payne C. D. Adherence of Candida species to human epidermal corneocytes and buccal mucosal cells: correlation with cutaneous pathogenicity. J Invest Dermatol. 1984 Jul;83(1):37–41. doi: 10.1111/1523-1747.ep12261661. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Payne C. D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect Immun. 1988 Aug;56(8):1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. L., Wuepper K. D. Activation of the alternative (properdin) pathway of complement by Candida albicans and related species. J Invest Dermatol. 1976 Dec;67(6):700–703. doi: 10.1111/1523-1747.ep12598581. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Wuepper K. D. Experimental cutaneous candidiasis in rodents. J Invest Dermatol. 1976 Jan;66(1):29–33. doi: 10.1111/1523-1747.ep12478053. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Wuepper K. D. Experimental cutaneous candidiasis in rodents; II. Role of the stratum corneum barrier and serum complement as a mediator of a protective infalmmatory response. Arch Dermatol. 1978 Apr;114(4):539–543. doi: 10.1001/archderm.114.4.539. [DOI] [PubMed] [Google Scholar]
- Remold H., Fasold H., Staib F. Purification and characterization of a proteolytic enzyme from Candida albicans. Biochim Biophys Acta. 1968 Oct 8;167(2):399–406. doi: 10.1016/0005-2744(68)90219-2. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Böning B. Detection of Candida proteinase by enzyme immunoassay and interaction of the enzyme with alpha-2-macroglobulin. J Immunol Methods. 1983 Jun 24;61(1):107–116. doi: 10.1016/0022-1759(83)90014-5. [DOI] [PubMed] [Google Scholar]
- Rüchel R. On the role of proteinases from Candida albicans in the pathogenesis of acronecrosis. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):524–536. [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Tegeler R., Trost M. A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia. 1982 Sep;20(3):233–244. doi: 10.1080/00362178285380341. [DOI] [PubMed] [Google Scholar]
- Staib F. Proteolysis and pathogenicity of Candida albicans strains. Mycopathol Mycol Appl. 1969 May 28;37(4):345–348. doi: 10.1007/BF02129881. [DOI] [PubMed] [Google Scholar]
- Staib F. Serum-proteins as nitrogen source for yeastlike fungi. Sabouraudia. 1965 Oct;4(3):187–193. doi: 10.1080/00362176685190421. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]