Abstract
Periciliary fluid balance is maintained by the coordination of sodium and chloride channels in the apical membranes of the airways. In the absence of the cystic fibrosis transmembrane regulator (CFTR), chloride secretion is diminished and sodium reabsorption exaggerated. ClC-2, a pH- and voltage-dependent chloride channel, is present on the apical membranes of airway epithelial cells. We hypothesized that ClC-2 agonists would provide a parallel pathway for chloride secretion. Using nasal potential difference (NPD) measurements, we quantified lubiprostone-mediated Cl− transport in sedated cystic fibrosis null (gut-corrected), C57Bl/6, and A/J mice during nasal perfusion of lubiprostone (a putative ClC-2 agonist). Baseline, amiloride-inhibited, chloride-free gluconate-substituted Ringer with amiloride and low-chloride Ringer plus lubiprostone (at increasing concentrations of lubiprostone) were perfused, and the NPD was continuously recorded. A clear dose-response relationship was detected in all murine strains. The magnitude of the NPD response to 20 μM lubiprostone was −5.8 ± 2.1 mV (CF, n = 12), −8.1 ± 2.6 mV (C57Bl/6 wild-type, n = 12), and −5.3 ± 1.2 mV (AJ wild-type, n = 8). A cohort of ClC-2 knockout mice did not respond to 20 μM lubiprostone (n = 6, P = 0.27). In C57Bl/6 mice, inhibition of CFTR with topical application of CFTR inhibitor-172 did not abolish the lubiprostone response, thus confirming the response seen is independent of CFTR regulation. RT-PCR confirmed expression of ClC-2 mRNA in murine lung homogenate. The direct application of lubiprostone in the CF murine nasal airway restores nearly normal levels of chloride secretion in nasal epithelia.
Keywords: nasal potential difference, murine, prostone
cystic fibrosis (CF) is one of the most common autosomal recessive life-span shortening diseases that affects almost 30,000 individuals in the United States. CF results from mutations in the gene that encodes the membrane glycoprotein CFTR (cystic fibrosis transmembrane regulator). In CF, mutant CFTR impairs epithelial apical chloride secretion and fails to inhibit ENaC-mediated sodium reabsorption (15), leading to dehydrated viscous mucus that obstructs respiratory, gastrointestinal, and reproductive tracts (18). Nasal and bronchial airway epithelial cells also coexpress other apical chloride channels (2) that may be therapeutic targets in CF. ClC-2, a member of the pH- and voltage-activated chloride channel family, is highly expressed in fetal airways and reduced at birth (20, 21) similarly to CFTR (6, 28). Lipecka et al. (17) demonstrated significant expression of ClC-2 at the apex of ciliated cells in both rat and human airways. We (25) previously demonstrated that overexpression of ClC-2 corrected the chloride transport defect in immortalized CF airway cells. No human lung disease has yet been attributed to mutations in ClC-2; however, we previously demonstrated functional response to acidic pH in adult human nasal epithelia in vivo by nasal potential difference (NPD) consistent with ClC-2 (29). Therefore, we propose that pharmaceutical activation of ClC-2-mediated chloride transport may compensate for the chloride transport defect in CF. Several novel prostones or PGE series-derived molecules developed by Sucampo Pharmaceuticals (Bethesda, MD), including SPI-0211 (lubiprostone), target ClC-2 channels (4, 7). Lubiprostone was recently approved by the U.S. Food and Drug Administration for the treatment of idiopathic constipation (16). The proposed mechanism of action is stimulation of intestinal chloride channels and resultant fluid secretion into the gut.
In this paper, we hypothesized that activation of ClC-2 with topical lubiprostone in CF murine nasal airways would activate sufficient chloride secretion to restore near normal levels of epithelial chloride transport. CFTRtm1Unc-Tg(FABPCFTR)1Jaw/J, gut corrected bitransgenic, hereafter known as CFKO, wild-type C57Bl/6, and A/J mice underwent a modified NPD protocol designed to quantify the contribution of lubiprostone-mediated activation of chloride transport in murine airway epithelia. We also employed a transgenic ClC-2 knockout mouse as an important negative control. We demonstrated that chloride transport can be restored in CF mice with topical application of a putative ClC-2 agonist. Activation of ClC-2 persists in wild-type mice in the presence of CFTR inhibitor. Finally, using RT-PCR techniques, we examined whole lung homogenates of CFKO, C57Bl/6, A/J, and ClC-2 KO mice strains. We confirmed that ClC-2 mRNA is expressed in lungs from the three mouse models that responded to lubiprostone, but not in the ClC-2 knockout.
METHODS
Animals.
All studies were approved by the Johns Hopkins University Animal Care and Use Committee. The double transgenic CFKO mice (31) were obtained from the Pediatric Animal Core at Case Western Reserve University (JAX #002364). The fatty acid binding protein promoter drives human CFTR expression in the mouse gastrointestinal tract, which improves survival. C57Bl/6 (JAX #000664) and A/J (JAX #000646) strains of female mice were obtained from JAX Mice (Bar Harbor, ME). The ClC-2 null mice (22) were generated onsite from breeding pairs generously provided by Dr. James Melvin (Univ. of Rochester, Rochester, NY). Because we have previously demonstrated ClC-2 expression is downregulated after birth (21), only mature female mice, aged between 16 and 28 wk at the time of study, were employed. The weights of each cohort appear in the tables. All mice were maintained in the Johns Hopkins University mouse core facility until study in the laboratory. Mice that later underwent tissue harvest were euthanized by anesthetic overdose and cervical dislocation in accordance with university guidelines.
Genotyping.
ClC-2 null genotype was determined from murine tail DNA. Primers were forward 5′-ATGTATGGCCGGTACACTCAGGAACTC-3′, reverse 5′-ACACCCAGGTCCCTGCCCCAATCTGG-3′, and reverse 5′-CCTGGAAGGTGCCACTCCCACTGTCC-3′ to amplify a 380-bp region. A 25-μl reaction mixture containing diluted genomic DNA, 10 μM dNPT (dATP, dGTP, dCTP, and dTTP), primers, MgCl, Taq DNA polymerase, and 10× buffer from Qiagen PCR kit was assembled. The DNA was initially denatured at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 30 s, primer annealing at 68°C for 15 s, and extension at 72°C for 30 s. In the last cycle, extension at 72°C was performed for 7 min. Positive, negative, and heterozygous controls were included with each run. Amplified DNA underwent electrophoresis on a 1.5% ethidium bromide-stained agarose gel along with DNA markers.
CFKO PCR was performed on mouse tail DNA using primers forward 5′-GAGAACTGGAAGCTTCAGAGG-3′, reverse 5′-TCCATGTAGTGGTGTGAACG-3′, and neo 5′-TCCATCTTGTTCAATGGCC-3′ to amplify a 357-bp region. Amplification of the gene was done in a 20-μl reaction mixture containing diluted genomic DNA, 10 μl of REDExtract-N-Amp Readymix (Sigma, St. Louis, MO), 4 μl of sterile H2O, and 1 μl each forward, neo, and reverse primers. DNA was initially denatured at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 30 s, primer annealing at 58°C for 45 s, and extension at 72°C for 45 s. In the last cycle, extension at 72°C was allowed for 2 min. Positive, negative, and heterozygous controls were run with each experiment. Amplified DNA underwent electrophoresis on a 1.5% ethidium bromide-stained agarose gel along with DNA molecular mass markers and was photographed under UV transillumination.
NPD.
All mice were anesthetized with an intraperitoneal injection of a ketamine and xylazine mixture (100 μg/10 μg per gram body wt). After reaching a steady plane of anesthesia (absent toe pinch), oral intubation was performed beginning with direct visualization of the vocal folds with an otoscope with a 2-mm speculum (model no. 20200; Welch Allyn, Skaneateles Falls, NY). A flexible guide wire was advanced through the vocal folds, and a 20 gauge (GA) intravenous catheter was passed over the wire (BD Medical, Franklin, NJ). Spontaneous ventilation via a breathing circuit containing free flow oxygen at 0.25 ml/min occurred throughout the procedure. Mice were placed head down on a 15° incline. Body temperature was monitored rectally (TH-5; Physiotemp, Clifton, NJ) and maintained with a phase change heating pad (Braintree Scientific, Braintree, MA) and heat lamp as needed. NPD measurements were undertaken using a modification of methods originally described by Grubb et al. (10). A high impedance voltmeter (World Precision Instruments, Sarasota, FL) was connected by silver-chloride pellet electrodes to an exploring nasal bridge and a reference subcutaneous bridge. The nasal bridge, a single polyethylene tube (PE10, 0.28-mm ID; Clay-Adams, BD, Sparks, MD), was pulled to approximately one-half its original diameter and cut at an acute angle to maximize surface area. The resulting orifice was ∼0.7 mm in diameter. The tubing was marked at 3 and 5 mm from the tip. The tubing was inserted into the naris to 3 mm, and, after steady state, was advanced to the point of maximum voltage, but never beyond 5 mm. The subcutaneous bridge was a 25 GA Butterfly (Abbott, Chicago, IL) needle containing Ringer solution inserted subcutaneously in the right abdominal wall. Each solution was warmed to 37°C and perfused to the naris for at least 3 min at 8 μl/min using a perfusion pump. Following the procedure, the oral cavity was gently suctioned of perfusate, and the mice were recovered in their cages.
Reagents.
Baseline Ringer (solution 1) was composed of 135 mM NaCl, 2.25 mM CaCl2 × 2H2O, 1.2 mM MgCl2 × 6H2O, 2.4 mM K2HPO4, and 0.4 mM KH2PO4, pH 7.4. The 100 μM amiloride (solution 2) was generated by adding amiloride directly to solution 1. The amiloride containing zero chloride (solution 3) solution was a gluconate substituted, balanced Ringer produced by sequential substitution with 135 mM sodium gluconate, 1.2 mM MgSO4 × 7H2O, 2.2 mM calcium gluconate, 2.4 mM K2HPO4, and 0.4 mM K2H2PO4 adjusted to pH 7.4. The lubiprostone-containing solutions (solutions 4–6) utilized stock drug supplied as aliquots of 2 mM lubiprostone in DMSO (Sigma, Japan) as well as a stock bottle of the same DMSO [provided by Materials Transfer Agreement with Sucampo Pharmaceuticals (Bethesda, MD)]. Lubiprostone was added to a stock of solution 3 and underwent serial dilution to final 1, 10, and 20 μM concentrations. The resulting DMSO concentration was less than 1% by volume. The pH remained >7.1 following addition of lubiprostone to stock solutions. Isoproterenol (solution 7) containing solution was prepared fresh with isoproterenol hydrochloride (1:5,000) added to a stock of solution 3, to a final concentration of 100 μM and protected from light. The 100 μM ATP (solution 8) was diluted in solution 7 just before use. The CFTR channel inhibitor-172 (24) (CalBioChem, San Diego, CA) was added to a stock of solution 3 to reach 50 μM concentration with final DMSO concentration <0.1%. Control solutions were prepared with 1% DMSO (Sigma). Solutions were sterilized through a 0.22-μm filter (Millipore, Bedford, MA).
Data collection and analysis.
Voltmeter data (in mV) were recorded by a PowerLab A/D converter connected to a PC running Chart software at 20 samples per second (AD Instruments, Colorado Springs, CO). A minimum of 400 data points (∼20 s) was used to calculated the mean voltage values for Ringer, Δ Cl−, (the voltage difference between peak low Cl− mV and peak amiloride mV), and Δ Lubi (the voltage difference between peak lubiprostone mV and peak zero Cl− mV). Statistical analysis was performed with SPSS software for Windows (SPSS, Chicago, IL) and included paired t-test, one-way ANOVA, and Tukey post hoc multiple comparisons. Significance was set at P < 0.05 for all tests. Voltage tracings were generated by exporting raw data from Chart software at a reduction ratio of 1:25 and importing to Microsoft Excel.
Total RNA isolation and RT-PCR.
Lungs were harvested by a ventral chest incision. The trachea was identified, and then using careful cranial-to-caudal dissection, the trachea, heart, and lung were removed as one unit. Each lung was then divided at the main stem bronchus, immediately placed in RNAlater (Ambion, Austin, TX), and stored at −80°C for later homogenization. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Following isolation, the RNA was purified twice using RNeasy spin columns with an on-column DNase I treatment to remove genomic DNA (Qiagen, Valencia, CA). A one-step RT-PCR reaction to detect ClC-2 mRNA was performed using the SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen). Prevalidated Quantitect primers were used for the specific amplification of murine ClC-2 (Qiagen, qt00141876, product size: 90 bp), and β-actin control primers were (+) 5′-TGGACTTCGAGCAAGAGATG-3′ and (−) 5′-GAAGGAAGGCTGGAAGAGTG-3′ (product size: 137 bp). A touchdown PCR approach was used for the ClC-2 amplification and included a 5-min incubation at 50°C (cDNA synthesis) followed by 10 cycles with annealing temperatures of 65–60°C (decreasing 0.5° per cycle) and 30 cycles at 59°C. The one-step amplification of actin included a 5-min incubation at 50°C followed by 35 cycles at 60°C. A no-RT control was performed by replacing SuperScript III with 5 units of Taq polymerase (Invitrogen). Twenty microliters of each PCR reaction was separated on a 2% agarose gel, and products were examined under UV fluorescence.
RESULTS
Baseline electrical properties of murine nasal epithelium resemble human NPD responses.
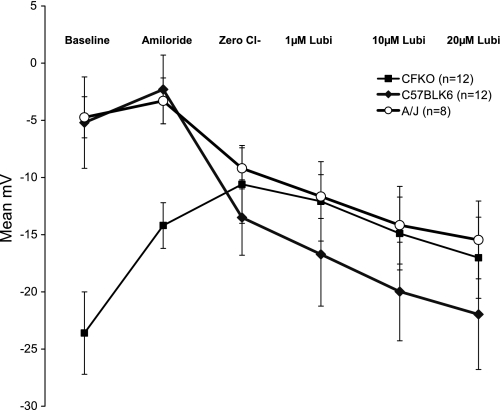
The baseline potential difference (PD) during perfusion with solution 1 (Ringer) is primarily a measure of the sodium potential difference across the nasal epithelium. The PDs of both non-CF strains, C57Bl/6 (n = 12), −5.2 ± 4.2 mV, and A/J (n = 8), −4.74 ± 1.8 mV, are significantly less polarized than the CFKO PD (−23.6 ± 3.6 mV), P < 0.05 (Table 1). Although we observed slightly lower baseline voltages than previously reported in these strains of mice (5, 10), the expected depolarization with amiloride blockade and subsequent hyperpolarization with zero chloride and isoproterenol solutions faithfully replicated both the human NPD waveform and previous reports in the two wild-type strains of mice. Depolarization induced with solution 2 (amiloride) was significantly greater in the CF mice than either wild-type strain, P < 0.05 (Table 1). Replacement of chloride with gluconate in the continued presence of amiloride (solution 3) increases the chemical gradient for chloride exit, and the PD will hyperpolarize if chloride channels are open. Multiple chloride channel species may participate, including the calcium-activated chloride channel CaCC, the outwardly rectifying chloride channel ORCC, ClC species, and CFTR, if present. Wild-type C57Bl/6 mice (n = 12) repolarized to a mean of −13.5 (±2.3) mV, whereas CFKO mice (n = 12) continued to depolarize to a mean of −10.6 (±3.1) mV, which is consistent with other reports. The A/J mouse strain has been reported to demonstrate lower polarization compared with C57Bl/6 in response to zero Cl− solution, yet shares a nearly identical forskolin response (5). We also observed a lower mean zero Cl− response in the A/J mice of −9.2 (±1.8) mV (vs. −13.5 mV), suggesting a possible reduction in the population of one or more chloride channel species in A/J mice.
Table 1.
Effect of topical lubiprostone on nasal potential difference in 3 mouse strains
| Genotype (n) | Weight, g | Baseline, mV | Amiloride, mV | Zero Cl−, mV | Δ Lubi 1 μM, mV | Δ Lubi 10 μM, mV | Δ Lubi 20 μM, mV | Δ Iso, mV |
|---|---|---|---|---|---|---|---|---|
| CFKO (12) | 24.0±3.1 | −23.6±3.6 | −14.2±3.0 | −10.6±3.4 | −1.2±1.1 | −3.7±1.5 | −5.8±2.1 | |
| Significance | P = 0.01 | P = 0.0001 | P = 0.0001 | |||||
| C57Bl/6 (12) | 23.6±2.9 | −5.2±4.0 | −2.3±2.0 | −13.5±3.3 | −3.4±2.5 | −6.2±2.1 | −8.1±2.6 | −9.7±3.9 |
| Significance | P = 0.001 | P = 0.0001 | P = 0.0001 | P = 0.001* | ||||
| A/J (8) | 21.2±2.3 | −4.7±1.8 | −3.3±2.0 | −9.2±1.8 | −2.2±1.6 | −4.1±1.2 | −5.3±1.2 | |
| Significance | P = 0.005 | P = 0.0001 | P = 0.0001 |
| Genotype (n) | Weight, g | Baseline, mV | Amiloride, mV | Zero Cl−, mV | 1% DMSO, mV | Zero Cl−, mV | 1% DMSO, mV |
|---|---|---|---|---|---|---|---|
| C57Bl/6 (3) | 22.8±1.9 | −3.8±0.5 | −0.8±0.6 | −12.3±1.8 | −12.2±2.0 | −12.2±1.8 | −12.1±1.9 |
Data are means ± SD. Δ Zero Cl− is the difference between the maximal change in lubiprostone (Lubi) and the stable amiloride-inhibited voltage. Δ Lubi 1, 10, or 20 μM is the difference between the maximal change in Lubi and the stable zero Cl− voltage. Δ Iso is the difference between the maximal change in Iso and the stable zero Cl− potential difference (PD).
Paired t-test for Lubi 20 μM and Iso was significant at P = 0.001. The DMSO vehicle control data set is at the bottom.
Lubiprostone activates chloride transport in a dose-dependent relationship in wild-type and CF mice.
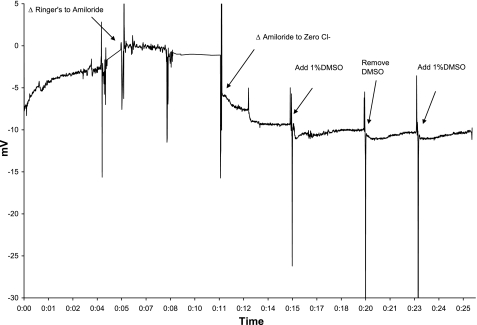
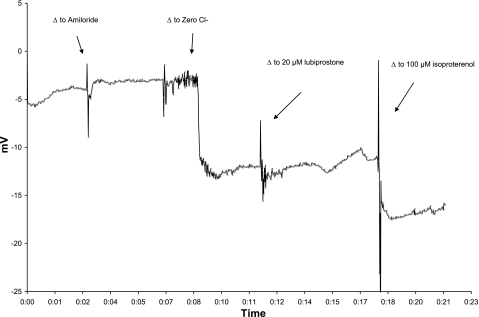
There has been controversy regarding the subcellular localization of ClC-2 protein, particularly in gastrointestinal epithelia (23). We sought to confirm an apical location in respiratory epithelia by measuring activation of ClC-2 through a non-absorbable putative ClC-2 agonist, lubiprostone. Nanomolar concentrations of lubiprostone in solution 4 that had shown activity in vitro (7) did not lead to detectable hyperpolarization in murine NPD (data not shown). However, 20 μM concentrations of lubiprostone led to consistently significant polarization of the NPD (Table 1 and Fig. 1) in all mouse strains. Separate observations in two CFKO mice showed that following topical lubiprostone, reperfusion with non-lubiprostone containing solution 3 resulted in a slow depolarization, suggesting that the agent must remain in contact with the channel to activate chloride conductance (Fig. 2). The vehicle control for lubiprostone (1% DMSO in solution 3) did not affect the PD (n = 3) in C57Bl/6 mice tested (Fig. 3). Perfusion of isoproterenol (solution 7) after the lubiprostone escalation in C57Bl/6 mice resulted in further repolarization Table 1, which was significant (P = 0.01) compared with the voltage generated by 20 μM lubiprostone.
Fig. 1.
Effect of transgenic mouse strain on the nasal potential difference (NPD) response to lubiprostone (Lubi). The standard NPD protocol was modified to replace the isoproterenol perfusion with a dose escalation of lubiprostone. Data are expressed as mean millivolts of potential difference (y-axis) in each perfusate (x-axis). Error bars mark the SD. CFTRtm1Unc-Tg(FABPCFTR)1Jaw/J, gut corrected bitransgenic (CFKO), n = 12; C57Bl/6, n = 12; A/J, n = 8. The A/J and CFKO strains have nearly identical lubiprostone responses.
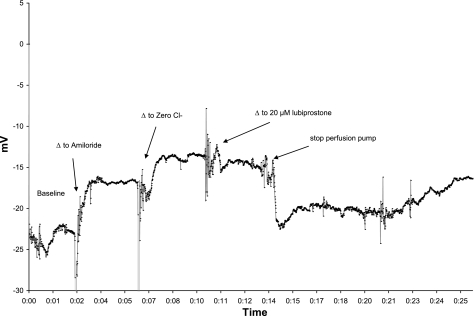
Fig. 2.
Lubiprostone-mediated chloride transport is sustained after 4 min of perfusion (x-axis units in hours:minutes elapsed) but runs down when perfusion of the compound is halted. The NPD in a CFKO mouse (1 of 2 experiments) is shown to illustrate that perfusion with lubiprostone sustained chloride secretion (∼10 min) before diminishing.
Fig. 3.
Effect of 1% DMSO vehicle control on an NPD in C57Bl/6 mouse. DMSO was added to the zero Cl− (solution 3) and run for at least 3 min. DMSO-free solution 3 was then applied for 3 min, and then 1% DMSO in solution 3 was reapplied. No significant voltage difference was seen in 3 mice tested. X-axis units in hours:minutes elapsed.
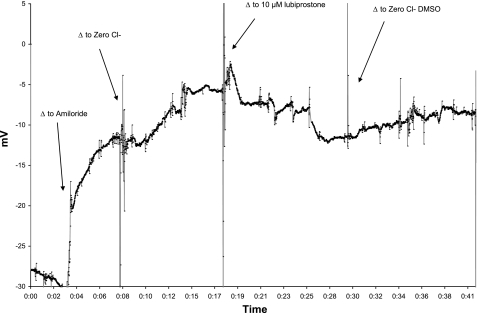
Mean lubiprostone responses in C57Bl/6 mice (n = 12) were: −3.4 (± 2.5) mV at 1 μM, −6.2 (± 2.1) mV for 10 μM, and −8.1 (± 2.6) mV for 20 μM. Analysis by one-way ANOVA was significant at P = 0.0001; however, post hoc Tukey analysis demonstrated significance only for the 10- and 20-μM lubiprostone concentrations compared with zero Cl−. In CFKO mice (n = 12), the mean lubiprostone responses were: 1.2 (±1.1) mV for 1 μM, −3.7 (±1.5) mV for 10 μM, and −5.8 (±2.1) mV for 20 μM. Analysis by one-way ANOVA was significant at P = 0.0001; however, post hoc Tukey analysis demonstrated significance only for the 10- and 20-μM lubiprostone concentrations compared with zero Cl−. Mean lubiprostone responses in A/J mice (n = 8) are included in Table 1. Analysis by one-way ANOVA was significant at P = 0.0001, and post hoc Tukey analysis demonstrated significance for all three lubiprostone concentrations compared with zero Cl− (P = 0.035, P = 0.0001, P = 0.0001). In data not shown, CFKO mice generated increased response at a higher lubiprostone concentration (100 μM). Separate observations in two CFKO mice showed that removal of lubiprostone led to rundown of chloride transport over 10 min, again suggesting that the compound must remain in contact with the epithelium to continue the response (Fig. 4).
Fig. 4.
Effect of washout of lubiprostone. X-axis units in hours:minutes elapsed. Lubiprostone (10 μM) (solution 5) supplied to a CFKO mouse produces the characteristic hyperpolarization (n = 2). However, substitution with zero Cl− (solution 3) containing 1% DMSO (vehicle control) results in a loss of effect or washout. This can be repeated several times in the same mouse.
Topical lubiprostone has no effect on NPD in ClC-2 null mice.
If the majority of the chloride transport response elicited by topical lubiprostone is through ClC-2, then mice lacking ClC-2 should not have a significant response to lubiprostone. We challenged six ClC-2 (n = 6) null mice by NPD with 20-μM concentrations of lubiprostone and observed no significant additional chloride transport following the low chloride (solution 3) (Table 2 and Fig. 5). These mice exhibited a significant response to isoproterenol (solution 7) (Table 2), suggesting CFTR was further activated following lubiprostone perfusion. One-way ANOVA was significant at P = 0.01. Tukey post hoc analysis was significant when comparing 20 μM lubiprostone and isoproterenol (P = 0.013).
Table 2.
Absence of chloride response to 20 μM lubiprostone in ClC-2 knockout mice
| Genotype (n) | Weight, g | Baseline, mV | Amiloride, mV | Zero Cl−, mV | Lubi 20 μM, mV | Isoproterenol 100 μM, mV | Δ Lubi 20 μM, mV | Δ Iso, mV |
|---|---|---|---|---|---|---|---|---|
| ClC-2 knockout (6) | 25.4±2.1 | −3.2±1.0 | −0.9±0.7 | −11.0±2.5 | −10.3±2.8 | −15.9±3.4 | 0.7±2.0 | −4.6±1.6 |
| P = 0.413* | P = 0.001* |
Data are means ± SD.
Paired t-test was not significant between zero Cl− PD and lubiprostone (P = 0.413); however, it was significant between zero Cl− PD and Iso PD (P = 0.001). Data are consistent with the absence of ClC-2 and the presence of CFTR.
Fig. 5.
Lubiprostone challenge in ClC-2 knockout mouse. X-axis units in hours:minutes elapsed. After baseline NPD was recorded, amiloride superfusion led to a modest depolarization consistent with the presence of wild-type CFTR. Shortly after switching to solution 3, chloride transport was induced, consistent with the presence of functional CFTR. Addition of 20 μM lubiprostone did not activate further chloride transport, consistent with the absence of ClC-2. Confirmation of CFTR function occurred with the subsequent exposure to 100 μM isoproterenol in solution 7.
CFTR is not required for lubiprostone-activated chloride transport.
Lubiprostone stimulates chloride transport in CFTR null mice; however, the magnitude of the response is less than in wild-type C57Bl/6. C57Bl/6 mice respond to both lubiprostone and isoproterenol, suggesting that CFTR can respond to lubiprostone at high doses (4) or increase the response of other Cl− channels to the compound. To confirm that functional CFTR is not an absolute requirement for lubiprostone activity in the C57Bl/6 strain, CFTR inhibitor-172 was included in solution 3 in a subset of experiments to inhibit CFTR before application of lubiprostone. In C57Bl/6 mice (n = 7), topical inhibition of CFTR did not block subsequent lubiprostone activity (Table 3). We observed a lack of polarization of the PD with the addition of CFTR inhibitor-172 to the zero Cl− (solution 3) perfusate (Table 3). In earlier reports of murine NPDs, CFTR inhibitor-172 was applied after hyperpolarization was induced with chloride-free Ringer and resulted in only minor depolarization (24). This demonstrates in vivo a requirement for CFTR Cl− activation potential being present for apical Cl− transport in response to a Cl− ion gradient even if CFTR is not the principal anion channel in mouse NPDs as the authors suggest. Further addition of 100 μM ATP to the final perfusate resulted in continued polarization (Table 3), suggesting further activation of calcium-activated channels.
Table 3.
Inhibition of CFTR does not abolish the chloride transport activated by lubiprostone or ATP
| Genotype (n) | Weight, g | Baseline, mV | Amiloride, mV | Zero Cl−, mV +50 μM CFTRinh 172* | Δ Lubi 20 μM, mV | Δ 100 μM ATP, mV |
|---|---|---|---|---|---|---|
| C57Bl/6 (7) | 24.7±0.5 | −4.0±0.71 | −1.6±0.51 | −0.7±0.84 | −6.5±1.8 | −10.0±3.7 |
| P = 0.0001 | P = 0.003 | |||||
| P = 0.007† |
C57Bl/6 mice (n = 7) underwent NPD testing with zero Cl− solution containing CFTRinh 172 before lubiprostone stimulation.
Application of CFTR inhibitor before the zero Cl− response resulted in near isoelectric PD. The value Δ Lubi 20 μM reflects the difference of the maximal change induced by lubiprostone and the stable zero Cl− value. The Δ 100 μM ATP value is the difference of maximal ATP response and the stable zero Cl− value, which represents the calcium-activated chloride conductance. Significance was tested by paired t-test for each response compared with zero Cl− containing CFTRinh 172.
Paired t-test result for lubiprostone and ATP.
ClC-2 mRNA is expressed in murine whole lung homogenates.
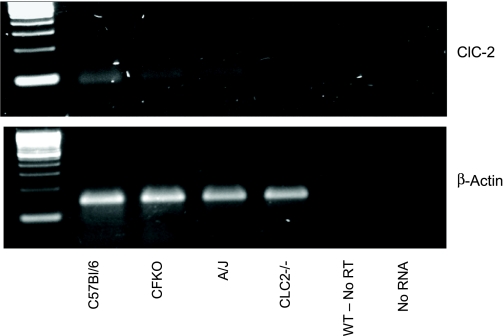
Figure 6 demonstrates the expression of ClC-2 mRNA (157 bp) by RT-PCR in mature mice of all three genotypes that had a positive response to lubiprostone. The ClC-2 knockout mouse lacked detectable ClC-2 mRNA expression.
Fig. 6.
Top: photograph of RT-PCR gel examining ClC-2 mRNA (90 bp). Bottom: β-actin control (130 bp).
DISCUSSION
Physiologically active concentrations of lubiprostone are in the micromolar range when applied to the murine nasal luminal surface.
Previous studies of lubiprostone in cultured cell systems suggested that chloride secretion could be induced with nanomolar concentrations in the cell medium (7, 19). We anticipated a similar dose-response profile in the murine nose; however, in our study of murine nasal airways in vivo, micromolar concentrations were required. Cuppoletti et al. (7) employed T84 colon carcinoma cells, which are known to have significantly increased expression of ClC-2 mRNA compared with the immortalized human bronchial epithelial cell line IB3-1 (25). T84 monolayers were treated with 1-ethyl-2-benzimidazolinone followed by permeabilization of the basal lateral membrane with nystatin, eliminating basolateral potassium exchange transport as a limit to apical chloride secretion. In intact airway epithelial Calu-3 monolayers, lubiprostone stimulated short-circuit current at roughly twice the maximum concentration of the T84 experiments (19). However, the location and amount of expression of ClC-2 in Calu-3 is unclear (8). The T84 and Calu-3 cell lines employed by the previous authors are both primary secretory epithelia, without detectable levels of sodium absorption via ENaC. Mouse respiratory epithelium has active ENaC, and chloride secretion detection by NPD requires the presence of amiloride. Amiloride reduces the influx of sodium so that a repolarization due to chloride exit is detectable as a change in NPD in the negative direction, and is not necessary during measurements of whole cell currents in vitro. However, as we have shown experimentally, the nasal potential difference in CFKO following amiloride is not zero and remains significantly polarized compared with non-CF mice, suggesting a higher gradient and more drug needed to make effective measurements. We do not expect amiloride will be required therapeutically and only use it here to make the electrical measurements. In fact, the opposite therapeutic maneuver, aerosolization of hypertonic sodium chloride to the lumen, has been shown to improve lung function in CF patients. In that study, amiloride added no further benefit (9). Furthermore, not all tissues affected in CF depend on epithelial sodium reabsorption, making lubiprostone potentially useful in CF gut-related distal intestinal obstruction syndrome. To date, lubiprostone has only been critically evaluated in adult functional constipation (12).
The nasal potential difference measurement is a dynamic process requiring constant perfusion of drug to maintain electrical contact with the epithelium. We demonstrated that when lubiprostone perfusion is removed from the NPD circuit (Fig. 4), the effect runs down. Additionally, time of drug exposure was severely limited in our model due to constraints of multiple doses and limited duration of animal anesthesia.
Although the precise mechanism of action of lubiprostone is unclear, MacVinish et al. (19) noted that ion channel properties, when studied in vitro, often differ when assayed in vivo. Variability is introduced when the drug must be solubilized at the final concentrations before placement in the syringe and tubing. It is possible that solubilization, even with DMSO, is not identical in perfusion solution as cell culture medium to the process used in vitro. A reduction in potency is also expected when moving from a single cell layer in vitro to a living host in vivo with intact airway defenses. An editorial by Widdicombe (30) reviews the pitfalls of airway drug delivery, including penetration and diffusion of the airway surface layer as well as drug-mucin interactions, supporting the observations that higher concentrations are required for the mouse nasal airway. Our data clearly demonstrate a dose-related response to topical lubiprostone in three distinct mouse strains, suggesting it may be useful as an airway hydration agent.
CFTR is not required for ClC-2-mediated chloride secretion.
Lubiprostone had no significant effect on chloride secretion in ClC-2 null mice, and inhibition of CFTR in C57Bl/6 mice with CFTR inhibitor-172 did not prevent lubiprostone-mediated repolarization of the NPD. However, the magnitude of the chloride secretory change in NPD in CFKO mice was lower than in C57Bl/6 mice, but similar to the A/J strain. This suggests a strain or genetic variation in zero Cl− PD (5) and subsequent strain-related lubiprostone responses. The CFKO double transgenic CF mouse (31) used here is a robust animal; however, it is in a mixed-strain background (mostly C57Bl/6 and SVJ129), making differences due to strain difficult to quantify. Other explanations for these observed voltage differences might include: 1) lubiprostone-induced chloride secretion may lead to increased cellular cAMP and indirect stimulation of CFTR in wild-type mice. However, in our data, when CFTR was inhibited before 20 μM lubiprostone, the response was similar: −7.4 mV vs. −8.1 mV without CFTR inhibition in C57Bl/6 mice. Also, Bao et al. (4) have demonstrated that lubiprostone does not lead to increased cAMP. Lubiprostone might induce calcium entry into the cell, activating the CaCC pathway; however, to our knowledge this has not been shown. We are actively examining ClC-2-specific antagonists (27) to examine lubiprostone channel selectivity. 2) Our lung homogenate data suggest reduced expression of ClC-2 mRNA in CFKO and A/J mice by PCR compared with the C57Bl/6 mice; however, quantitative studies would be required to verify this hypothesis. 3) Lubiprostone-induced chloride secretion might further downregulate ENaC activity in the CFKO mouse, thus blunting the negative change. This last explanation would be desirable in CF but would require additional experiments to confirm the effect on ENaC. König et al. (13) demonstrate inhibition of ENaC in oocytes by coexpression of ClC-0, as well as high extracellular Na+ and Cl− in partially permeabilized oocytes. They conclude that inhibition of ENaC is not specific to CFTR and seems to be mediated by intracellular Cl− levels. Bachhuber et al. (3) showed that activation of ClC-0 in oocytes increased intracellular Cl− concentration leading to ENaC inhibition. Kunzelmann (14) had similar findings. Mo and Wills (19a) concluded coexpression of ClC-5 and ENaC in oocytes resulted in decreased trafficking activity of ENaC.
Continuous exposure to lubiprostone appears to be desirable since either removal of lubiprostone from the nasal perfusion or washout led to rundown in the chloride secretory PD (Figs. 2 and 4). Lubiprostone is not absorbed from the GI tract when given for idiopathic constipation, and, therefore, is not likely absorbed through the airways either. Airway delivery of a prostone for CF might benefit from a slow release formulation or dry powder aerosol to sustain the effect in the airway lumen.
ClC-2 is expressed in tissues affected by CF.
Given the coexpression of CFTR and ClC-2 in airways and gastrointestinal epithelia, including the biliary tract (17, 26), specific ClC-2 agonists that are under development for liver and biliary tract disease (1) might also have some utility in CF. The NPD is widely accepted as a surrogate marker of tracheal and bronchial ion transport. However, measurement of ion transport in other affected tissues is much more difficult, and with the exception of the sweat chloride test, there are no surrogate measures for CFTR function in other locations in the body. CFTR is normally expressed in the sweat gland secretory coil and duct, the sinuses, airway submucosal glands, liver, reproductive tract, and small intestines. Aerosol delivery would only assist the lung epithelium and possibly penetrate to the submucosal glands, but would not treat sweat glands, the sinuses, or gastrointestinal tracts. Topical delivery of a ClC-2 agonist would be inaccessible in tissues such as the pancreas, liver, where ClC-2 is expressed in the biliary epithelium, and the small intestines (26). Realistically, the pancreas and male reproductive tract are likely to be resistant to therapies that increase chloride secretion because the damage from dysfunctional CFTR begins early in utero and results in irreversible structural changes before birth. Fortunately, systemic delivery of a prostone is feasible. One example, SPI-8811, is a novel prostone developed by Sucampo Pharmaceuticals for treatment of biliary cirrhosis via activation of ClC-2 (1). This investigational agent has oral bioavailability and has been studied in a phase I trial in CF.
ClC-2 function is not required for normal lung development or airway defense.
ClC-2 mutations have been identified in people suffering from idiopathic generalized seizures (11). These people do not have a history of lung disease. In our experience, the ClC-2 knockout mouse does not develop these seizures or spontaneous lung disease; however, the male mice are sterile. We have previously shown in the rat that ClC-2 is abundant in fetal lung airways, and, that in the perinatal period, there is a transition from predominantly chloride secretion to sodium absorption across airway epithelial cells (21). ClC-2 immunoreactivity is strongly positive in the fetal lung and is markedly downregulated at birth. The fetal lung liquid is acidic, and acidic pH on the luminal surface of fetal airway cells activates chloride secretion. Thus, it is likely that ClC-2 contributes to the driving force for lung liquid generation in utero. Surprisingly, the ClC-2 knockout mouse undergoes normal lung development, probably due to the presence of multiple alternative chloride channel conductance pathways including the CFTR, the ORCC, and the CaCC, at a minimum.
In summary, our data demonstrate lubiprostone mediated chloride channel activation in murine nasal epithelium that is independent of the function of CFTR. This work shows in vivo the potential of prostones as a new therapeutic class for cystic fibrosis.
GRANTS
K. MacDonald was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant T32-HL-72748 and a Cystic Fibrosis Foundation Fellowship Grant. P. Zeitlin was supported by NHLBI Grant R01-HL-59410.
Acknowledgments
We thank Eric Brownhill and Steve Mazur for excellent technical assistance. Lubiprostone was provided by Sucampo Pharmaceuticals (Bethesda, MD) under a Materials Transfer Agreement with Johns Hopkins University.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Sucampo Pharmaceuticals. In the Pipeline (Online). http://www.sucampo.com/inthepipeline.html [2008].
- 2.Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol Lung Cell Mol Physiol 263: L1–L14, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Bachhuber T, Konig J, Voelcker T, Murle B, Schreiber R, Kunzelmann K. Cl− interference with the epithelial Na+ channel ENaC. J Biol Chem 280: 31587–31594, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G234–G251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady KG, Kelley TJ, Drumm ML. Examining basal chloride transport using the nasal potential difference response in a murine model. Am J Physiol Lung Cell Mol Physiol 281: L1173–L1179, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Broackes-Carter FC, Mouchel N, Gill D, Hyde S, Bassett J, Harris A. Temporal regulation of CFTR expression during ovine lung development: implications for CF gene therapy. Hum Mol Genet 11: 125–131, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cuppoletti J, Tewari KP, Sherry AM, Kupert EY, Malinowska DH. ClC-2 Cl− channels in human lung epithelia: activation by arachidonic acid, amidation, and acid-activated omeprazole. Am J Physiol Cell Physiol 281: C46–C54, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 354: 241–250, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca2+-mediated Cl− secretion in nasal epithelia of CF mice. Am J Physiol Cell Physiol 266: C1478–C1483, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Haug K, Warnstedt M, Alekov AK, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H, Heils A. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet 33: 527, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol 103: 170–177, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Konig J, Schreiber R, Voelcker T, Mall M, Kunzelmann K. The cystic fibrosis transmembrane conductance regulator (CFTR) inhibits ENaC through an increase in the intracellular Cl− concentration. EMBO Rep 2: 1047–1051, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunzelmann K ENaC is inhibited by an increase in the intracellular Cl− concentration mediated through activation of Cl− channels. Pflügers Arch 445: 504–512, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Kunzelmann K, Schreiber R, Nitschke R, Mall M. Control of epithelial Na+ conductance by the cystic fibrosis transmembrane conductance regulator. Pflügers Arch 440: 193–201, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Lacy BE, Campbell Levy L. Lubiprostone: a chloride channel activator. J Clin Gastroenterol 41: 345–351, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 282: C805–C816, 2002. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald KD, McKenzie KR, Zeitlin PL. Cystic fibrosis transmembrane regulator protein mutations: ‘class’ opportunity for novel drug innovation. Pediatr Drugs 9: 1–10, 2007. [DOI] [PubMed] [Google Scholar]
- 19.MacVinish LJ, Cope G, Ropenga A, Cuthbert AW. Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol 150: 1055–1065, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Mo L, Wills NK. ClC-5 chloride channel alters expression of epithelial sodium channel (ENaC). J Membr Biol 202: 21–37, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Murray CB, Chu S, Zeitlin PL. Gestational and tissue-specific regulation of C1C-2 chloride channel expression. Am J Physiol Lung Cell Mol Physiol 271: L829–L837, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol 12: 597–604, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Nehrke K, Arreola J, Nguyen HV, Pilato J, Richardson L, Okunade G, Baggs R, Shull GE, Melvin JE. Loss of hyperpolarization-activated Cl− current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem 277: 23604–23611, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci 118: 4243–4252, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Salinas DB, Pedemonte N, Muanprasat C, Finkbeiner WF, Nielson DW, Verkman AS. CFTR involvement in nasal potential differences in mice and pigs studied using a thiazolidinone CFTR inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L936–L943, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Schwiebert EM, Cid-Soto LP, Stafford D, Carter M, Blaisdell CJ, Zeitlin PL, Guggino WB, Cutting GR. Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells. Proc Natl Acad Sci USA 95: 3879–3884, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356: 57, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Thompson CH, Fields DM, Olivetti PR, Fuller MD, Zhang ZR, Kubanek J, McCarty NA. Inhibition of ClC-2 chloride channels by a peptide component or components of scorpion venom. J Membr Biol 208: 65–76, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Tizzano EF, Chitayat D, Buchwald M. Cell-specific localization of CFTR mRNA shows developmentally regulated expression in human fetal tissues. Hum Mol Genet 2: 219–224, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Uwaifo O, Bamford P, Zeitlin PL, Blaisdell CJ. Acidic pH hyperpolarizes nasal potential difference. Pediatr Pulmonol 41: 151–157, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Widdicombe JG Airway liquid: a barrier to drug diffusion? Eur Respir J 10: 2194–2197, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 266: 1705–1708, 1994. [DOI] [PubMed] [Google Scholar]