Abstract
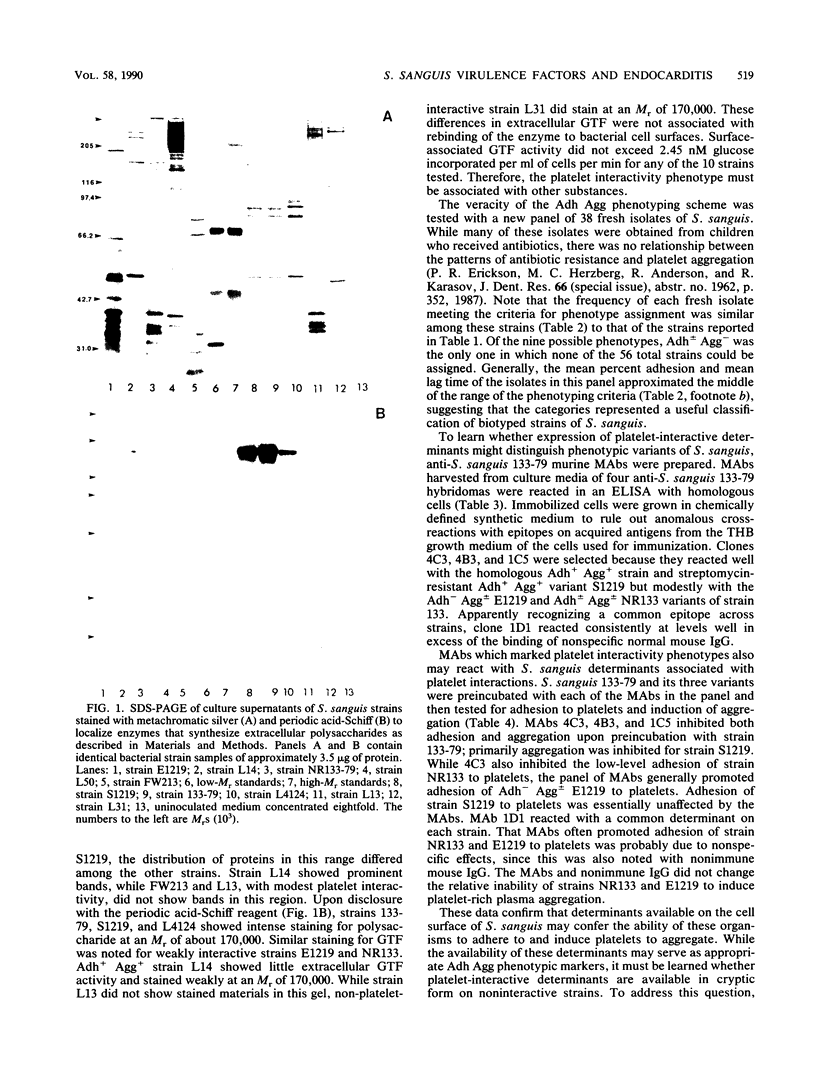
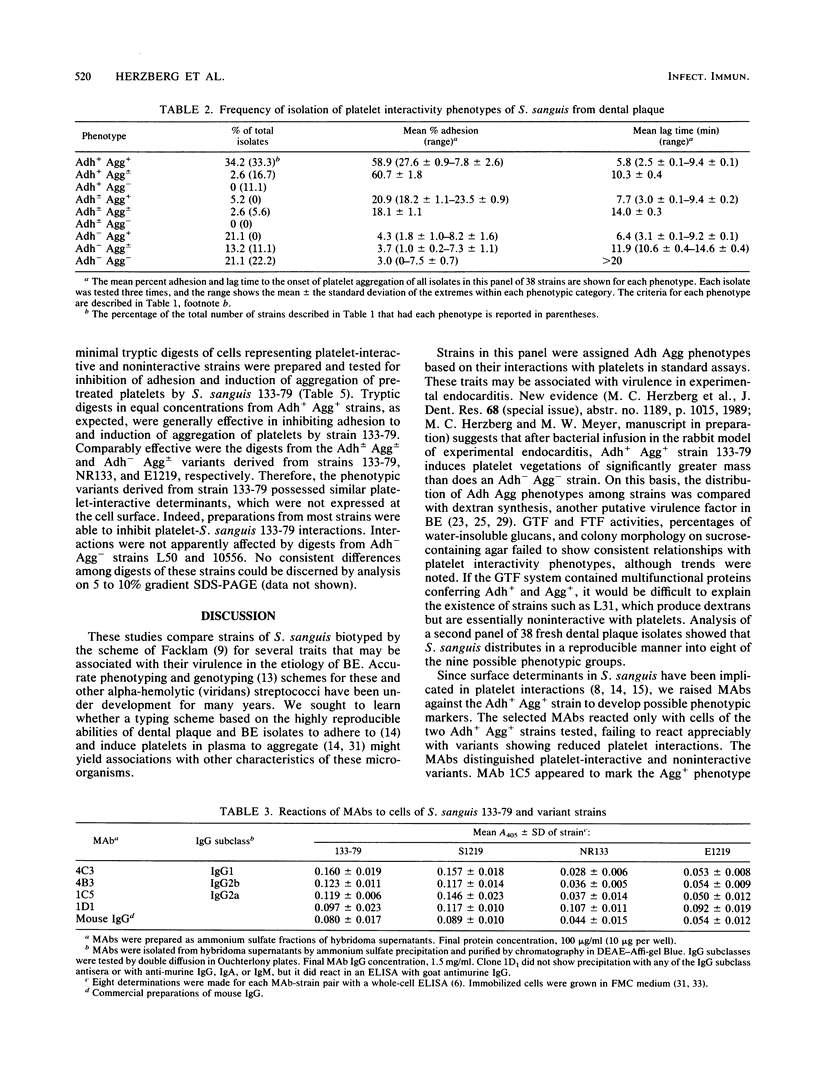
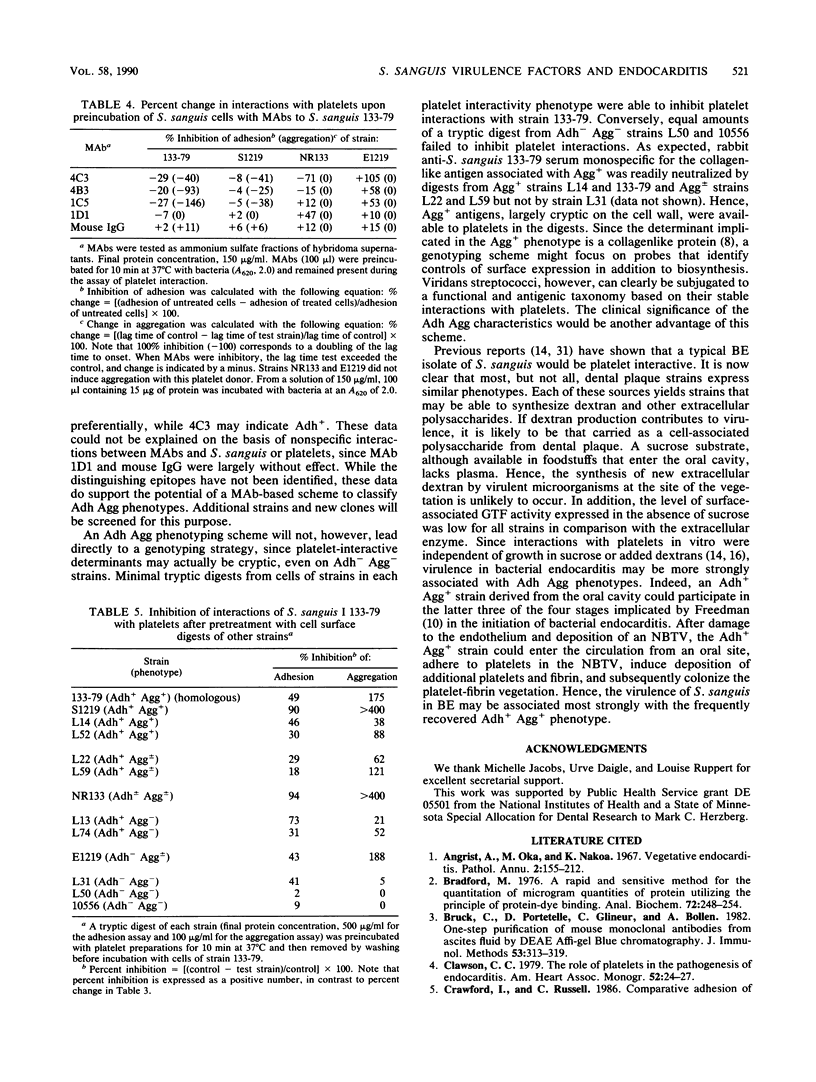
Certain strains of Streptococcus sanguis adhere (Adh+) selectively to human platelets and, in plasma, induce them to aggregate (Agg+) into in vitro thrombi. In this study, we examined 18 recent endocarditis and dental plaque isolates of microorganisms that were biotyped as S. sanguis for coexpression of platelet interactivity phenotypes with another possible virulence factor in bacterial endocarditis, dextran synthesis. Detectable production of extracellular glucosyltransferase ranged from 0.2 to 66 mU/mg of culture fluid for 10 representative strains tested. Production of extracellular or cell-associated glucosyltransferase, fructosyltransferase, and soluble or insoluble dextrans was not necessarily coexpressed with platelet interactivity phenotypes, since the levels of production of soluble and insoluble dextrans varied among representative Adh+ Agg+ and Adh- Agg- strains. Analysis of a second panel of 38 fresh dental plaque isolates showed that S. sanguis distributes in a reproducible manner into the possible phenotype groups. Strains with different platelet interactivity phenotypes were distinguished with a panel of four murine monoclonal antibodies (MAbs) raised against Adh+ Agg+ strain 133-79 and screened to rule out artifactual reactions with antigenic components in culture media. The MAbs reacted selectively with Adh+ Agg+ strains in a direct-binding, whole-cell, enzyme-linked immunosorbent assay and also inhibited their interactions with platelets. Analysis of minimal tryptic digests of many strains, including variants that failed to bind the MAbs, suggested that some noninteractivity phenotypes possess cryptic surface determinants. Since the ability to adhere to platelets and induce them to aggregate is relatively stable, these traits may be useful in a phenotyping scheme for these Lancefield nontypeable streptococci.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Elder B. L., Boraker D. K., Fives-Taylor P. M. Whole-bacterial cell enzyme-linked immunosorbent assay for Streptococcus sanguis fimbrial antigens. J Clin Microbiol. 1982 Jul;16(1):141–144. doi: 10.1128/jcm.16.1.141-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder B. L., Fives-Taylor P. Characterization of monoclonal antibodies specific for adhesion: isolation of an adhesin of Streptococcus sanguis FW213. Infect Immun. 1986 Nov;54(2):421–427. doi: 10.1128/iai.54.2.421-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C. A collagen-like immunodeterminant on the surface of Streptococcus sanguis induces platelet aggregation. J Immunol. 1987 May 15;138(10):3360–3366. [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman L. R. The pathogenesis of infective endocarditis. J Antimicrob Chemother. 1987 Sep;20 (Suppl A):1–6. doi: 10.1093/jac/20.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Freedman L. R., Valone J., Jr Experimental infective endocarditis. Prog Cardiovasc Dis. 1979 Nov-Dec;22(3):169–180. doi: 10.1016/0033-0620(79)90021-5. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gilmour M. N., Whittam T. S., Kilian M., Selander R. K. Genetic relationships among the oral streptococci. J Bacteriol. 1987 Nov;169(11):5247–5257. doi: 10.1128/jb.169.11.5247-5257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Cell-free released components of Streptococcus sanguis inhibit human platelet aggregation. Infect Immun. 1983 Oct;42(1):394–401. doi: 10.1128/iai.42.1.394-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lineberger L. T., De Marco T. J. Evaluation of transient bacteremia following routine periodontal procedures. J Periodontol. 1973 Dec;44(12):757–762. doi: 10.1902/jop.1973.44.12.757. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Ross R. S., Fishbein M. C., Siegel R. J. Nonbacterial thrombotic endocarditis: a review. Am Heart J. 1987 Mar;113(3):773–784. doi: 10.1016/0002-8703(87)90719-8. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Shimamura A., Tsumori H. Direct activity stains for glycosidase and glucosyltransferase after isoelectric focusing in horizontal polyacrylamide gel layers. Anal Biochem. 1982 Jul 1;123(2):276–284. doi: 10.1016/0003-2697(82)90446-8. [DOI] [PubMed] [Google Scholar]
- Ness P. M., Perkins H. A. Transient bacteremia after dental procedures and other minor manipulations. Transfusion. 1980 Jan-Feb;20(1):82–85. doi: 10.1046/j.1537-2995.1980.20180125046.x. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J Clin Invest. 1978 Oct;62(4):805–814. doi: 10.1172/JCI109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Effects of molecular weight of dextran on the adherence of Streptococcus sanguis to damaged heart valves. Infect Immun. 1980 Jul;29(1):1–7. doi: 10.1128/iai.29.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Krieger A. G., Schiller N. L., Gross K. C. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis. 1979 Nov-Dec;1(6):955–966. doi: 10.1093/clinids/1.6.955. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H., Rognum T. O., Brandtzaeg P. Performance testing of antigen-coated polystyrene microplates for ELISA measurements of serum antibodies to bacterial and dietary antigens. Acta Pathol Microbiol Immunol Scand C. 1985 Jun;93(3):117–123. doi: 10.1111/j.1699-0463.1985.tb02932.x. [DOI] [PubMed] [Google Scholar]
- Soberay A. H., Herzberg M. C., Rudney J. D., Nieuwenhuis H. K., Sixma J. J., Seligsohn U. Responses of platelets to strains of streptococcus sanguis: findings in healthy subjects, Bernard-Soulier, Glanzmann's, and collagen-unresponsive patients. Thromb Haemost. 1987 Apr 7;57(2):222–225. [PubMed] [Google Scholar]
- Terleckyj B., Shockman G. D. Amino acid requirements of Streptococcus mutans and other oral streptococci. Infect Immun. 1975 Apr;11(4):656–664. doi: 10.1128/iai.11.4.656-664.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Reyn C. F., Levy B. S., Arbeit R. D., Friedland G., Crumpacker C. S. Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med. 1981 Apr;94(4 Pt 1):505–518. doi: 10.7326/0003-4819-94-4-505. [DOI] [PubMed] [Google Scholar]
- Young S. E. Aetiology and epidemiology of infective endocarditis in England and Wales. J Antimicrob Chemother. 1987 Sep;20 (Suppl A):7–15. doi: 10.1093/jac/20.suppl_a.7. [DOI] [PubMed] [Google Scholar]



